Analysis of Sirtuin 1 and Sirtuin 3 at Enzyme and Protein Levels in Human Breast Milk during the Neonatal Period
Abstract
1. Introduction
2. Results
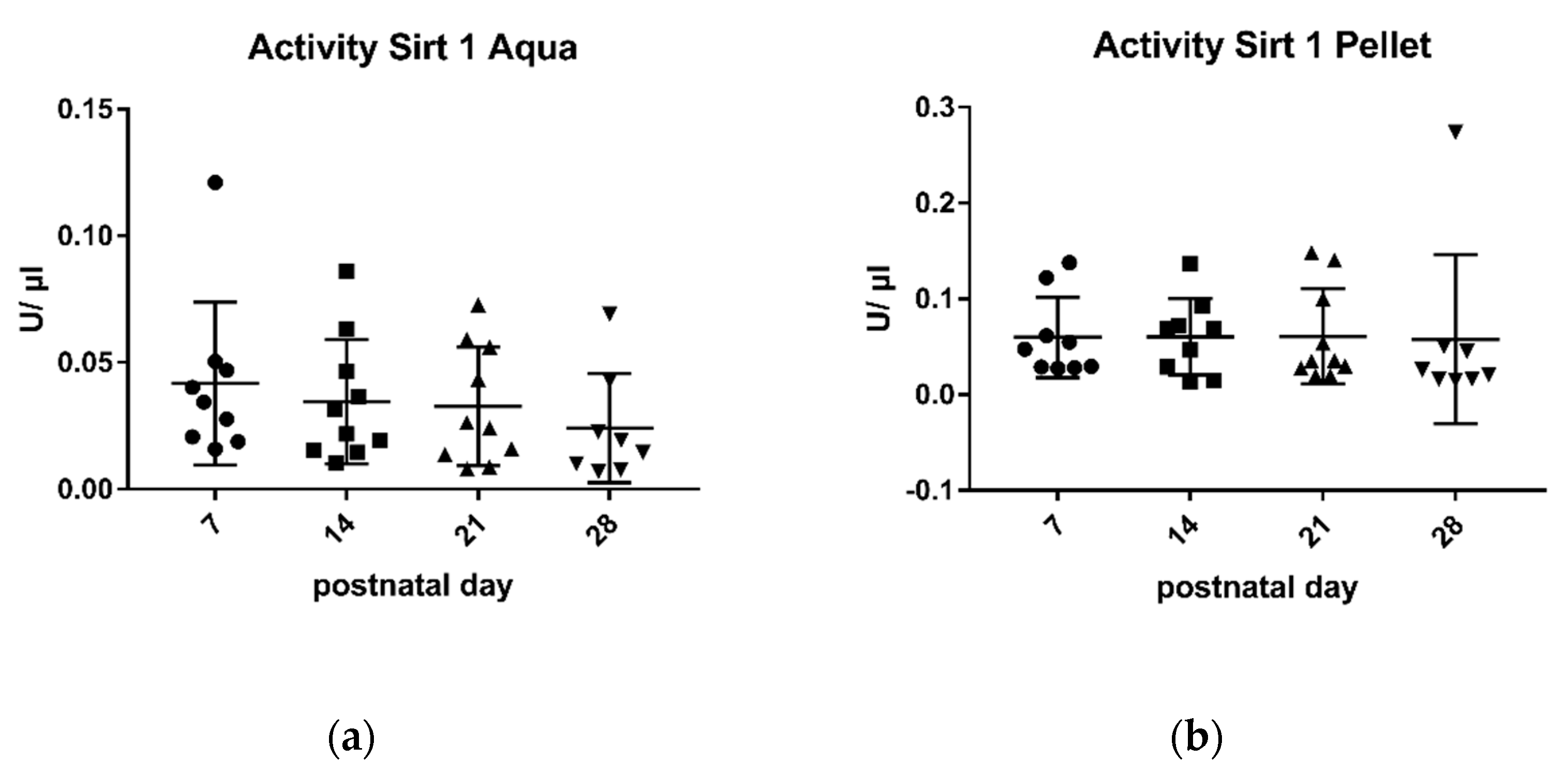
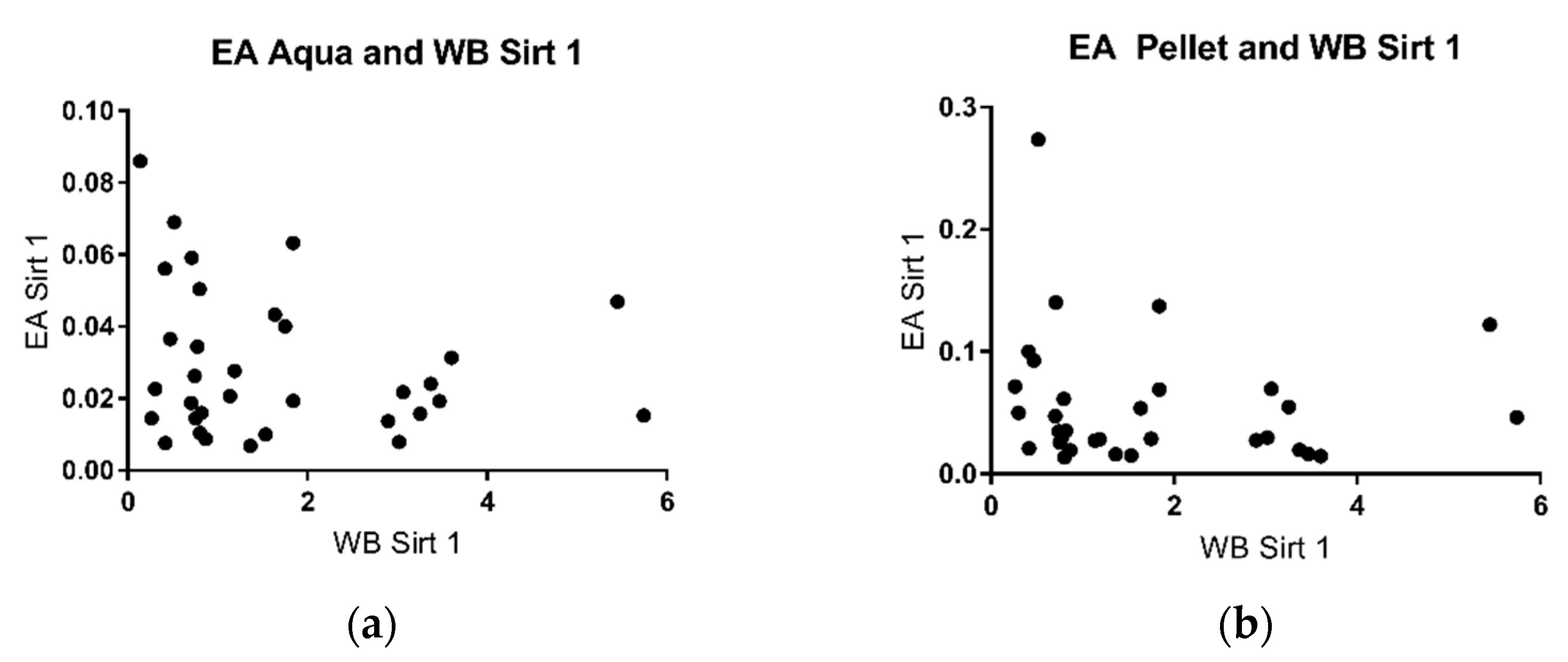
2.1. Sirtuin 1 Enzymatic Activity
2.2. Sirtuin 3 Enzymatic Activity
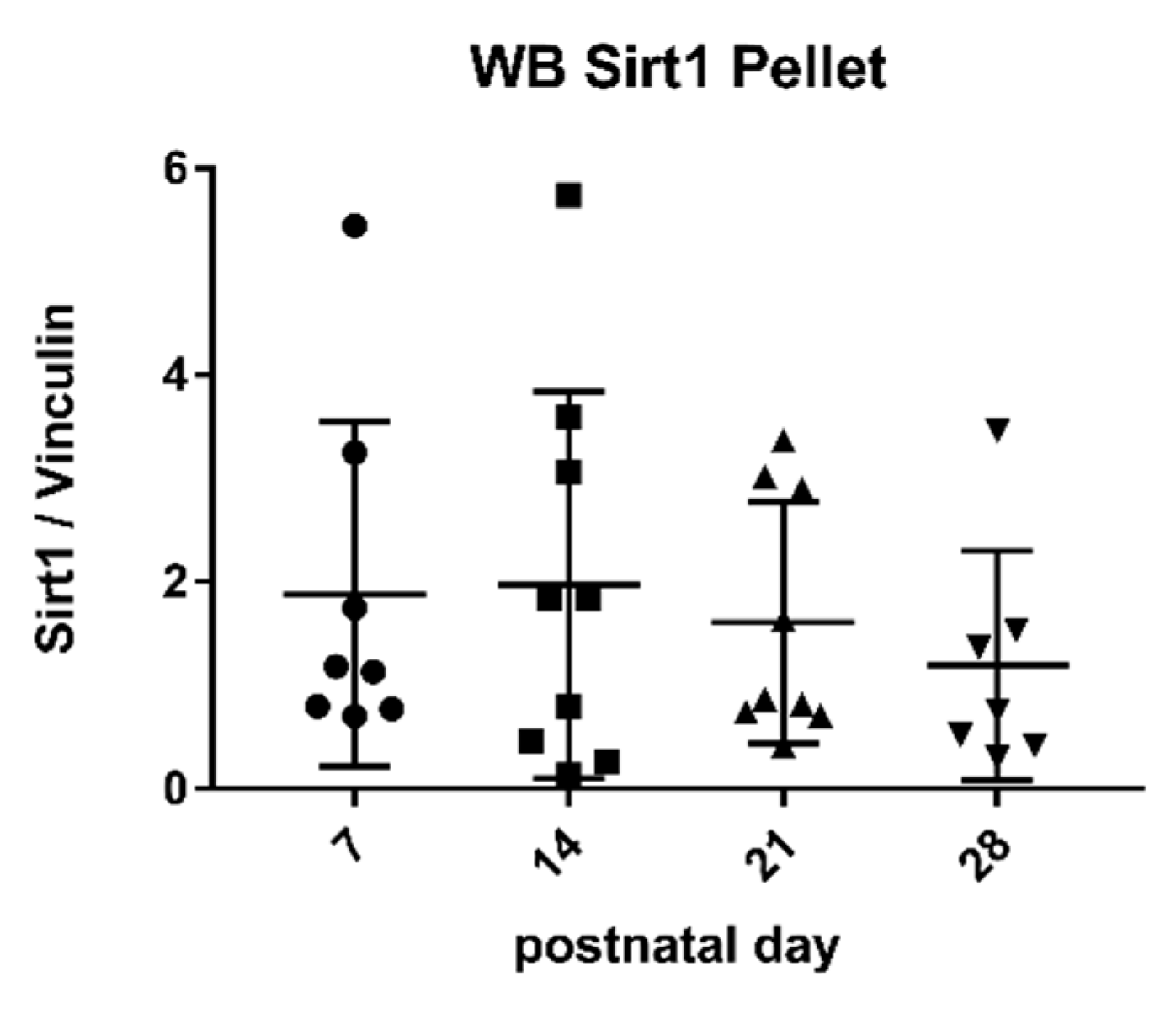
2.3. Sirtuin 1 Western Blot
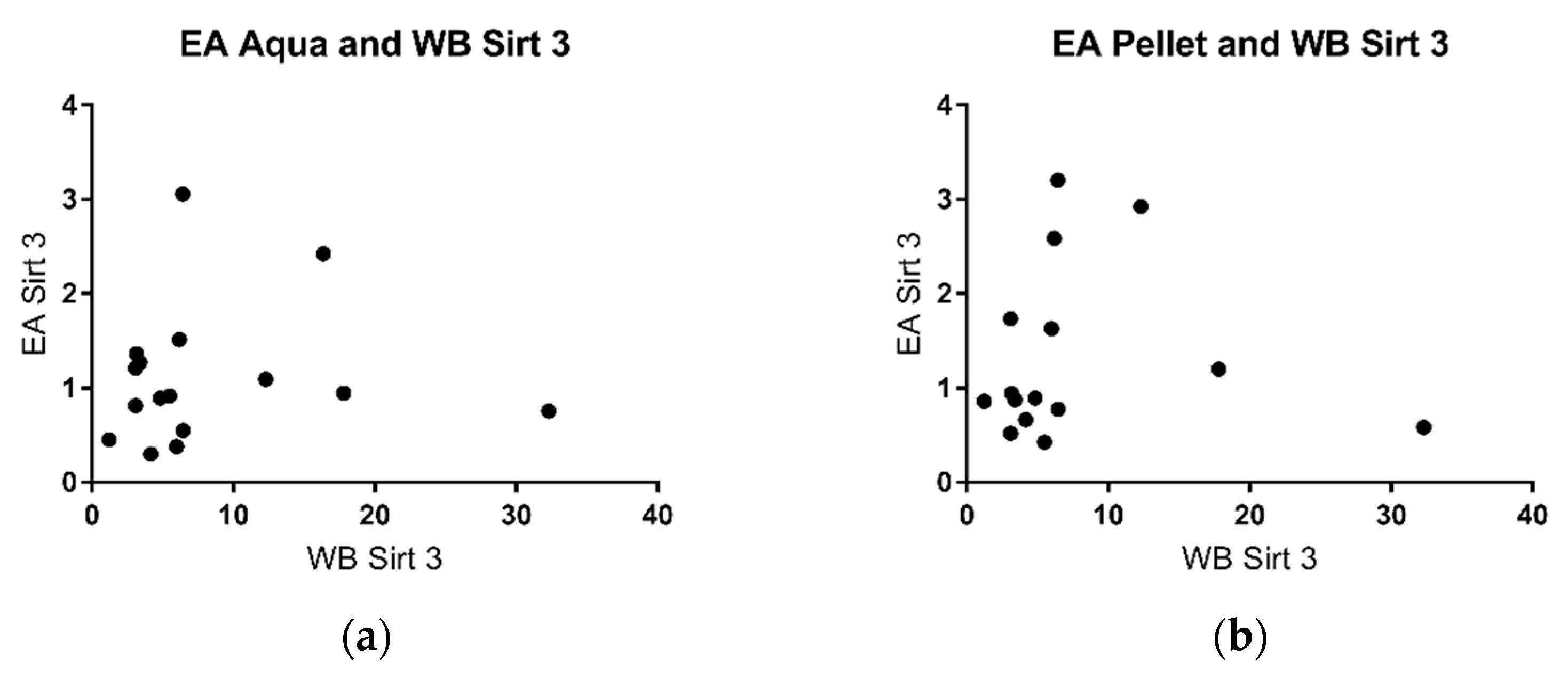
2.4. Sirtuin 3 Western Blot
3. Discussion
4. Materials and Methods
4.1. Enzymatic Activity of Sirtuins
4.2. Protein Expression
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weltgesundheitsorganisation; Unicef. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef]
- Hanson, L.A.; Korotkova, M.; Lundin, S.; Håversen, L.; Silfverdal, S.; Mattsby-Baltzer, I.; Strandvik, B.; Telemo, E. The transfer of immunity from mother to child. Ann. New York Acad. Sci. 2003, 987, 199. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Beluska-Turkan, K.; Korczak, R.; Hartell, B.; Moskal, K.; Maukonen, J.; Alexander, D.E.; Salem, N.; Harkness, L.; Ayad, W.; Szaro, J.; et al. Nutritional Gaps and Supplementation in the First 1000 Days. Nutrients 2019, 11, 2891. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. (Regul. Ed.) 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Structure and Functions. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 115–123. [Google Scholar] [PubMed]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092–22096. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Samuel, T.M.; Zhou, Q.; Munblit, D.; Thakkar, S.K.; Verhasselt, V.; Giuffrida, F. