Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking
Abstract
1. Introduction
2. Results
2.1. Global Metabolic Profiling of Plasma from Healthy Controls, E-Cig Users, and Cigarette Smokers Analyzed by UPLC-MS
2.2. Nicotine Degradation-Related Metabolites Increased in Both Plasma from Cigarette Smokers and E-Cig Users
2.3. Metabolites Associated with TCA Cycles Dysregulated in Plasma from E-Cig Users
2.4. Dysregulated Sphingolipid Metabolites Found in Plasma from Cigarette Smokers
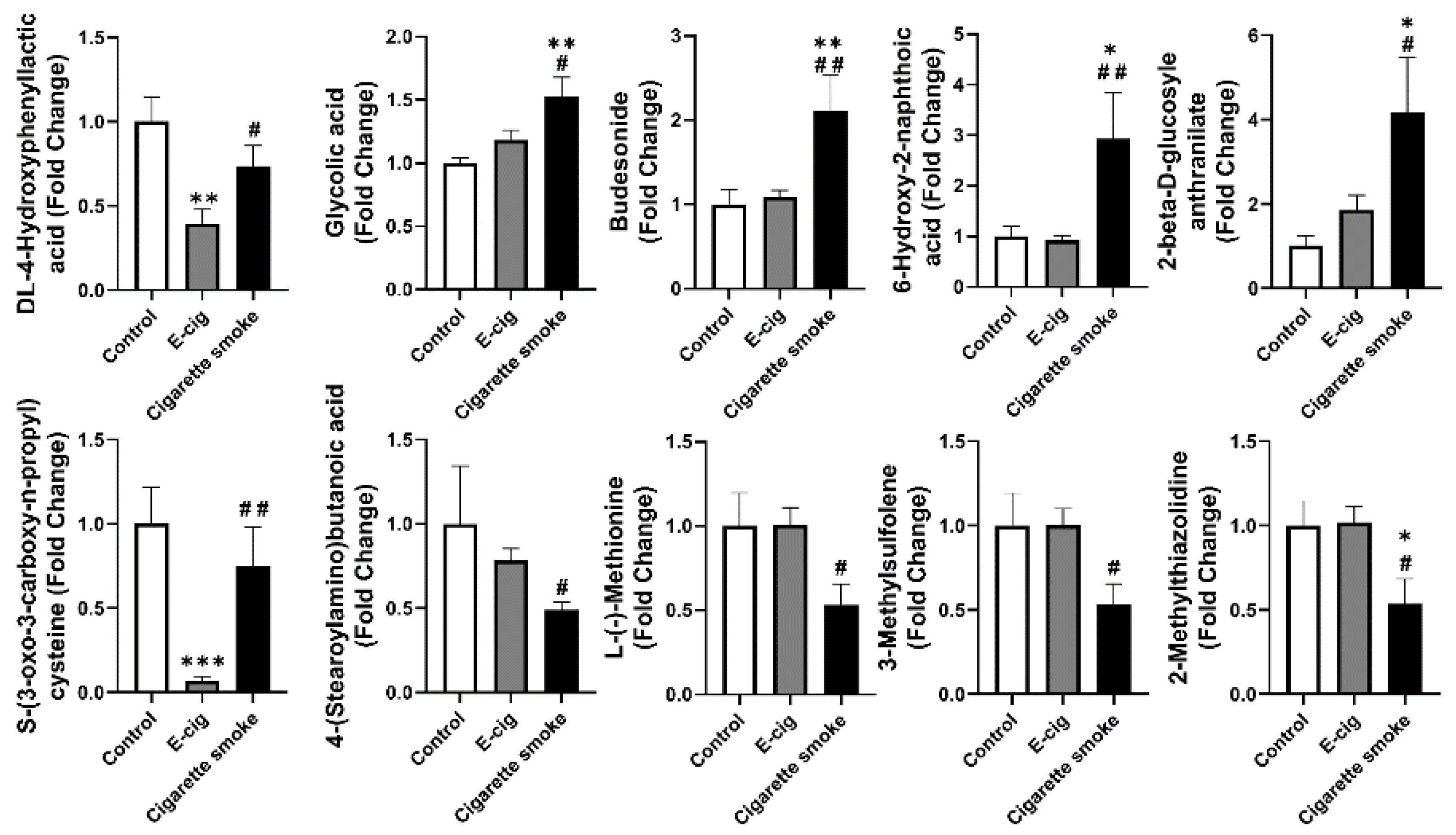
2.5. Other Dysregulated Metabolites in Plasma Identified from E-Cig Users or Cigarette Smokers
3. Discussion
4. Methods and Materials
4.1. Human Subjects
4.2. Plasma Samples Collection
4.3. Chemicals
4.4. UPLC-MS Analysis
4.5. Data Processing
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, D.; Davis, K.C.; Cox, S.; Bradfield, B.; King, B.A.; Shafer, P.; Caraballo, R.; Bunnell, R. Reasons for current E-cigarette use among U.S. adults. Prev. Med. 2016, 93, 14–20. [Google Scholar] [CrossRef]
- Madison, M.C.; Landers, C.T.; Gu, B.-H.; Chang, C.-Y.; Tung, H.-Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.-Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. Nicotine Levels in Electronic Cigarettes. Nicotine Tob. Res. 2012, 15, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.A.; Popova, L.; Ling, P.M. A Longitudinal Analysis of Electronic Cigarette Use and Smoking CessationElectronic Cigarette Use and Smoking CessationLetters. JAMA Intern. Med. 2014, 174, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Friedman, M.R.; McGraw, M.D.; Ginsberg, G.; Friedman, A.E.; Rahman, I. Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 2020, 8, 25. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Publications and Reports of the Surgeon General. In How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010. [Google Scholar]
- Górna, I.; Napierala, M.; Florek, E. Electronic Cigarette Use and Metabolic Syndrome Development: A Critical Review. Toxics 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Zhou, B.; Zhao, Y.; Ressom, H.W.; Cheema, A.K.; Pickworth, W.; Shields, P.G. Feasibility of identifying the tobacco-related global metabolome in blood by UPLC-QTOF-MS. J. Proteome Res. 2013, 12, 679–691. [Google Scholar] [CrossRef]
