Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome
Abstract
:1. Introduction
2. Results
2.1. Study Population
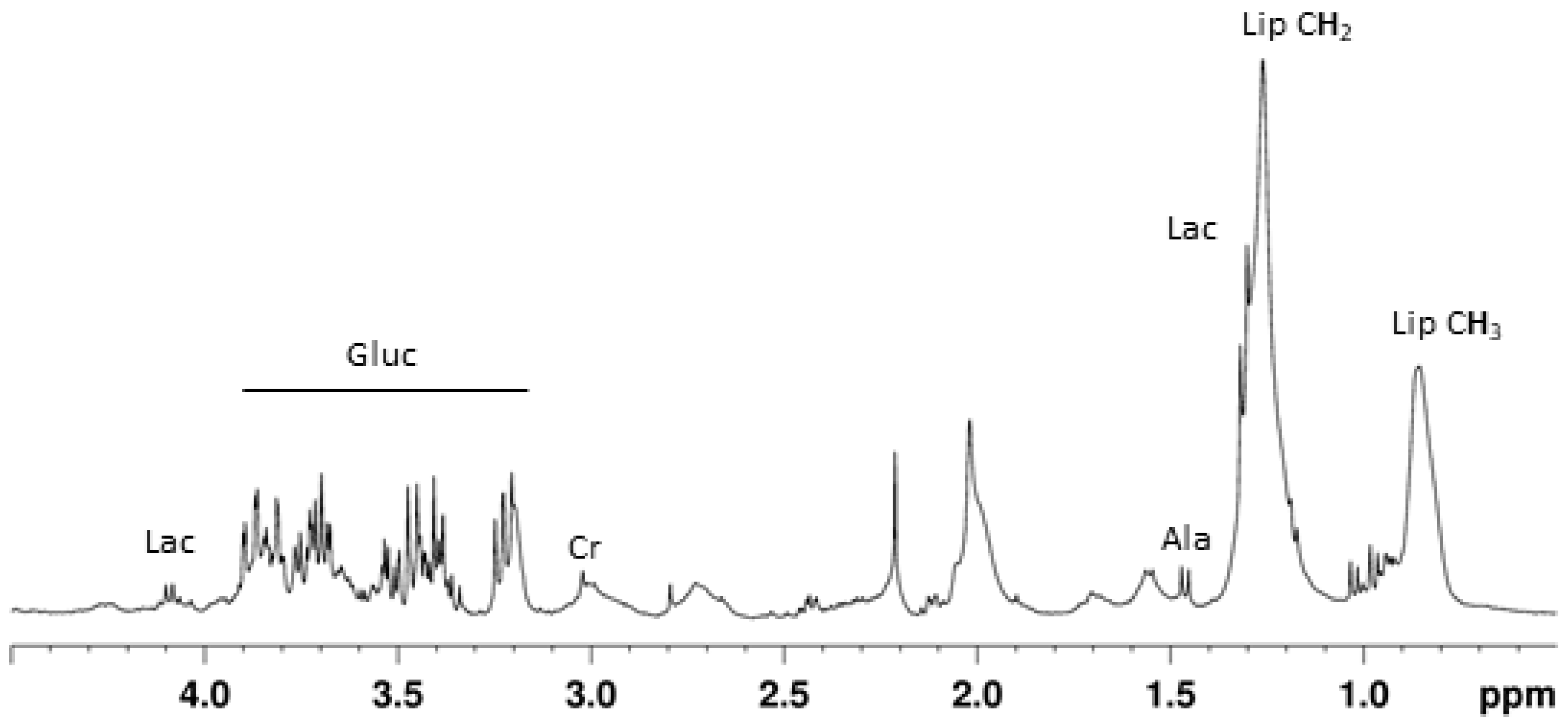
2.2. Visual Inspection
2.3. Pattern Recognition
2.3.1. The Acute Phase: Takotsubo vs. AMI
2.3.2. Evolution of Takotsubo Metabolic Profile: Acute vs. Subacute Phases
3. Discussion
3.1. The Acute Phase: Takotsubo vs. AMI
3.2. Differences between Acute and Subacute Phases
4. Material and Methods
4.1. Patients
4.2. NMR Spectroscopy
4.3. Pattern Recognition
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Hear. J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K.; Ueshima, K.; Uchida, T.; Oh-Mura, N.; Kimura, K.; Owa, M.; Yoshiyama, M.; Miyazaki, S.; Haze, K.; Ogawa, H.; et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Hurst, R.T.; Askew, J.W.; Reuss, C.S.; Lee, R.W.; Sweeney, J.P.; Fortuin, F.D.; Oh, J.K.; Tajik, A.J. Transient Midventricular Ballooning Syndrome: A New Variant. J. Am. Coll. Cardiol. 2006, 48, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Gil, I.J.; Molina, M.; Bernardo, E.; Ibañez, B.; Ruiz-Mateos, B.; Garcia-Rubira, J.C.; Vivas, D.; Feltes, G.; Luaces, M.; Alonso, J.; et al. Síndrome de tako-tsubo e insuficiencia cardiaca: Seguimiento a largo plazo. Rev. Española Cardiol. 2012, 65, 996–1002. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, S.W.; Windenburg, D.C.; Lesser, J.R.; Maron, M.S.; Hauser, R.G.; Lesser, J.N.; Haas, T.S.; Hodges, J.S.; Maron, B.J. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gil, I.J.; Almendro-Delia, M.; Andrés, M.; Sionis, A.; Martin, A.; Bastante, T.; Córdoba-Soriano, J.G.; Linares, J.A.; Sucarrats, S.G.; Sánchez-Grande-Flecha, A.; et al. Secondary forms of Takotsubo cardiomyopathy: A whole different prognosis. Eur. Hear. J. Acute Cardiovasc. Care 2016, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Rentería, H.D.; Núñez-Gil, I.J. Takotsubo syndrome: Advances in the understanding and management of an enigmatic stress cardiomyopathy. World J. Cardiol. 2016, 8, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.I.; Dunn, W.; Griffin, J.L.; Allwood, J.; Goodacre, R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics 2007, 8, 1243–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Basak, T.; Varshney, S.; Hamid, Z.; Ghosh, S.; Seth, S.; Sengupta, S. Identification of metabolic markers in coronary artery disease using an untargeted LC-MS based metabolomic approach. J. Proteom. 2015, 127, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.A.A.D.G.D.I.; Andrés, M.; Garcia-Dorado, D. Metabolomics and Heart Diseases: From Basic to Clinical Approach. Curr. Med. Chem. 2019, 26, 46–59. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Griffin, J.L.; Atherton, H.J.; Shockcor, J.P.; Atzori, L. Metabolomics as a tool for cardiac research. Nat. Rev. Cardiol. 2011, 8, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J. Mol. Cell. Cardiol. 