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. (Lausanne) 2020, 7, 576133. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Denu, J.M. Sirtuin catalysis and regulation. J. Biol. Chem. 2012, 287, 42419–42427. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: From chemistry to the clinic. Clin. Epigenet. 2016, 8, 1–21. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jesko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef]
- Dabke, P.; Das, A.M. Mechanism of Action of Ketogenic Diet Treatment: Impact of Decanoic Acid and Beta-Hydroxybutyrate on Sirtuins and Energy Metabolism in Hippocampal Murine Neurons. Nutrients 2020, 12, 2379. [Google Scholar] [CrossRef]
- Buler, M.; Andersson, U.; Hakkola, J. Who watches the watchmen? Regulation of the expression and activity of sirtuins. FASEB J. 2016, 30, 3942. [Google Scholar] [CrossRef]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging (Albany NY) 2013, 5, 144–150. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Nyárády, K.; Turai, R.; Funke, S.; Györgyi, E.; Makai, A.; Prémusz, V.; Bódis, J.; Sulyok, E. Effects of perinatal factors on sirtuin 3, 8-hydroxy-2′- deoxyguanosine, brain-derived neurotrophic factor and serotonin in cord blood and early breast milk: An observational study. Int. Breastfeed. J. 2020, 15, 1–57. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Potthast, A.B.; Nebl, J.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Hahn, A.; Das, A. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients 2020, 12, 1004. [Google Scholar] [CrossRef]