- Gu, F.; Derkach, A.; Freedman, N.D.; Landi, M.T.; Albanes, D.; Weinstein, S.J.; Mondul, A.M.; Matthews, C.E.; Guertin, K.A.; Xiao, Q.; et al. Cigarette smoking behaviour and blood metabolomics. Int. J. Epidemiol. 2016, 45, 1421–1432. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P., III; Benowitz, N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef]
- Cross, A.J.; Boca, S.; Freedman, N.D.; Caporaso, N.E.; Huang, W.Y.; Sinha, R.; Sampson, J.N.; Moore, S.C. Metabolites of tobacco smoking and colorectal cancer risk. Carcinogenesis 2014, 35, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Lawyer, G.; McDonough, S.; Wang, Q.; Kassem, N.O.; Kas-Petrus, F.; Ye, D.; Singh, K.P.; Kassem, N.O.F.; Rahman, I. Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tob. Control. 2020, 29 (Suppl. S2), s102. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Maremanda, K.P.; Li, D.; Rahman, I. Exosomal microRNAs are novel circulating biomarkers in cigarette, waterpipe smokers, E-cigarette users and dual smokers. BMC Med. Genom. 2020, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Solanki, H.S.; Babu, N.; Jain, A.P.; Bhat, M.Y.; Puttamallesh, V.N.; Advani, J.; Raja, R.; Mangalaparthi, K.K.; Kumar, M.M.; Prasad, T.S.K.; et al. Cigarette smoke induces mitochondrial metabolic reprogramming in lung cells. Mitochondrion 2018, 40, 58–70. [Google Scholar] [CrossRef]
- Agarwal, A.R.; Yin, F.; Cadenas, E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am. J. Respir. Cell Mol. Biol. 2014, 51, 284–293. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Li, J.; Huynh, L.; Cornwell, W.D.; Tang, M.-S.; Simborio, H.; Huang, J.; Kosmider, B.; Rogers, T.J.; Zhao, H.; Steinberg, M.B.; et al. Electronic Cigarettes Induce Mitochondrial DNA Damage and Trigger TLR9 (Toll-Like Receptor 9)-Mediated Atherosclerosis. Arter. Thromb. Vasc. Biol. 2021, 41, 839–853. [Google Scholar] [CrossRef]
- Fowles, J.R.; Banton, M.I.; Pottenger, L.H. A toxicological review of the propylene glycols. Crit. Rev. Toxicol. 2013, 43, 363–390. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Ghidoni, R.; Caretti, A.; Signorelli, P. Role of Sphingolipids in the Pathobiology of Lung Inflammation. Mediators Inflamm. 2015, 2015, 487508. [Google Scholar] [CrossRef]
