Abstract
Metabolic reprogramming is an emerging hallmark of cancer and is driven by abnormalities of oncogenes and tumor suppressors. Accelerated metabolism causes cancer cell aggression through the dysregulation of rate-limiting metabolic enzymes as well as by facilitating the production of intermediary metabolites. However, the mechanisms by which a shift in the metabolic landscape reshapes the intracellular signaling to promote the survival of cancer cells remain to be clarified. Recent high-resolution mass spectrometry-based proteomic analyses have spotlighted that, unexpectedly, lysine residues of numerous cytosolic as well as nuclear proteins are acetylated and that this modification modulates protein activity, sublocalization and stability, with profound impact on cellular function. More importantly, cancer cells exploit acetylation as a post-translational protein for microenvironmental adaptation, nominating it as a means for dynamic modulation of the phenotypes of cancer cells at the interface between genetics and environments. The objectives of this review were to describe the functional implications of protein lysine acetylation in cancer biology by examining recent evidence that implicates oncogenic signaling as a strong driver of protein acetylation, which might be exploitable for novel therapeutic strategies against cancer.
1. Introduction
Metabolic reprogramming is an emerging hallmark of cancer and is driven by abnormalities of oncogenes and tumor suppressors [1]. Accelerated metabolism translates into cancer cell aggression through the dysregulation of rate-limiting metabolic enzymes as well as by facilitating the production of intermediary metabolites [2], but it remains to be fully clarified how a shift in the metabolic landscape reshapes the intracellular signaling to promote the survival of cancer cells [3,4]. Post-translational modification (PTM) of proteins is an essential phenomenon that dynamically regulates cellular functions in an appropriate spatio-temporal manner and which is responsive to a drastic shift in the microenvironment [5,6]. Recent studies have demonstrated that lysine acetylation, one of the major protein PTMs, is prevalent for a variety of enzymes that catalyze intracellular metabolism [7], suggesting that protein acetylation plays a major role in cellular functions including synchronous metabolic cascades [7,8]. Advanced proteomics using mass spectrometry have further enabled the global identification and characterization of thousands of acetylation sites which are involved in the regulation of protein function by affecting protein interactions with nucleic acids and other proteins, the catalytic activity of proteins, and protein sublocalization [8,9]. More importantly, cancer cells exploit the PTM of proteins with acetylation for adapting to the microenvironment, suggesting that acetylation may dynamically modulate the phenotypes of cancer cells at the interface of genetics and the environment [5,10,11,12]. Here, the purpose of the review carried out was to describe the functional implications of protein lysine acetylation in different cellular compartments, highlighting its biological role and prognostic value in cancer, by evaluating recent evidence implicating oncogenic signaling as a strong driver of protein acetylation, which could be exploitable for novel therapeutic strategies against cancer.
2. Regulatory Mode of Protein Lysine Acetylation
2.1. Acetylation Enzymes: Writers, Erasers and Readers of Lysine Acetylation
2.1.1. Writers of Lysine Acetylation
Protein acetylation, represented by the status of N-epsilon acetyl lysine, is controlled by the combinatory action of both lysine acetyltransferases (KATs) and lysine deacetylases (KDACs) [13]. KATs transfer the acetyl group from the intermediary metabolite, acetyl coenzyme A (acetyl-CoA), to the epsilon NH3+ side chain of lysines within the targeted protein, that is to say, work as a “writer of lysine acetylation” (Figure 1). The transfer of the acetyl group eventually neutralizes the positive charge on the lysine residue and changes the structure of the R group on the amino acid, affecting multifaceted aspects of the targeted protein [14]. An array of mammalian proteins have been reported to possess endogenous KAT activity, but KATs are basically subclassified into three families based on phylogenetic sequence similarities: (1) the GNAT (GCN5-related N-acetyltransferases) family that includes GCN5 (KAT2A) and PCAF (p300/CBP-associated factor, KAT2B), (2) the p300 (KAT3A)/CBP (CREBBP) [cAMP-responsive element-binding protein (CREB)-binding protein, KAT3B] family, and (3) the MYST family for MOZ (monocytic leukemia zinc finger protein, KAT6A), Ybf2, Sas2 and Tip60 (KAT5) (Table 1) [15]. KATs are usually incorporated within the unique molecular complexes that increase capacity for target specificity and facilitate interactions with a range of other proteins at a subset of enhancer and promoter elements as well as in gene bodies of transcriptionally active genes [14,15]. Of interest, the sublocalization and enzymatic activity of KATs can also be regulated by PTMs that include phosphorylation and acetylation, and in the example of p300 and PCAF, can be auto-acetylated for activation and stability, suggesting the intricate reciprocal interaction among the subcellular components by acetylation [16]. Importantly, some of these regulatory mechanisms of KATs rely upon oncogene activation, and it is not surprising that several KATs are associated with oncogenesis, in addition to other important functions in cellular differentiation and embryonic development [17].