- Maissan, P.; Mooij, E.J.; Barberis, M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Biology 2021, 10, 194. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 1368. [Google Scholar]
- Ratsika, A.; Codagnone, M.C.; O’mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation during Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology (N.Y. 1943) 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Rychahou, P.; Weiss, H.L.; Lee, E.Y.; Perry, C.L.; Barrett, T.A.; Wang, Q.; Evers, B.M. SIRT2 Contributes to the Regulation of Intestinal Cell Proliferation and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, X.; Lv, A.; Fan, S.; Zhang, J. Saccharomyces boulardii modulates necrotizing enterocolitis in neonatal mice by regulating the sirtuin 1/NF-κB pathway and the intestinal microbiota. Mol. Med. Rep. 2020, 22, 671–680. [Google Scholar] [CrossRef]
- Sheen, J.; Yu, H.; Tain, Y.; Tsai, W.; Tiao, M.; Lin, I.; Tsai, C.; Lin, Y.; Huang, L. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Li, S.; Yu, H.; Sheen, J.; Tiao, M.; Tain, Y.; Lin, I.; Lin, Y.; Chang, K.; Tsai, C.; Huang, L. A maternal high-fat diet during pregnancy and lactation, in addition to a postnatal high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: Beneficial effects of resveratrol. Oncotarget 2017, 8, 111998. [Google Scholar] [CrossRef]
- Czosnykowska-Lukacka, M.; Lis-Kuberka, J.; Krolak-Olejnik, B.; Orczyk-Pawilowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8, 428. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Geddes, D.; Perrella, S. Breastfeeding and Human Lactation. Nutrients 2019, 11, 802. [Google Scholar] [CrossRef]