- Petrache, I.; Medler, T.R.; Richter, A.T.; Kamocki, K.; Chukwueke, U.; Zhen, L.; Gu, Y.; Adamowicz, J.; Schweitzer, K.S.; Hubbard, W.C.; et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L44–L53. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Justice, M.J.; Schweitzer, K.S.; Krishnan, S.; Bronova, I.; Berdyshev, E.V.; Hubbard, W.C.; Pewzner-Jung, Y.; Futerman, A.H.; Choi, A.M.K.; et al. Sphingolipid regulation of lung epithelial cell mitophagy and necroptosis during cigarette smoke exposure. FASEB J. 2018, 32, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Petrusca, D.N.; Gu, Y.; Adamowicz, J.J.; Rush, N.I.; Hubbard, W.C.; Smith, P.A.; Berdyshev, E.V.; Birukov, K.G.; Lee, C.H.; Tuder, R.M.; et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J. Biol. Chem. 2010, 285, 40322–40332. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Armeson, K.; Pierce, J.S.; Bielawski, J.; Bielawska, A.; Visvanathan, K.; Hill, E.G.; Ogretmen, B. Plasma sphingolipids and lung cancer: A population-based, nested case-control study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Jacobson, S.; Cruickshank, C.; Hughes, G.J.; Siska, C.; Ory, D.S.; Petrache, I.; Schaffer, J.E.; Reisdorph, N.; Kechris, K. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am. J. Respir. Crit. Care Med. 2015, 191, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., III. Nicotine chemistry, metabolism, kinetics and biomarkers. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–60. [Google Scholar]
- Tegin, G.; Mekala, H.M.; Sarai, S.K.; Lippmann, S. E-Cigarette Toxicity? South Med. J. 2018, 111, 35–38. [Google Scholar] [CrossRef]
- Wang, Q.; Sundar, I.K.; Li, D.; Lucas, J.H.; Muthumalage, T.; McDonough, S.R.; Rahman, I. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: Role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res. 2020, 21, 154. [Google Scholar] [CrossRef]
- Wang, Q.; Ahmad Khan, N.; Muthumalage, T.; Lawyer, G.R.; McDonough, S.R.; Chuang, T.-D.; Gong, M.; Sundar, I.K.; Rehan, V.K.; Rahman, I. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019, 1, 609–623. [Google Scholar] [CrossRef]