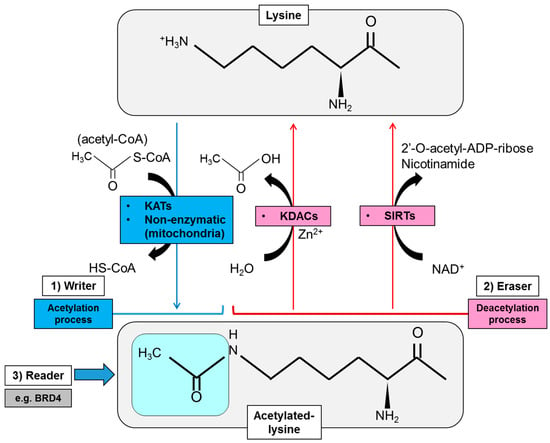
Figure 1.
Regulatory mode of protein lysine acetylation by modification enzymes including writers, erasers and readers of lysine acetylation. Writers of protein acetylation (KATs) transfer the acetyl group from an intermediary metabolite acetyl-CoA to the epsilon NH3+ side chain of lysines of the targeted protein. Acetylation in mitochondrial protein can also be processed non-enzymatically. Acetylation eraser (deacetylase) activity is mediated by Zn2+-dependent KDACs [class I, II and IV histone deacetylases (HDACs)], and class III HDAC or sirtuins (SIRTs) depending on NAD+. The acetylation marks on lysine residues of the histone protein are read by small protein modules called the bromodomain and extra-terminal (BET) proteins including BRD4. ADP, adenosine diphosphate; BRD4, bromodomain-containing protein 4; KAT, lysine acetyltransferase; KDAC, lysine deacetylase; NAD, nicotinamide adenine dinucleotide; SIRT, sirtuin.

Table 1.
Lysine acetyltransferases (KATs) family and aberration in cancer.
Lysine acetylation can also be achieved in a non-enzymatic fashion (Figure 1), notably in the acetylation of calf thymus nuclear histone protein with acetyl-CoA, that is dependent on pH, period of incubation, ionic strength and ionic species [35]. Recent studies also suggested that, under basal conditions, “non-enzymatic lysine acetylation” is maintained at a very low stoichiometry in mitochondria [36,37]. Acetyl-CoA and acetyl-glutathione reversibly acetylate protein cysteine residues, and non-enzymatic N-acetylation of lysine residues by acetyl-CoA occurs via proximal S-acetylated thiol intermediates that are sensitive to glyoxalase II in mitochondria [36]. Further, a large-scale lysine acetylation study has revealed that only approximately 20% of non-enzymatic acetylation sites are targeted by mitochondrial protein deacetylases, and there are sites at which acetylation does not alter the activity of the protein, while others can irreversibly inhibit the targeted enzyme [38]. Currently, less is known about the role of non-enzymatic acetylation in cancer in comparison with that by KATs, but several lines of evidence suggest that targeting non-enzymatic acetylation could be an important niche for future development of therapies in targeting cancer metabolism [39].
2.1.2. Erasers of Lysine Acetylation
Erasers of protein lysine acetylation are a group of protein called KDACs or HDACs (histone deacetylases) because this group mainly deacetylates the epsilon-amino group of lysine (K) residues on the nuclear histone (H) proteins that constitute the core octamers for nucleosomal complexes (Figure 1). Histone deacetylation results in the restoration of their positive charge, which increases their ability to bind to negatively charged DNA and eventually hinder the access of transcriptional complexes. In mammalian HDACs, 18 highly conserved genes were noted [40], and they are subdivided into Class I (HDAC1, HDAC2, HDAC3, HDAC8), Class IIa (HDAC4, HDAC5, HDAC7, HDAC9), Class IIb (HDAC 6, HDAC 10), Class III (sirtuin, or SIRT1-7) and Class IV (HDAC11) on the basis of phylogenetic analysis and sequence similarity to yeast factors (Table 2) [41]. The catalytic domain of HDACs is similar to a pocket and consists of two adjacent histidine residues, two aspartate residues and one tyrosine residue with Zn2+ ions as the core [42], whereas the deacetylase activity of class III or sirtuins (SIRTs) depends on nicotinamide adenine dinucleotide (NAD+) rather than on Zn2+-dependent enzymes (Figure 1) [43]. Similar to KATs, HDACs act on multiprotein complexes which ensure specific mechanisms of active repression of transcription in the promoter regions of the gene [44]. Notably, HDACs were also reported to deacetylate non-histone nuclear/cytoplasmic protein to regulate the sublocalization and activity of the targeted protein, which is involved in physiological, as well as abnormal, conditions including metabolic diseases and cancer [45,46,47].