- Pereira, C.V.; Lebiedzinska, M.; Wieckowski, M.R.; Oliveira, P.J. Regulation and protection of mitochondrial physiology by sirtuins. Mitochondrion 2012, 12, 66–76. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef]
- Twigger, A.; Hepworth, A.R.; Lai, C.T.; Chetwynd, E.; Stuebe, A.M.; Blancafort, P.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci. Rep. 2015, 5, 12933. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K.; et al. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar]
- Melnik, B.C.; Kakulas, F.; Geddes, D.T.; Hartmann, P.E.; John, S.M.; Carrera-Bastos, P.; Cordain, L.; Schmitz, G. Milk miRNAs: Simple nutrients or systemic functional regulators? Nutr. Metab. 2016, 13, 42. [Google Scholar] [CrossRef]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv. Nutr. (Bethesda Md.) 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Sandvoß, M.; Potthast, A.B.; von Versen-Höynck, F.; Das, A.M. HELLP Syndrome. Reprod. Sci. (Thousand Oaks Calif.) 2017, 24, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Tie, W.J.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human Milk Casein and Whey Protein and Infant Body Composition over the First 12 Months of Lactation. Nutrients 2018, 10, 1332. [Google Scholar] [CrossRef]
- Madden, L.R.; Nguyen, T.V.; Garcia-Mojica, S.; Presnell, S.C.; Nguyen, D.G.; Retting, K.N. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/ Tox Functions. IScience 2018, 2, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delince, M.; Di Caprio, G.; Upadhyayula, S.; De Nola, G. Design and validation of a human brain endothelial microvessel-on-a-chip open microfluidic model enabling advanced optical imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Cho, S. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef]