- Schick, S.F.; Blount, B.C.; Jacob, P.; Saliba, N.A.; Bernert, J.T.; El Hellani, A.; Jatlow, P.; Pappas, R.S.; Wang, L.; Foulds, J.; et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L425–L452. [Google Scholar] [CrossRef]
- Jacob, P.; St Helen, G.; Yu, L.; Nardone, N.; Havel, C.; Cheung, P.; Benowitz, N.L. Biomarkers of Exposure for Dual Use of Electronic Cigarettes and Combustible Cigarettes: Nicotelline, NNAL, and Total Nicotine Equivalents. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2020, 22, 1107–1113. [Google Scholar] [CrossRef]
- Xu, X.; Su, Y.; Fan, Z.H. Cotinine concentration in serum correlates with tobacco smoke-induced emphysema in mice. Sci. Rep. 2014, 4, 3864. [Google Scholar] [CrossRef]
- Wang, L.; Bernert, J.T.; Benowitz, N.L.; Feng, J.; Jacob, P., III; McGahee, E.; Caudill, S.P.; Scherer, G.; Scherer, M.; Pluym, N.; et al. Collaborative Method Performance Study of the Measurement of Nicotine, Its Metabolites, and Total Nicotine Equivalents in Human Urine. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- von Weymarn, L.B.; Thomson, N.M.; Donny, E.C.; Hatsukami, D.K.; Murphy, S.E. Quantitation of the Minor Tobacco Alkaloids Nornicotine, Anatabine, and Anabasine in Smokers’ Urine by High Throughput Liquid Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2016, 29, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Woodall, M.; Jacob, J.; Kalsi, K.K.; Schroeder, V.; Davis, E.; Kenyon, B.; Khan, I.; Garnett, J.P.; Tarran, R.; Baines, D.L. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L957–L967. [Google Scholar] [CrossRef]
- Lerner, C.A.; Rutagarama, P.; Ahmad, T.; Sundar, I.K.; Elder, A.; Rahman, I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016, 477, 620–625. [Google Scholar] [CrossRef]
- Su, R.; Dong, L.; Li, C.; Nachtergaele, S.; Wunderlich, M.; Qing, Y.; Deng, X.; Wang, Y.; Weng, X.; Hu, C.; et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m6A/MYC/CEBPA Signaling. Cell 2018, 172, 90–105.e23. [Google Scholar] [CrossRef]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef]
- Tibboel, J.; Reiss, I.; de Jongste, J.C.; Post, M. Ceramides: A potential therapeutic target in pulmonary emphysema. Respir. Res. 2013, 14, 96. [Google Scholar] [CrossRef]