Table 2.
Lysine deacetylases (KDACs/HDACs) family and aberration in caner.
2.1.3. Readers of Lysine Acetylation
One of the primary proteins that are targeted by KAT/HAT (histone acetyltransferase) and KDAC/HDAC are histone proteins, whose dynamic modification is involved in various biological processes and is correlated with several human diseases, including cancer. The acetylation marks on lysine residues of the histone proteins are read by small protein modules called the bromodomain and extra-terminal (BET) proteins (namely BRD2, BRD3, BRD4, and BRDT) that utilize tandem bromodomain (BRD) modules to recognize and dock themselves on the acetylated lysines (Figure 1) [68]. BET protein BRD4 binds particularly to acetylated histones at enhancers and promoters via its bromodomains where it regulates transcriptional elongation through interaction with transcriptional complexes including P-TEFb (positive transcription elongation factor b) and mediator [69]. Of interest, BRD4 is highly enriched in super-enhancers that drive the expression of oncogenic transcription factors such as c-Myc, suggesting that targeting BET family proteins could be a promising approach for cancer treatment [70]. Additionally, a recent report suggests a mechanism by which enhancer-directed transcripts (eRNAs) are directly associated with gene regulation by modulating enhancer interactions and transcriptional functions of BRD4 [71], further expanding the role of BET proteins in the normal physiology, as well as cancer biology. Together with the writers and erasers of protein lysine acetylation, readers of lysine acetylation thus are of equal importance for the phenotypes of cancer cells and provide the promising opportunity to develop a novel type of therapeutics to specifically target cancer transcriptomes.
2.2. Donor Substrate for Acetylation: Production of Intermediary Metabolites for Protein Acetylation
Many enzymes that play important roles in epigenetic gene regulation harness intermediary metabolites as co-substrates yielded by cellular metabolic reprogramming [1,72]. The methyl-donor SAM (S-adenosylmethionine) which is derived from methionine is utilized by methyltransferases, and its metabolism can profoundly affect epigenetic changes including DNA and histone methylation status [73,74]. As for protein acetylation, acetyl-CoA is the substrate used to modify histone tails as well as non-histone proteins and can be produced through a variety of metabolic pathways [75]. The acetyl donor, acetyl-CoA, can be obtained from a number of sources, primarily through the conversion of pyruvate from glycolysis and citrate from the tricarboxylic acid (TCA) cycle. Acetyl-CoA is also released from the breakdown of fatty acids and amino acids in the mitochondria while pyruvate derived from glucose can be converted into acetyl-CoA by the pyruvate dehydrogenase complex (PDC) (Figure 2). In addition, recent studies have demonstrated that the dynamic translocation of mitochondrial pyruvate dehydrogenase (PDH) to the nucleus provides a pathway for nuclear acetyl-CoA synthesis required for histone acetylation and epigenetic regulation [76,77,78]. Importantly, the nuclear translocation of PDH is facilitated by the stimulation of growth factor receptor and mTOR (mechanistic target of rapamycin) pathway signaling [77]. ATP citrate lyase (ACLY) is another key enzyme responsible for generating cytosolic acetyl-CoA and oxaloacetate which are important metabolites for cancer cells (Figure 2). ACLY can be regulated by growth factor stimulation, which is also required for histone acetylation and gene expression [79], and inhibition of ACLY results in tumor growth arrest [80]. Of interest, acetyl-CoA can be produced by PDH from glucose and by acyl-coenzyme A synthetase short-chain family member 2 (ACSS2) from acetate, both of which are closely associated with the biology of the malignant brain tumor, glioblastoma (GBM), through acetylation of cytosolic proteins (Figure 2) [81]. In addition to glucose, acetate is also an emerging target nutrient of interest for cancer biology. Acetate can be used by tumor cells as an important bioenergetic fuel or as a nutritional source to support lipid biosynthesis as well as a precursor for acetylation of histones and other proteins and hence can serve as an epigenetic and post-translational modifier [82]. Of note, despite its low circulating concentration in plasma, acetate could still exert its effects through intra- and intercellular recycling of acetate molecules within the tumor microenvironment, leading to the role of acetate as a positron emission tomography (PET) imaging probe for cancer as well as an exploitable metabolite for future anti-ACSS2 therapy against cancer [83].