- Aldewachi, H.; Al-Zidan, R.N.; Conner, M.T.; Salman, M.M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8. [Google Scholar]
- Salman, M.M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.M.; Wade-Martins, R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human Milk from Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]


| (a) | |||||
| Mother 1 | Mother 2 | Mother 3 | Mother 4 | Mother 5 | |
| Age | 31 | 28 | 37 | 30 | 35 |
| Week of gestation | 31 + 3 | 37 + 5 | 38 + 5 | 33 + 6 | 28 + 1 |
| Height in m | 1.73 | 1.60 | 1.67 | 1.68 | 1.76 |
| Weight in kg | 75 | 54 | 70 | 75 | 95 |
| BMI in kg/m2 | 25 | 21 | 25 | 27 | 31 |
| Pregnancy complications | early rupture of membranes, premature labour | -- | polyhydramnion | twin pregnancy, no TTTS | preterm birth |
| Pre-existing illness | spondylarthritis | -- | pelvic vein thrombosis in previous pregnancy | sinus tachycardia, incomplete RBBB | -- |
| Drugs | sulfasalazine | iron | heparin | heparin, iron | -- |
| Smoking in general | no | no | no | no | no |
| Smoking during pregnancy | no | no | no | no | no |
| Diet | OV | OV | OV | OV | OV |
| (b) | |||||
| Mother 6 | Mother 7 | Mother 8 | Mother 9 | Mother 10 | |
| Age | 31 | 39 | 33 | 30 | 42 |
| Week of gestation | 29 + 3 | 29 + 2 | 29 + 1 | 33 + 2 | 32 + 2 |
| Height in m | 1.56 | 1.89 | 1.68 | 1.74 | 1.71 |
| Weight in kg | 67 | 88 | 49 | 82 | 75 |
| BMI in kg/m2 | 28 | 25 | 17 | 27 | 26 |