- Liu, D.; Meister, M.; Zhang, S.; Vong, C.I.; Wang, S.; Fang, R.; Li, L.; Wang, P.G.; Massion, P.; Ji, X. Identification of lipid biomarker from serum in patients with chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Bodas, M.; Pehote, G.; Silverberg, D.; Gulbins, E.; Vij, N. Autophagy augmentation alleviates cigarette smoke-induced CFTR-dysfunction, ceramide-accumulation and COPD-emphysema pathogenesis. Free Radic. Biol. Med. 2019, 131, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Harikumar, K.B. Sphingosine 1-Phosphate: A Novel Target for Lung Disorders. Front. Immunol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, N.D.; Grimmer, J.A.; Tanwar, V.; Schwieterman, N.; Mohler, P.J.; Wold, L.E. Cardiovascular risk of electronic cigarettes: A review of preclinical and clinical studies. Cardiovasc. Res. 2019, 116, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pittilo, M. Cigarette smoking, endothelial injury and cardiovascular disease. Int. J. Exp. Pathol. 2000, 81, 219–230. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric Acid and l-malic Acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Alternat. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, T.; Ji, F.; Wang, H.; Pang, J. Effect of GABA on blood pressure and blood dynamics of anesthetic rats. Int. J. Clin. Exp. Med. 2015, 8, 14296–14302. [Google Scholar]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef]
- Yu, D.; Yang, S.E.; Miller, B.R.; Wisinski, J.A.; Sherman, D.S.; Brinkman, J.A.; Tomasiewicz, J.L.; Cummings, N.E.; Kimple, M.E.; Cryns, V.L.; et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J. 2018, 32, 3471–3482. [Google Scholar] [CrossRef]
- Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Nevill, A.M.; Douglas, K.M.; Koutedakis, Y.; Kitas, G.D. Cigarette smoking significantly increases basal metabolic rate in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 70–73. [Google Scholar] [CrossRef]
- Dhar, I.; Lysne, V.; Seifert, R.; Svingen, G.F.T.; Ueland, P.M.; Nygård, O.K. Plasma methionine and risk of acute myocardial infarction: Effect modification by established risk factors. Atherosclerosis 2018, 272, 175–181. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press (US): Washington, DC, USA, 2018. [Google Scholar]