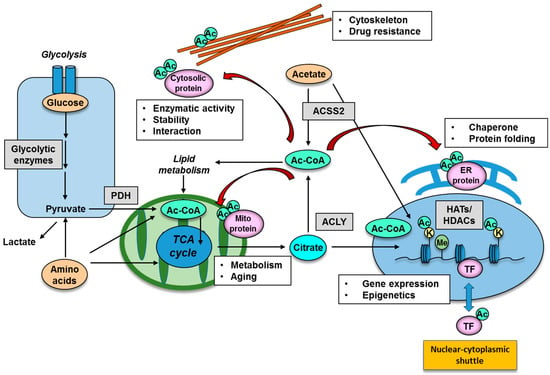
Figure 2.
Functional significance of lysine acetylation in different cellular organelle. Protein acetylation is mediated by an intermediary metabolite acetyl-CoA, produced by the enzymes of PDH, ACLY and ACSS2. Representative nuclear protein which could be regulated by acetylation is histone protein and transcription factors, the acetylation of which has an impact on gene expression and epigenetic changes. Acetylation of cytoplasmic organelle is represented by that of cytoskeleton, mitochondria and ER protein, and acetylation in these organelle could be involved in intracellular metabolism, aging, protein chaperone and drug resistance. Ac, acetyl group; Ac-CoA, acetyl-CoA; ACLY, ATP citrate lyase; ACSS2, acyl-coenzyme A synthetase short-chain family member 2; ER, endoplasmic reticulum; HAT, histone acetyltransferase; HDAC, histone deacetylase; K, lysine residue; Me, methyl group; PDH, pyruvate dehydrogenase; TF, transcription factor.
3. Functional Significance of Lysine Acetylation in Different Cellular Organelle
3.1. Acetylation of Nuclear Proteins: Implication for Epigenetics
One of the essential constituents in the nucleosomal structure are the histone proteins, where their N-terminal tails can undergo a variety of posttranslational covalent modifications including methylation, acetylation, ubiquitination, sumoylation and phosphorylation on specific residues [84]. These modifications eventually affect regional or whole chromatin structure and regulate key biological processes such as transcription, replication and repair, leading to either promotion or suppression of gene expression, depending upon the spatio-temporal patterns of the modification [84]. For example, lysine acetylation is correlated with transcriptional activation, whereas lysine methylation results in transcriptional activation or repression depending upon the modified residue species and the degree (i.e., mono-, di-, tri-) of methylation [85]. Histone H3 acetylation at the lysine 27 residue (H3K27ac) locates to the promoter and enhancer of the gene in specific loci and H3K27ac modification has a key role in regulating genome conformation by establishing TADs (topologically associating domains) which fold genome DNA into separate domains with specific functions [86]. Enhancer regions are often primed by the monomethylation of histone H3 at lysine 4 (H3K4me1) mark and are fully activated upon deposition of H3K27ac, where DNA accessibility for transcription factors and activators can be augmented [87,88]. Conversely, enhancers are decommissioned by the release of transcription factors, which is accompanied by removal of the H3K4me1 and H3K27ac histone marks and reduced chromatin accessibility. Furthermore, a recent study demonstrated the presence of bivalent chromatin domains marked by both activating and repressive chromatin modifications which could be associated with subtype-specific signatures in developmental or neoplastic cells [89].
