Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia
Abstract
1. Introduction
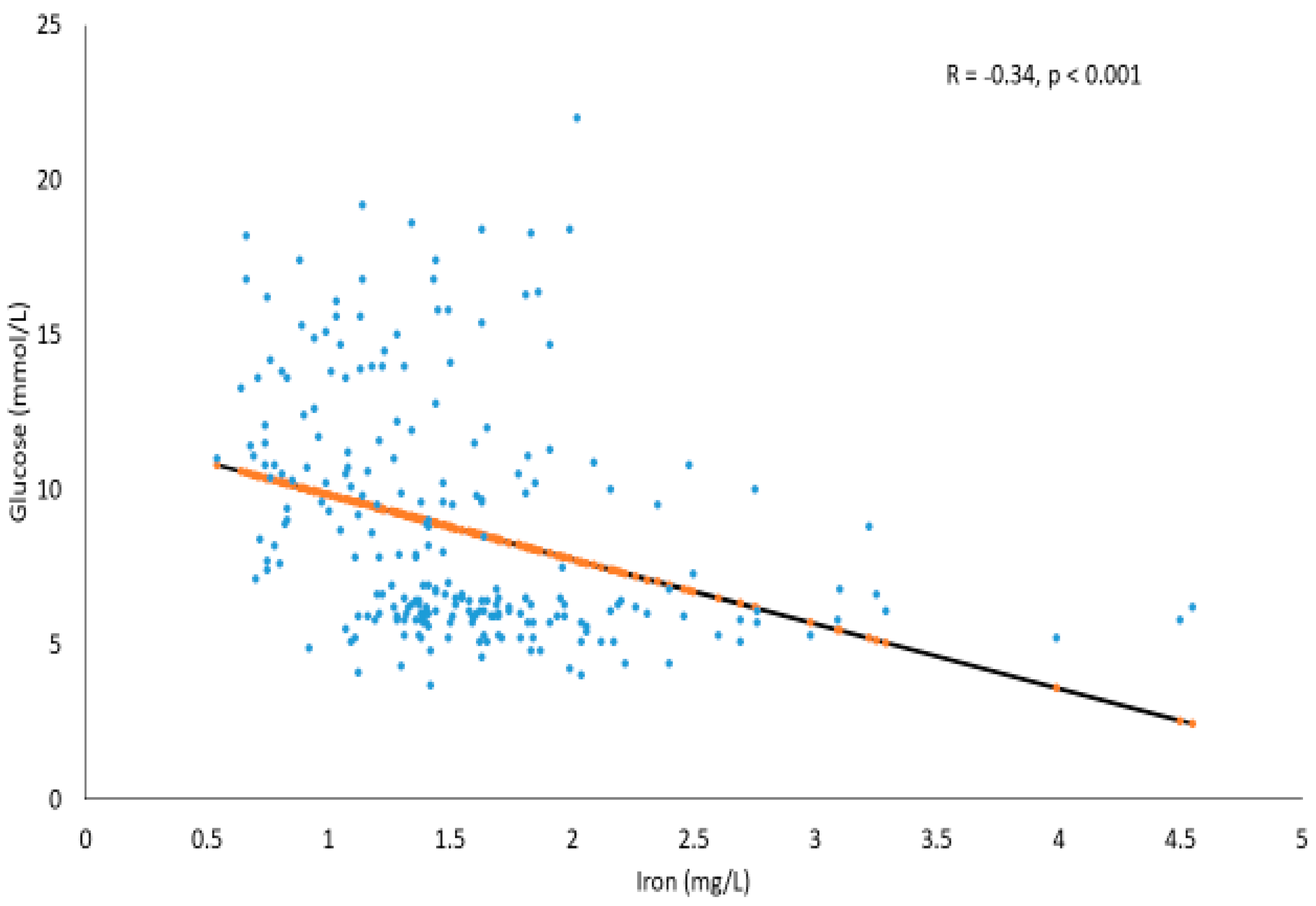
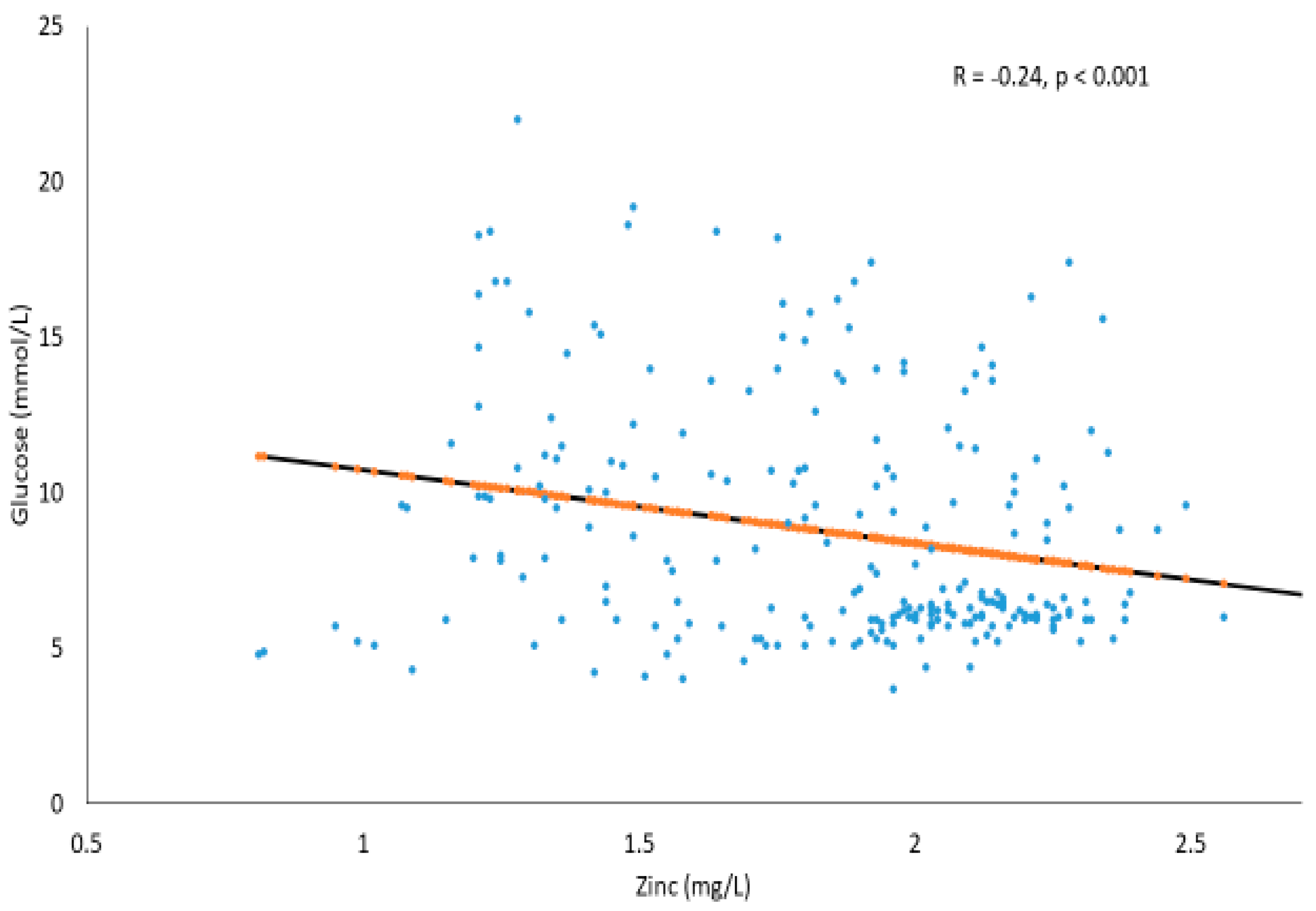
2. Results
Correlations between Serum Glucose and Trace Elements in T2DM and Prediabetes Subjects
3. Discussion
4. Materials and Methods
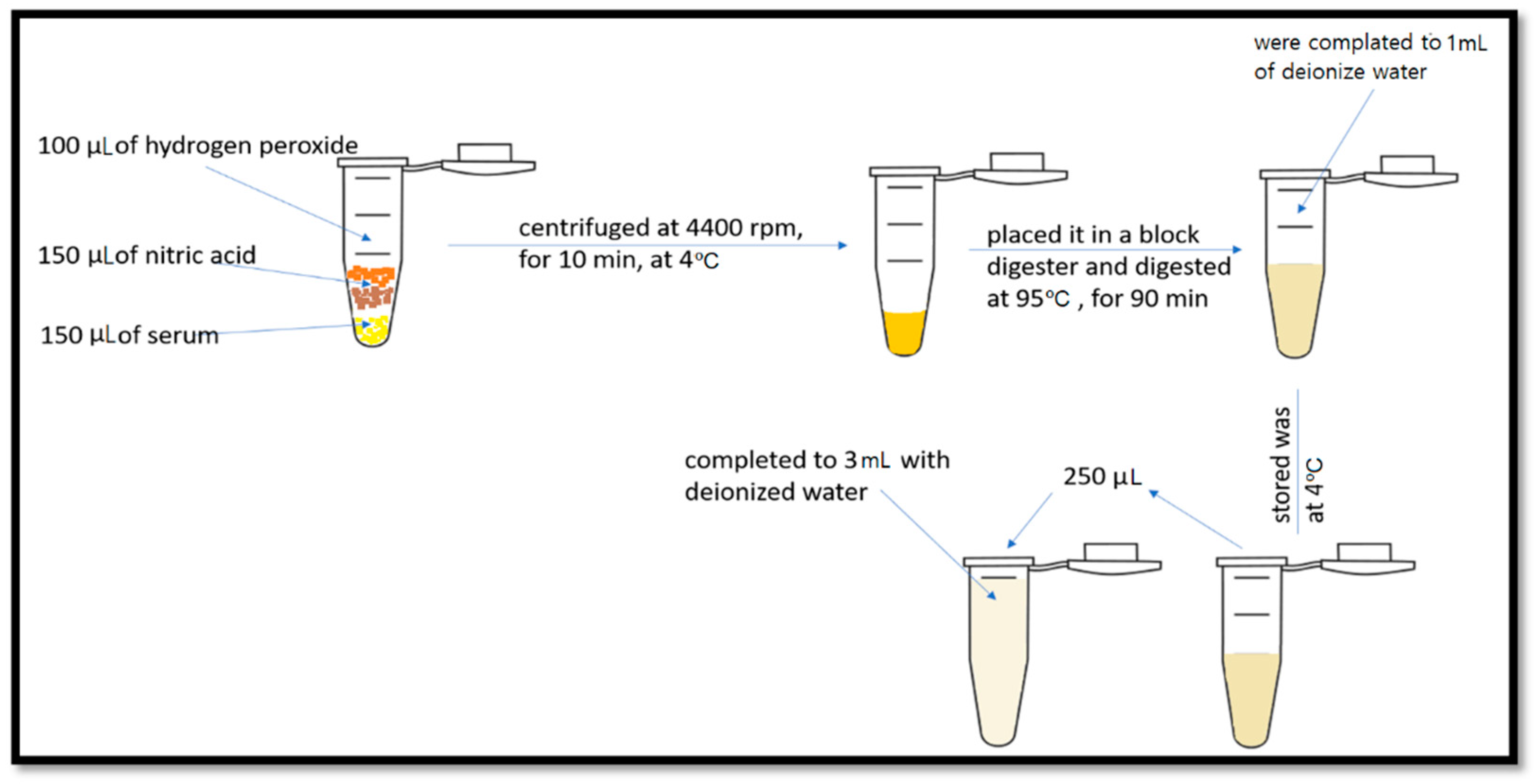
4.1. Trace Element Determination
4.2. Statistical Analysis
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2009, 1, 169–176. [Google Scholar] [CrossRef]
- International Diabetes Federation. Idf diabetes atlas. Bruss. Int. Diabetes Fed. 2013, 128, 40–50. [Google Scholar]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes Mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Clerici, M.; Al-Attas, O.; Forni, D.; Alokail, M.S.; Alkharfy, K.M.; Sabico, S.; Mohammed, A.K.; Cagliani, R.; Sironi, M. A nonsense polymorphism (R392X) in TLR5 protects from obesity but predisposes to diabetes. J. Immunol. 2013, 190, 3716–3720. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Alokail, M.S.; Alenad, A.M.; Al-Attas, O.S.; Mohammed, A.K.; Sabico, S.; Albagha, O.M. Assessing the contribution of 38 genetic loci to the risk of type 2 diabetes in the Saudi Arabian Population. Clin. Endocrinol. 2014, 80, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Al-Attas, O.; Al-Daghri, N.; Alokail, M.; Abd-Alrahman, S.; Vinodson, B.; Sabico, S. Metabolic Benefits of Six-month Thiamine Supplementation in Patients With and Without Diabetes Mellitus Type 2. Clin. Med. Insights Endocrinol. Diabetes 2014, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Daghri, N.M.; Sabico, S.B.; Al-Othman, A.