- Zhu, S.-H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control. 2014, 23 (Suppl. S3), iii3. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metab. Off. J. Metab. Soc. 2007, 3, 211–221. [Google Scholar]
- Singh, K.P.; Lawyer, G.; Muthumalage, T.; Maremanda, K.P.; Khan, N.A.; McDonough, S.R.; Ye, D.; McIntosh, S.; Rahman, I. Systemic biomarkers in electronic cigarette users: Implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. 2019, 5, 00182–2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Gaul, D.A.; Nan, H.; Kim, J.; Fernández, F.M. Deep Metabolomics of a High-Grade Serous Ovarian Cancer Triple-Knockout Mouse Model. J. Proteome Res. 2019, 18, 3184–3194. [Google Scholar] [CrossRef] [PubMed]
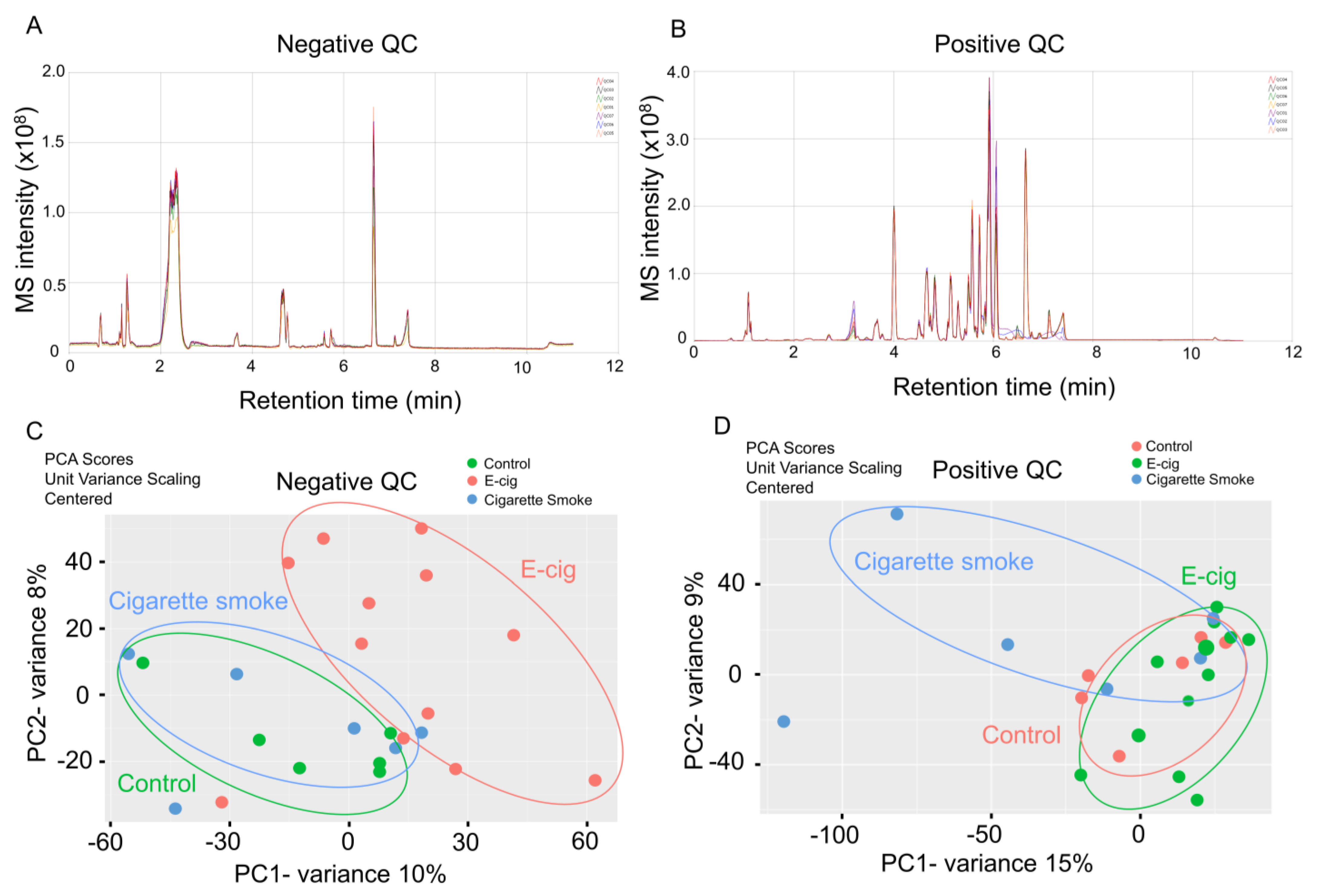

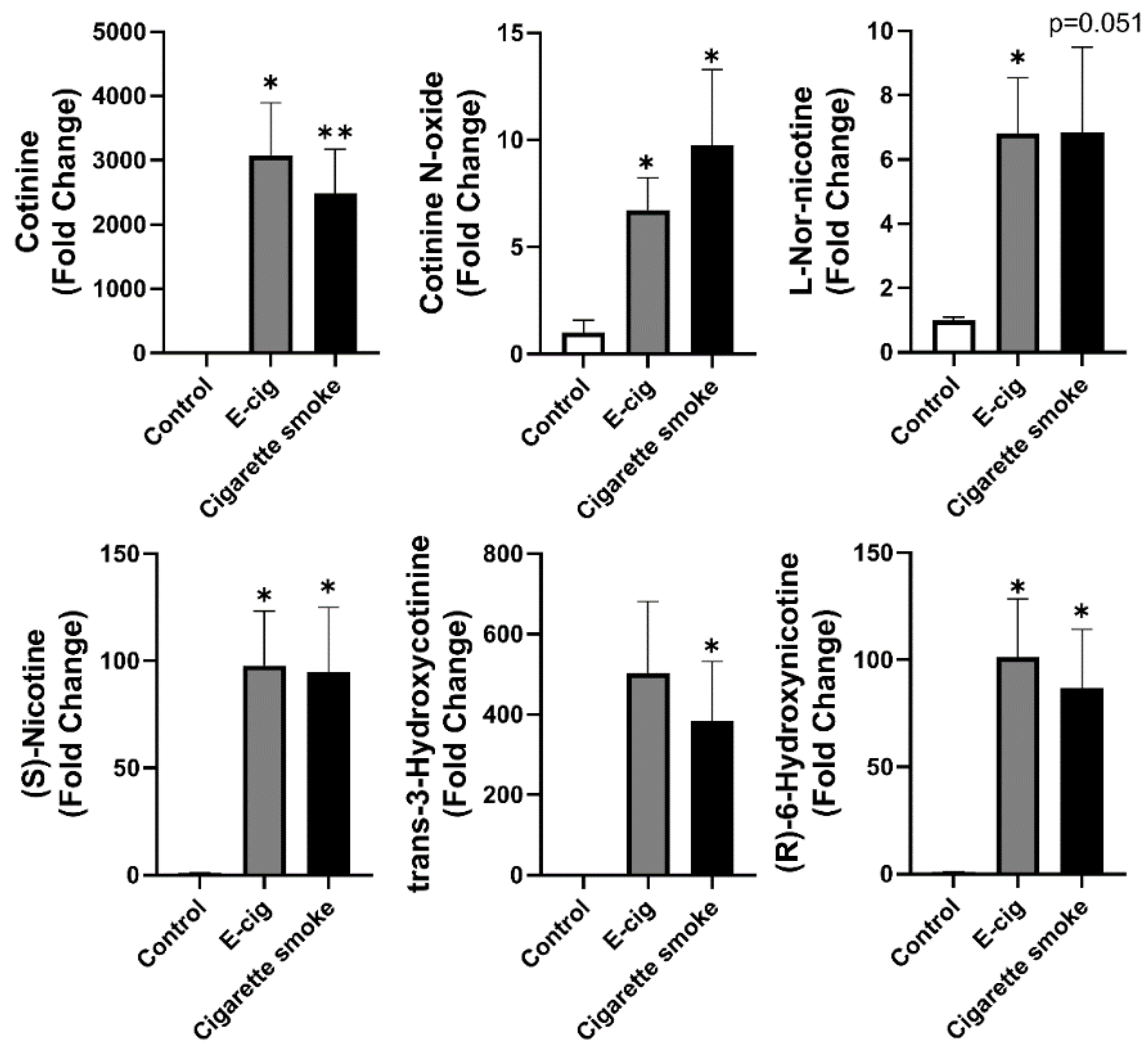
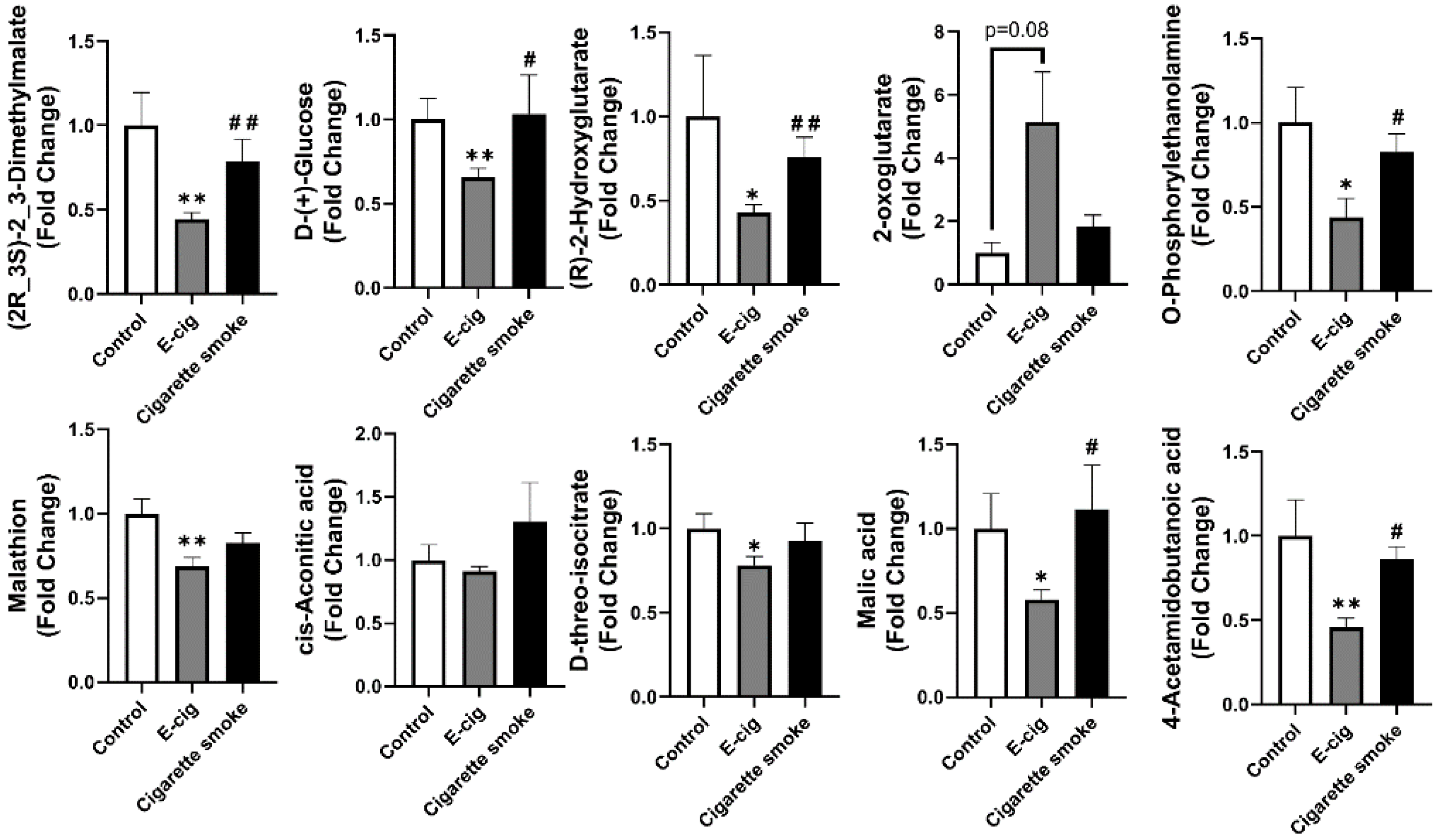
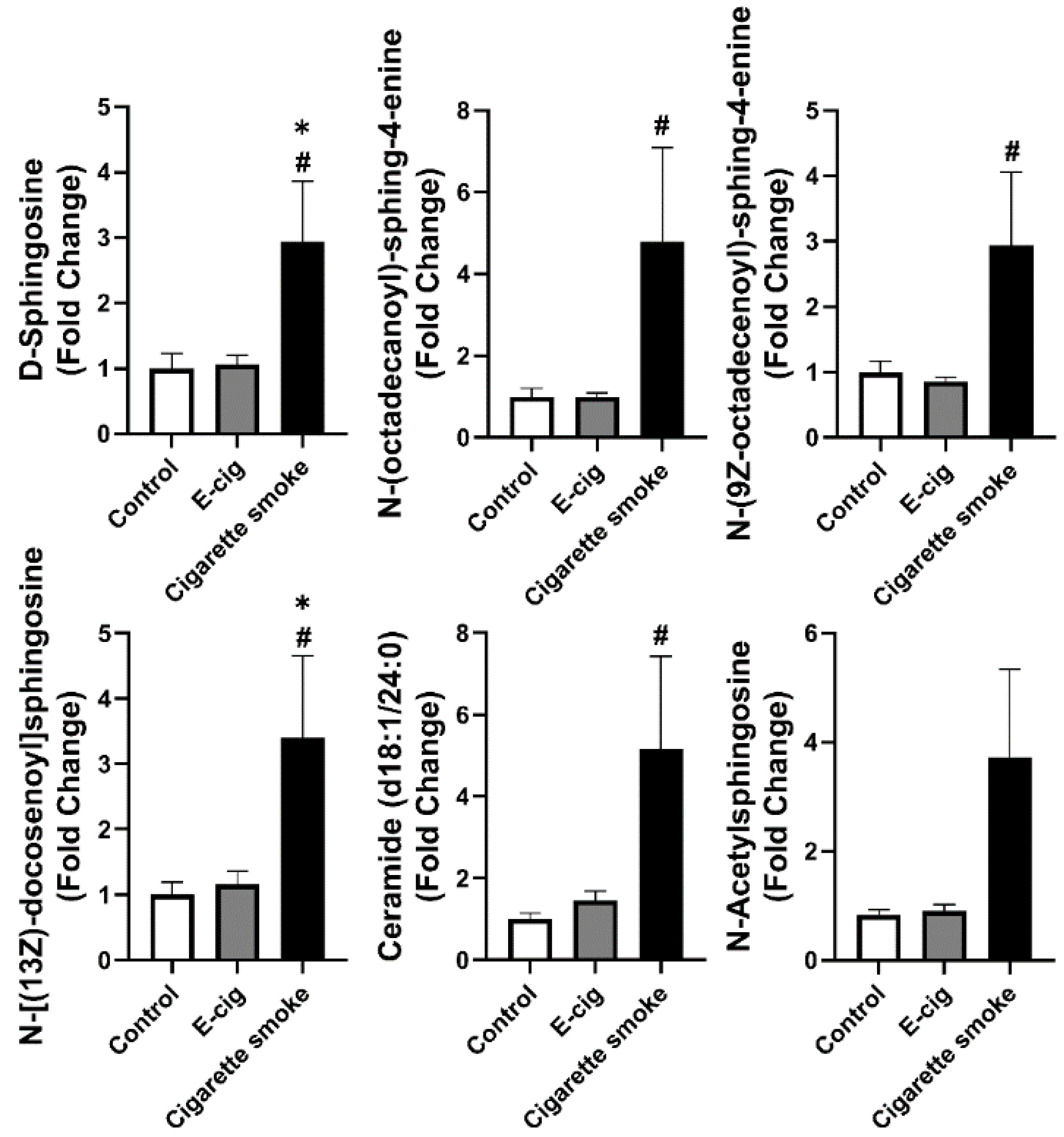
| Control vs. E-Cigarette | Control vs. Cigarette Smoke | ||||||
|---|---|---|---|---|---|---|---|
| Pathways | Overlap Size | Pathway Size | p-Value | Pathways | Overlap Size | Pathway Size | p-Value |
| Nicotine degradation III | 7 | 17 | 0.00361 | Nicotine degradation III | 6 | 17 | 0.00042 |
| Serotonin degradation | 5 | 7 | 0.00106 | Serotonin degradation | 3 | 7 | 0.00106 |
| Gluconeogenesis | 5 | 9 | 0.00138 | Gluconeogenesis | 4 | 9 | 0.00094 |
| TCA cycle | 8 | 9 | 0.00086 | nicotine degradation IV | 4 | 15 | 0.0016 |
| d-galactose degradation V | 6 | 6 | 0.00087 | ||||
| UDP-N-acetyl-d-galactosamine biosynthesis II | 6 | 7 | 0.00088 | ||||
| Group | Non-Smokers | E-Cigarette Users | Cigarette Smokers |
|---|---|---|---|
| Age | 43.17 ± 7.00 | 40.50 ± 4.24 | 44.00 ± 4.59 |
| Sex (Male/Female) | 3/3 | 6/6 | 3/3 |
| Ethnicity | |||
| Caucasian/White | 66.67% | 41.67% | 83.33% |
| African American | 16.67% | 25.00% | 16.67% |
| Asian | 16.67% | 8.33% | 0 |
| N/A | 0.00% | 25.00% | 0 |
| Name | Confidence Level * | Neutral Elemental Formula | Average Neutral MW | Average RT | Ion Type Detected | Theoretical Neutral Mass | Mass Error (ppm) | Description |
|---|---|---|---|---|---|---|---|---|