Histone modification patterns are dynamically regulated by enzymes that add and remove covalent modifications to histone proteins. Histone acetyltransferases (HATs) and histone methyltransferases (HMTs) add acetyl and methyl groups, whereas histone deacetylases (HDACs) and histone demethylases (HDMs) remove acetyl and methyl groups, respectively (Figure 2) [90]. Aberrant patterns of histone modifications are observed in several types of cancer and might be therapeutically exploitable [91]; for example, the heterogeneity of malignant brain tumors across the entire age spectrum was demonstrated in terms of histone modifications on the tumor epigenomic signatures [92]. In addition to histone modifications, the maintenance DNA methyltransferase (DNMT1) can be regulated through Tip60-mediated acetylation, which targets DNMT1 for proteasomal degradation [93], suggesting that DNA methylation may also be affected by nutrient status and protein PTMs.
Another example of nuclear proteins that could be regulated by acetylation are the transcription factors (Figure 2). The stability of transcription factors, such as p53, FoxO (forkhead box-containing protein, O subfamily), and c-Myc, can be affected by acetylation, achieved by blocking ubiquitination of the same residues, which then targets the protein for proteasomal degradation [94,95,96]. Acetylation of FoxO also regulates its function through altering its affinity with target DNA and its sensitivity for phosphorylation [97]. Importantly, p53, FoxO and c-Myc are transcription factors with major roles for pro- or anti-tumorigenic potential, depending on the context of tumor types, and suggests that PTM by acetylation could be associated with tumorigenesis through the regulation of oncogenic transcription factors. The function of DNA repair enzymes can also be regulated in the nucleus through PTMs. ATM (ataxia telangiectasia mutated) is a serine/threonine protein kinase that is recruited and activated by DNA double-strand breaks. It phosphorylates several key proteins that initiate activation of the DNA damage checkpoint, leading to cell cycle arrest, DNA repair or apoptosis, which is an essential mechanism of genome DNA protection. ATM kinase activity is tightly regulated by Tip60-dependent acetylation at K3016, affecting the ATM-dependent phosphorylation of p53 and CHK2 (checkpoint kinase 2) proteins, the dysregulation of which is associated with the formation of various types of cancer [98].
3.2. Acetylation of Cytosolic Proteins in Specific Organelles
Acetylation of non-histone, cytoplasmic proteins also has many biological implications, and non-histone acetylation plays a role in protein stability, DNA binding, gene expression, protein interactions, localization, messenger-ribonucleic acid (mRNA) stability and enzymatic activity [99]. A representative functional example for cytoplasmic protein acetylation is that of cytoskeletal components (Figure 2). α-tubulin, that together with β-tubulin forms the heterodimeric building block of microtubules, was the first cytoplasmic protein described to be acetylated [100]. Acetylation of α-tubulin, a well established marker of microtubule stability [101], is induced on lysine 40 (K40) by the α-tubulin acetyltransferase 1 (ATAT-1) [102], and can be reversed by histone deacetylase 6 (HDAC6) and sirtuin 2 (SIRT2) [103]. Of interest, acetylation of cytoskeletons is associated with cancer biology, where high HDAC6 and low levels of acetylated α-tubulin are associated with good prognosis and increased disease-free survival of breast cancer patients [104].
Interestingly, an extensive proteomic survey of cellular proteins has revealed that a large number of mitochondrial proteins are subject to reversible lysine acetylation [105]. Indeed, acetylation is an abundant modifications of mitochondrial protein: 277 acetylation sites were identified in 133 proteins, and at least 20% of all mitochondrial proteins are lysine-acetylated [105]. It is well known that three mitochondrial deacetylases (SIRT3, SIRT4, SIRT5) mediate mitochondrial protein acetylation levels. Recent reports have shown that a series of targeted proteins are involved in metabolic pathways such as the TCA cycle, oxidative phosphorylation, β-oxidation of lipids, amino acid metabolism, carbohydrate metabolism, nucleotide metabolism and the urea cycle (Figure 2) [8,106,107]. Eventually, alterations in mitochondrial acetylation states, and, hence, alterations in carbon substrate utilization, may contribute to the unusual preference for aerobic glycolysis and glutaminolysis, the emerging features frequently observed in numerous forms of cancer [108].
Other intracellular components which could be regulated by lysine acetylation are the endoplasmic reticulum (ER) and Golgi apparatus. Proteomic studies have assessed the ER acetylome, and predicted wide-ranging biological implications of this pathway [109]. The list of ER-resident proteins includes chaperones and enzymes involved with PTM and protein folding (Figure 2) [10]. ER acetylation has been reported to be catalyzed by the two ER-based KATs, AT-1 (also known as camello-like 2 and N-acetyltransferase 8B) and AT-2 (also known as camello-like 1 and N-acetyltransferase 8). Both of these members of the camello family belong to the GNAT superfamily (Table 1) [110]. Of interest, manipulation of AT-1 function in mice leads to the appearance of neurodegenerative features, inflammation and cancer [21]. Together, protein lysine acetylation appears to be an essential component of specific subcellular organelles, and its aberration could lead to cancer formation.