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation in patients with diabetes mellitus type 2 on different therapeutic regimens: A one-year prospective study. Cardiovasc. Diabetol. 2013, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Hajra, B.; Orakzai, S.A.; Faryal, U.; Hassan, M.; Rasheed, S.; Wazir, S. Insulin sensitivity to trace metals (chromium, manganese) in type 2 diabetic patients and non diabetic individuals. J. Ayub Med. Coll. Abbottabad 2016, 28, 534–536. [Google Scholar]
- Lu, S.-Y.; Zhang, H.-M.; Sojinu, S.O.; Liu, G.-H.; Zhang, J.-Q.; Ni, H.-G. Trace elements contamination and human health risk assessment in drinking water from shenzhen, china. Environ. Monit. Assess. J. 2015, 187, 1–8. [Google Scholar] [CrossRef]
- Al-Fartusie, F.S.; Mohssan, S.N. Essential trace elements and their vital roles in human body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar]
- Zhang, H.; Yan, C.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. Alterations of serum trace elements in patients with type 2 diabetes. J. Trace Elem. Med. Biol. 2017, 40, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Awan, F.R. Metals in the pathogenesis of type 2 diabetes. J. Diabetes Metab. Dis. 2014, 13, 1–6. [Google Scholar] [CrossRef]
- Hansen, A.F.; Simić, A.; Åsvold, B.O.; Romundstad, P.R.; Midthjell, K.; Syversen, T.; Flaten, T.P. Trace elements in early phase type 2 diabetes mellitus—A population-based study. The hunt study in norway. J. Trace Elem. Med. Biol. 2017, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Naqash, A.; Bader, G.N. Influence of iron deficiency anemia on hba1c. Curr. Res. Diabetes Obes. J. 2018, 5, 1–3. [Google Scholar]
- Brooks, A.; Metcalfe, J.; Day, J.; Edwards, M. Iron deficiency and glycosylated haemoglobin a1. Lancet 1980, 316, 141. [Google Scholar] [CrossRef]