2013, 55, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Van Der Westhuizen, J.; Mathew, J.P.; Schwinn, D.; Glower, D.D.; Newgard, C.B.; Podgoreanu, M. Metabolomic Profiling Reveals Distinct Patterns of Myocardial Substrate Use in Humans With Coronary Artery Disease or Left Ventricular Dysfunction During Surgical Ischemia/Reperfusion. Circulation 2009, 119, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Shen, A.; Huang, Y.; Su, L.; Lai, W.; Wang, P.; Xie, Z.; Xie, Z.; Zeng, Q.; Ren, H.; et al. 1H-NMR-Based Metabolic Analysis of Human Serum Reveals Novel Markers of Myocardial Energy Expenditure in Heart Failure Patients. PLoS ONE 2014, 9, e88102. [Google Scholar] [CrossRef]
- Mueller-Hennessen, M.; Sigl, J.; Fuhrmann, J.C.; Witt, H.; Reszka, R.; Schmitz, O.; Kastler, J.; Fischer, J.J.; Müller, O.J.; Giannitsis, E.; et al. Metabolic profiles in heart failure due to non-ischemic cardiomyopathy at rest and under exercise. ESC Hear. Fail. 2017, 4, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lema, C.; Andrés, M.; Aguadé-Bruix, S.; Consegal, M.; Rodriguez-Sinovas, A.; Benito, B.; Ferreira-Gonzalez, I.; Barba, I. 1H NMR serum metabolomic profiling of patients at risk of cardiovascular diseases performing stress test. Sci. Rep. 2020, 10, 17838. [Google Scholar] [CrossRef]
- Barba, I.; De León, G.; Martin, E.; Cuevas, A.; Aguadé, S.; Barrabés, J.A.; Candell-Riera, J.; Garcia-Dorado, D. Nuclear magnetic resonance-based metabolomics predicts exercise-induced ischemia in patients with suspected coronary artery disease. Magn. Reson. Med. 2008, 60, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Maron, B.J. Epidemiology and Clinical Profile of Takotsubo Cardiomyopathy. Circ. J. 2014, 78, 2119–2128. [Google Scholar] [CrossRef] [Green Version]
- Gil, I.J.N.; Andrés, M.; Delia, M.A.; Sionis, A.; Martin, A.; Bastante, T.; Soriano, J.G.C.; Vicente, J.A.L.; Sucarrats, S.G.; Flecha, A.S.-G. Characterization of Tako-tsubo Cardiomyopathy in Spain: Results from the RETAKO National Registry. Rev. Española Cardiol. 2015, 68, 505–512. [Google Scholar] [CrossRef]
- Randle, P.J.; England, P.J.; Denton, R.M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem. J. 1970, 117, 677–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [Green Version]
- Kirschenlohr, H.L.; Griffin, J.L.; Clarke, S.C.; Rhydwen, R.; Grace, A.A.; Schofield, P.M.; Brindle, K.M.; Metcalfe, J.C. Proton NMR analysis of plasma is a weak predictor of coronary artery disease. Nat. Med. 2006, 12, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Barba, I.; Andrés, M.; Picón, I.; Aguade-Bruix, S.; Garcia-Dorado, D. Sex differences in the 1H NMR metabolic profile of serum in cardiovascular risk patients. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, H.J.; Gulston, M.K.; Bailey, N.J.; Cheng, K.-K.; Zhang, W.; Clarke, K.; Griffin, J.L. Metabolomics of the interaction between PPAR-α and age in the PPAR-α-null mouse. Mol. Syst. Biol. 2009, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.K.; Neil, C.J.; Henning, A.; Cameron, D.; Jagpal, B.; Bruce, M.; Horowitz, J.; Frenneaux, M.P. Tako-Tsubo Cardiomyopathy: A Heart Stressed Out of Energy? JACC Cardiovasc. Imaging 2015, 8, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.M.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef]