| Cotinine | 2 | C10H12N2O | 176.0950 | 2.70 | [M+H]+ | 176.0950 | 0.0 | MS2 matched to MZCloud |
| Cotinine N-oxide | 2 | C10H12N2O2 | 192.0899 | 4.45 | [M+H]+ | 192.0899 | 0.0 | MS2 matched to MZCloud |
| l-Nornicotine | 3 | C9H12N2 | 148.1001 | 4.53 | [M+H]+ | 148.1000 | 0.7 | Two RT 4.530 and 5.374 |
| (S)-Nicotine | 2 | C10H14N2 | 162.1157 | 5.47 | [M+H]+ | 162.1157 | 0.0 | MS2 matched to MZCloud |
| trans-3-Hydroxycotinine | 2 | C10H12N2O2 | 192.0899 | 2.65 | [M+H]+ | 192.0899 | 0.0 | MS2 matched to MZCloud |
| (R)-6-Hydroxynicotine | 3 | C10H14N2O | 178.1106 | 6.00 | [M+H]+ | 178.1106 | 0.0 | MS2 matched to MZCloud |
| (2R,3S)-2,3-Dimethylmalate | 3 | C6H10O5 | 162.0526 | 1.35 | [M-H]− | 162.0528 | −1.2 | No MS/MS |
| d-(+)-Glucose | 2 | C6H12O6·H2CO2 | 226.0688 | 3.27 | [M+HCO2]− | 226.0689 | −0.4 | MS2 matched at 226.0689 M+H2CO2 |
| (R)-2-Hydroxyglutarate | 3 | C5H8O5 | 148.0371 | 1.41 | [M-H]− | 148.0372 | −0.7 | MS2 matched to MZCloud (two RT 1.409 and 2.769) |
| 2-Oxoglutarate | 2 | C5H6O5 | 146.0214 | 1.98 | [M-H]− | 146.0215 | −0.7 | MS2 matched to MZCloud |
| O-Phosphorylethanolamine | 2 | C2H8NO4P | 141.0190 | 6.47 | [M-H]− | 141.0191 | −0.7 | MS2 matched to MZCloud |
| Malathion | 3 | C10H19O6PS2 | 330.0361 | 7.44 | [M-H]− | 330.0361 | 0.0 | malathion is a man-made insecticide |
| cis-Aconitic acid | 2 | C6H6O6 | 174.0162 | 0.84 | [M-H]− | 174.0164 | −1.1 | MS2 matched to MZCloud (3 RT 0.843, 1.195, 2.169) |
| d-threo-Isocitrate | 4 | C6H8O7 | 192.02687 | 6.72 | [M-H]− | 192.0270 | - | 8 peaks 5.387–7.169 |
| Malic acid | 2 | C4H6O5 | 134.0216 | 1.36 | [M-H]− | 134.0215 | 0.7 | MS2 matched to MZCloud |
| 4-Acetamidobutanoic acid | 2 | C6H11NO3 | 145.0739 | 1.56 | [M+H]+ | 145.0739 | 0.0 | MS2 matched to MZCloud |
| d-Sphingosine | 2 | C18H37NO2 | 299.2826 | 3.84 | [M+H]+ | 299.2824 | 0.7 | MS2 matched to local Database and MzCloud |
| N-(Octadecanoyl)-sphing-4-enine | 3 | C36H71NO3 | 565.5437 | 1.12 | [M+H]+ | 565.5434 | 0.5 | No MS/MS |
| N-(9Z-Octadecenoyl)-sphing-4-enine | 3 | C36H69NO3 | 563.5281 | 1.12 | [M+H]+ | 563.5277 | 0.7 | No MS/MS |
| [SP(20:0)]N-(Eicosanoyl)-sphing-4-enine | 3 | C38H75NO3 | 593.575 | 1.12 | [M+H]+ | 593.5747 | 0.5 | No MS/MS |