- Devi, T.R.; Hijam, D.; Dubey, A.; Debnath, S.; Oinam, P.; Devi, N.T.; Singh, W.G. Study of serum zinc and copper levels in type 2 diabetes mellitus. Int. J. Contemp. Med. Res. 2016, 3, 1036–1040. [Google Scholar]
- Pushparani, D.S.; Anandan, S.N.; Theagarayan, P. Serum zinc and magnesium concentrations in type 2 diabetes mellitus with periodontitis. J. Indian Soc. Periodontol. 2014, 18, 187–193. [Google Scholar] [CrossRef]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef]
- Maher, M.; Ahmed, S. A study of serum magnesium, zinc, copper and glycohemoglobin in children with type 1 diabetes mellitus. Alex. J. Pediatr. 2002, 16, 285–289. [Google Scholar]
- Anderson, R.A.; Mertz, W. Glucose tolerance factor: An essential dietary agent. Trends Biochem. Sci. 1977, 2, 277–279. [Google Scholar] [CrossRef]
- Brun, J.-F.; Fons, C.; Fussellier, M.; Bardet, L.; Orsetti, A. Urinary zinc and its relationships with microalbuminuria in type i diabetics. Biol. Trace Elem. Res. 1992, 32, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Basaki, M.; Saeb, M.; Nazifi, S.; Shamsaei, H. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol. Trace Elem. Res. 2012, 148, 161–164. [Google Scholar] [CrossRef]
- Atalay, H.; Boyuk, B.; Guzel, S.; Altay, M.; Kiziler, A.R.; Aydemir, B. Serum trace elements in type 2 diabetes mellitus. Acta Med. 2017, 33, 795. [Google Scholar]
- Naidu, M.; Kumar, S.; Vali, S.; Madhav, D.; Subrahmanyam, G. Study of the role of copper, zinc and magnesium in diabetic nephropathy. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 72. [Google Scholar]
- Kumar, N.; Bhatnagar, V. A study of trace elements in serum of diabetic patients. J. Appl. Dent. Med. Sci. 2018, 17, 4–7. [Google Scholar]