- Griffin, J.L.; Nicholls, A.W. Metabolomics as a functional genomic tool for understanding lipid dysfunction in diabetes, obesity and related disorders. Pharmacogenomics 2006, 7, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.M.; Darzi, A.W.; Takats, Z.; Lindon, J.C. Metabolic phenotyping in clinical and surgical environments. Nature 2012, 491, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Frangieh, A.H.; Obeid, S.; Ghadri, J.; Imori, Y.; D’Ascenzo, F.; Kovac, M.; Ruschitzka, F.; Lüscher, T.F.; Duru, F.; Templin, C.; et al. ECG Criteria to Differentiate Between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J. Am. Hear. Assoc. 2016, 5, e003418. [Google Scholar] [CrossRef] [Green Version]
- Kosuge, M.; Ebina, T.; Hibi, K.; Iwahashi, N.; Tsukahara, K.; Endo, M.; Maejima, N.; Nagashima, Z.; Suzuki, H.; Morita, S.; et al. Differences in Negative T Waves Between Takotsubo Cardiomyopathy and Reperfused Anterior Acute Myocardial Infarction. Circ. J. 2012, 76, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Beltran, C.; Pardo, R.; Bou-Teen, D.; Ruiz-Meana, M.; Villena, J.A.; Ferreira-González, I.; Barba, I. Enhancing Glycolysis Protects against Ischemia-Reperfusion Injury by Reducing ROS Production. Metabolites 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.A.; Barba, I.; Fenoy-Alejandre, M.; López-López, P.; Baquero-Artigao, F.; Rodríguez-Molino, P.; Noguera-Julian, A.; Nicolás-López, M.; De La Fuente-Juárez, A.; Codina-Grau, M.G.; et al. 1H-NMR Urinary Metabolic Profile, A Promising Tool for the Management of Infants with Human Cytomegalovirus-Infection. Metabolites 2019, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]


| Cases | Controls | |
|---|---|---|
| Age (years) | 71.54 ± 10.41 | 67.75 ± 17.39 |
| Hypertension | 45% | 75% |
| Dyslipidemia | 50% | 75% |
| Diabetes | 30% | 25% |
| Smoker | 30% | 50% |
| Family History | 55% | 25% |
| Obesity | 4% | 50% |
| ASA | 89% | 0% |
| Statins | 72% | 0% |
| Anxiolytics | 4% | 0% |
| Antidepressant | 8% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez-Gil, I.J.; Andrés, M.; Benito, B.; Bernardo, E.; Vedia, O.; Ferreira-Gonzalez, I.; Barba, I. Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome. Metabolites 2021, 11, 439. https://doi.org/10.3390/metabo11070439
Nuñez-Gil IJ, Andrés M, Benito B, Bernardo E, Vedia O, Ferreira-Gonzalez I, Barba I. Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome. Metabolites. 2021; 11(7):439. https://doi.org/10.3390/metabo11070439
Chicago/Turabian StyleNuñez-Gil, Iván J., Mireia Andrés, Begoña Benito, Esther Bernardo, Oscar Vedia, Ignacio Ferreira-Gonzalez, and Ignasi Barba. 2021. "Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome" Metabolites 11, no. 7: 439. https://doi.org/10.3390/metabo11070439
APA StyleNuñez-Gil, I. J., Andrés, M., Benito, B., Bernardo, E., Vedia, O., Ferreira-Gonzalez, I., & Barba, I. (2021). Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome. Metabolites, 11(7), 439. https://doi.org/10.3390/metabo11070439