4. Aberrant Protein Acetylation in the Phenotypes of Cancer Cells
4.1. Driver Mutations of Lysine Acetylation/Deacetylation Genes in Cancer
Consistent with the importance of acetylation levels in cells, somatic mutations in KATs lead to malignancy, and KATs act as tumor suppressors or oncogenes in a context- and cell type-specific manner (Table 1). Mutations in cAMP-responsive element-binding protein (CREB)-binding protein (CREBBP) and EP300, that are the responsible genes for Rubinstein–Taybi syndrome of multiple congenital anomalies, could also be involved in hematological cancer including those leukemias where chromosome translocations disrupt the CREB-binding protein (CBP) gene function [24]. Pro-tumorigenic mutations in CBP and EP300 tend to be inactivating mutations, suggesting that they act as tumor suppressors [15]. The MYST family gene monocytic leukemia zinc finger protein (MOZ) was reported to be fused to CBP, discovered initially in acute myeloid leukemia (AML) [30], and chromosomal translocations in AML can also fuse MOZ to the CBP homologue p300 [111]. These findings highlight the importance of fusion of acetylation-related genes in hematological tumors. Chromosomal translocations involving MOZ appear to create bona fide oncogenes. In addition to hematological cancer, KATs mutations also contribute to tumorigenesis and solid tumor cancer stem cell (CSC) function [33]. The GNAT family PCAF missense variants with CBP truncations and intronic microdeletions have found in human epithelial cancer cell lines and primary tumors [19], and the deregulation of distinct GCN5/PCAF-containing complexes leads to the malignant transformation of the cells [18].
HDACs work as both oncogenes and tumor suppressor genes to contribute to tumorigenesis (Table 2). The frameshift mutation in exon 1 of the HDAC2 gene is largely confined to colon tumors with microsatellite instability (MSI), which produces a premature stop codon that results in loss of HDAC2 protein expression [48]. Importantly, HDAC2-deficient colon cancer cells are highly refractory to the apoptosis induced by HDAC inhibitors. Aberrations in other classes of HDACs are also associated with CSC function and tumor stratification [55,57]. Sirtuins are a particular type of HDACs, the function of which is to influence extension of lifespan (longevity). Sirtuin 2 (SIRT2) is a class III NAD+-dependent deacetylase, which regulates a broad range of biological functions, including aging, metabolism, differentiation, genome maintenance, and tumor suppression [43]. Of interest, naturally occurring cancer-associated SIRT2 mutations at evolutionarily conserved sites disrupt its deacetylation of DNA-damage response proteins by impairing SIRT2 catalytic activity or protein levels [60], supporting a model for SIRT2’s tumor-suppressive function which contributes to genomic stability. Additionally, SIRT family can also function as tumor suppressors, especially those residing in mitochondria, including SIRT3, SIRT4 and SIRT5 (Table 2).
As a reader of protein lysine acetylation, BRD4 is largely acknowledged in cancer for its role in super-enhancer (SE) organization and oncogene expression. Inhibition of BRD4 shortcuts the communication between SEs and target promoters with a subsequent cell-specific repression of oncogenes to which cancer cells are addicted [112]. Importantly, BRD4 itself is a target of mutation in cancer: NUT (nuclear protein in testis) carcinoma (formerly known as NUT midline carcinoma) is characterized by the presence of NUT fusion oncogenes, the most common being BRD4-NUT [113]. BRD4 genetic amplification also facilitates an oncogenic gene expression program in ovarian high-grade serous carcinomas and confers sensitivity to BET inhibitors [114]. Of interest, BRD4 inhibition induced homologous recombination deficiency (HRD) and sensitized cells across multiple tumor lineages to PARP inhibitors, regardless of BRCA1/2, TP53, RAS, or BRAF mutation status, through depletion of the DNA double-stand break resection protein CtIP (C-terminal binding protein interacting protein) [115]. All these findings indicate that lysine acetylation genes play a role in tumorigenesis as bona fide oncogenes and tumor suppressors and as exploitable therapeutic targets.