- Zabłocka-Słowińska, K.; Grajeta, H. Selenium and copper in type 2 diabetes mellitus-more doubt than certainty. J. Elementol. 2017, 22, 365–376. [Google Scholar] [CrossRef]
- Aljohani, A.H.; Alrubyyi, M.A.; Alharbi, A.B.; Alomair, A.M.; Alomair, A.A.; Aldossari, N.A.; Alghamdi, S.K.; Alawaji, S.A.; Alamri, S.A.S.; Tallab, O.T.M. The relation between diabetes type ii and anemia. Egypt. J. Hosp. Med. 2018, 70, 526–531. [Google Scholar] [CrossRef][Green Version]
- Kumar, C.M.; Nutakki, G. A prospective study of effect of iron deficiency anemia on hba1c levels in non-diabetics. Int. Arch. Med. 2017, 4, 137–146. [Google Scholar]
- Moulani, M.R.; Gawali, P.S. Effect of iron deficiency on glycation of hemoglobin in non diabetics. Ann. Clin. Chem. Lab. Med. 2016, 2, 4–7. [Google Scholar] [CrossRef][Green Version]
- Madhu, S.; Raj, A.; Gupta, S.; Giri, S.; Rusia, U. Effect of iron deficiency anemia and iron supplementation on hba1c levels-implications for diagnosis of prediabetes and diabetes mellitus in asian indians. Clin. Chim. Acta 2017, 468, 225–229. [Google Scholar] [CrossRef]
- Veeramalla, V.; Madas, S. Correlation of glycated hemoglobin and iron deficiency anemia among diabetic and non diabetic patients. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2669–2675. [Google Scholar] [CrossRef]
- Khalil, M.Y.; Abdrabo, A.A.; Ismail, A.M. Evaluation of zinc level among vitamin d deficient type2 diabetes mellitus patients. Br. J. Med. Health Res. 2015, 2, 53–61. [Google Scholar]