| Pregnancy complications | -- | early rupture of membranes | twin pregnancy, hyperemesis gravidarum, cervical insufficiency | -- | Vasa praevia |
| Pre-existing illness | -- | -- | Intestinal fructose intolerance | hypothyroidism | Hashimoto’s thyroiditis, factor V Leiden deficiency |
| Drugs | -- | unknown | -- | L-thyroxin | L-thyroxin |
| Smoking in general | yes | no | no | no | no |
| Smoking during pregnancy | yes | no | no | no | no |
| Diet | OV | OV | OV | OV | OV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hase, K.; Stahmer, L.; Shammas, H.; Peter, C.; Bohnhorst, B.; Das, A.M. Analysis of Sirtuin 1 and Sirtuin 3 at Enzyme and Protein Levels in Human Breast Milk during the Neonatal Period. Metabolites 2021, 11, 348. https://doi.org/10.3390/metabo11060348
Hase K, Stahmer L, Shammas H, Peter C, Bohnhorst B, Das AM. Analysis of Sirtuin 1 and Sirtuin 3 at Enzyme and Protein Levels in Human Breast Milk during the Neonatal Period. Metabolites. 2021; 11(6):348. https://doi.org/10.3390/metabo11060348
Chicago/Turabian StyleHase, Kristina, Laura Stahmer, Hadeel Shammas, Corinna Peter, Bettina Bohnhorst, and Anibh Martin Das. 2021. "Analysis of Sirtuin 1 and Sirtuin 3 at Enzyme and Protein Levels in Human Breast Milk during the Neonatal Period" Metabolites 11, no. 6: 348. https://doi.org/10.3390/metabo11060348
APA StyleHase, K., Stahmer, L., Shammas, H., Peter, C., Bohnhorst, B., & Das, A. M. (2021). Analysis of Sirtuin 1 and Sirtuin 3 at Enzyme and Protein Levels in Human Breast Milk during the Neonatal Period. Metabolites, 11(6), 348. https://doi.org/10.3390/metabo11060348