| [SP(22:0)]N-(Docosanoyl)-sphing-4-enine | 3 | C40H79NO3 | 621.6063 | 1.12 | [M+H]+ | 621.6060 | 0.5 | No MS/MS |
| N-[(13z)-Docosenoyl]sphingosine | 3 | C40H77NO3 | 619.5906 | 1.11 | [M+H]+ | 619.5903 | 0.5 | No MS/MS |
| Ceramide (d18:1/24:0) | 2 | C42H83NO3 | 649.6376 | 1.12 | [M+H]+ | 649.6373 | 0.5 | MS2 matched to local Database and MzCloud |
| dl-4-Hydroxyphenyllactic acid | 2 | C9H10O4 | 182.0579 | 1.188 | [M-H]− | 182.0579 | 0.0 | MS2 matched to local Database and MzCloud |
| S-(3-oxo-3-Carboxy-n-propyl) cysteine | 3 | C7H11NO5S | 221.0361 | 1.245 | [M-H]− | 221.0358 | 1.4 | MS2 does not match well to in silico prediction |
| Glycolic acid | 3 | C2H4O3 | 76.0160 | 1.368 | [M-H]− | 76.0160 | 0.0 | |
| 2-beta-d-Glucosyle anthranilate | 3 | C13H17NO7 | 299.1007 | 6.46 | [M+H]+ | 299.1005 | 0.7 | This structure has the amine group ortho, and there is also an isomer where amine is para. |
| 4-(Stearoylamino)butanoic acid | 4 | C22H43NO3 | 369.3246 | 1.082 | [M+H]+ | - | - | MS2 does not match well to in silico prediction |
| l-(−)-Methionine | 2 | C5H11NO2S | 149.0511 | 4.855 | [M+H]+ | 149.0511 | 0.0 | MS2 matched to MZCloud |
| 3-Methylsulfolene | 4 | C5H8O2S | 132.0246 | 4.862 | [M+H]+ | - | - | MS2 does not match well to in silico prediction, isotopic pattern did not match sulfur-containing formula |
| 2-Methylthiazolidine | 3 | C4H9NS | 103.0456 | 4.861 | [M+H]+ | 103.0456 | 0.0 | MS2 matches in silico prediction, isotopic pattern suggests sulfur-containing formula |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ji, X.; Rahman, I. Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking. Metabolites 2021, 11, 345. https://doi.org/10.3390/metabo11060345
Wang Q, Ji X, Rahman I. Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking. Metabolites. 2021; 11(6):345. https://doi.org/10.3390/metabo11060345
Chicago/Turabian StyleWang, Qixin, Xiangming Ji, and Irfan Rahman. 2021. "Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking" Metabolites 11, no. 6: 345. https://doi.org/10.3390/metabo11060345
APA StyleWang, Q., Ji, X., & Rahman, I. (2021). Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking. Metabolites, 11(6), 345. https://doi.org/10.3390/metabo11060345