- Zargar, A.H.; Shah, N.A.; Masoodi, S.R.; Laway, B.A.; Dar, F.A.; Khan, A.R.; Sofi, F.A.; Wani, A.I. Copper, zinc, and magnesium levels in non-insulin dependent diabetes mellitus. Postgrad. Med. J. 1998, 74, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.; Manjrekar, P.A.; Agarwal, A.; Ahmad, A.; Hegde, A.; Srikantiah, R.M. Association of serum selenium, zinc and magnesium levels with glycaemic indices and insulin resistance in pre-diabetes: A cross-sectional study from south india. Biol. Trace Elem. Res. 2017, 175, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; Islam, M.R.; Hancock, S.; Attia, J. Is serum zinc associated with pancreatic beta cell function and insulin sensitivity in pre-diabetic and normal individuals? Findings from the hunter community study. PLoS ONE 2014, 9, e83944. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.G.; Afridi, H.I.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Kandhro, G.A. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol. Trace Elem. Res. 2008, 122, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pompano, L.M.; Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Diabetes and zinc dyshomeostasis: Can zinc supplementation mitigate diabetic complications? Crit. Rev. Food Sci. Nutr. 2020, 1–16. [Google Scholar] [CrossRef]
- Fung, E.B.; Ahmad, T.; Killilea, D.W.; Hussain, R.; Lal, A. Zinc supplementation improves markers of glucose homeostasis in thalassaemia. Br. J. Haematol. 2020, 190, e162–e166. [Google Scholar] [CrossRef]

| Parameters | Non-T2DM | Prediabetes | T2DM | p-Value | p-Value * |
|---|---|---|---|---|---|
| N | 95 | 80 | 119 | -- | -- |
| Age (Years) | 40.9 ± 13.7 | 46.7 ± 10.9 | 52.2 ± 11.2 A,B | 0.000 | -- |
| Female/Male | 68/27 | 49/31 | 57/62 | 0.002 | -- |
| BMI (kg/m2) | 29.9 ± 6.0 | 32.6 ± 7.6 | 31.8 ± 6.0 | 0.017 | -- |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.003 | 0.13 |
| Systolic BP (mmHg) | 112.3 ± 10.3 | 124.3 ± 14.1 A | 128.5 ± 15.1 A | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 74.1 ± 7.0 | 78.5 ± 8.0 | 79.6 ± 9.0 | 0.004 | 0.06 |
| Trace Minerals | Non-T2DM | Prediabetes | T2DM | p-Value | p-Value * |
|---|---|---|---|---|---|
| Cu (mg/L) | 2.7 ± 1.3 | 1.5 ± 0.5 A | 0.9 ± 0.6 A,B | <0.001 | <0.001 |
| Fe (mg/L) | 1.8 ± 0.4 | 1.8 ± 0.7 | 1.3 ± 0.5 A,B | <0.001 | <0.001 |
| Zn (mg/L) | 1.7 ± 0.4 | 2.1 ± 0.1 A | 1.7 ± 0.4 B | <0.001 | <0.001 |
| Radio Frequency Power | 1600 W |
|---|---|
| Nebulizer gas flow | 0.65 L/min |
| Lens Voltage | 9.55 V |
| Analog Stage Voltage | −1745 V |
| Pulse Stage Voltage | 950 V |
| Number of Replicates | 3 |
| Reading/Replicates | 20 |
| Scan Mode | Peak Hopping |
| Dwell Time | 40 ms |
| Integration | 1200 ms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakout, S.; Faqeeh, F.; Al-Attas, O.; Hussain, S.D.; Al-Daghri, N.M. Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia. Metabolites 2021, 11, 297. https://doi.org/10.3390/metabo11050297
Yakout S, Faqeeh F, Al-Attas O, Hussain SD, Al-Daghri NM. Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia. Metabolites. 2021; 11(5):297. https://doi.org/10.3390/metabo11050297
Chicago/Turabian StyleYakout, Sobhy, Fatimah Faqeeh, Omar Al-Attas, Syed D. Hussain, and Nasser M. Al-Daghri. 2021. "Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia" Metabolites 11, no. 5: 297. https://doi.org/10.3390/metabo11050297
APA StyleYakout, S., Faqeeh, F., Al-Attas, O., Hussain, S. D., & Al-Daghri, N. M. (2021). Patterns and Associations of Essential Trace Elements (Cu, Fe and Zn) in Saudi Adults with Varying Levels of Glycemia. Metabolites, 11(5), 297. https://doi.org/10.3390/metabo11050297