4.2. Oncogenic Signaling and Protein Acetylation: Mechanistic Target of Rapamycin Complex 2 (mTORC2) as a Strong Acetylation Driver in Cancer
Accumulated evidence indicates that acetylation is an essential protein modification contributing to aggressive cancer cell phenotypes, making it important to unravel how an acetylation network is remodeled in the cancer cells in an oncogene-dependent manner. One of the major driver genes in cancer is a receptor-type tyrosine kinase (RTK), epidermal growth factor receptor (EGFR), and persistent growth factor receptor signaling including that from EGFR activates mechanistic target of rapamycin (mTOR) complex signaling, potentially affecting protein PTMs including acetylation [116]. Our recent work has demonstrated that one of the mTOR complexes, mTORC2, is a strong acetylation driver in cancer cells, especially in the context of EGFR-mutant genotypes [117].
We recently set out to determine the role of mTORC2 in metabolic reprogramming of the malignant brain tumor GBM, and an unexpected Akt-independent role for mTORC2 in inducing metabolic reprogramming in GBM was found [46]. mTORC2 renders GBM cells strongly addicted to glucose, and this is mediated by regulating the intracellular level of c-Myc, a crucial regulator of the Warburg effect or aerobic glycolysis. Of interest, modulation of an acetylation network of cytosolic protein by mTORC2 lies behind the cancer cell aggressiveness via metabolic reprogramming with c-Myc upregulation. mTORC2 executes an Akt-independent phosphorylation of class IIa HDACs (HDAC4, 5 and 7), which leads to the inactivating acetylation of FoxO, a negative regulator of c-Myc (Figure 3). The mechanism of FoxO inactivation relies on its acetylation to be tethered in the cytoplasm, hindering its transcriptional regulatory activity. As a result, the microRNA-dependent blockade of c-Myc is relieved, potently promoting glycolytic tumor growth. Importantly, the axis of mTORC2/acetylated FoxO/c-Myc expression confers an adverse prognostic impact to GBM patients, and it can be abrogated by dual PI3K/mTOR kinase inhibition, resulting in tumor cell death of xenograft tumor models using patient-derived GBM neurospheres. These results provide new insight into the role of mTORC2 in shaping cancer cell phenotypes through acetylation-dependent regulation of cytoplasmic and nuclear proteins (i.e., transcription factor FoxO). Additionally, an oncogenic transcription factor of c-Myc itself is known to be regulated by acetylation, providing further dimension to the acetylated web of cytoplasmic/nuclear protein interaction in cancer cells [95].
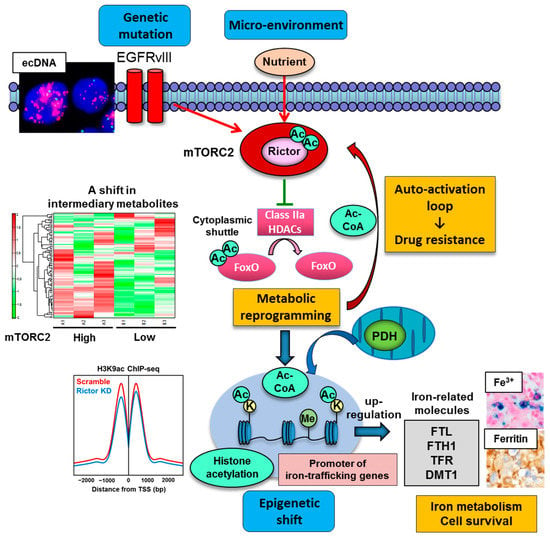
Figure 3.
mTORC2 as a strong acetylation driver in cancer. Genetic mutation including extrachromosomal DNA (ecDNA)-dependent EGFRvIII (epidermal growth factor receptor variant III) overexpression and nutrient in the microenvironment promote mTORC2 activity which facilitates protein acetylation including cytoplasmic protein (FoxO and Rictor) and nuclear histone protein. mTORC2-dependent protein acetylation eventually contributes to c-Myc-dependent metabolic reprogramming of glycolysis, drug resistance to molecular targeting therapies, and epigenetic shift in cancer cells. Of interest, the expression of iron-related genes (FLT, FTH1, TFR, DMT1) is epigenetically promoted by mTORC2-dependent histone acetylation at their promoters, driving iron metabolism and cell survival in cancer. The findings suggest that protein acetylation driven by mTORC2 is a key player to integrate genetics, epigenetics and environment in cancer. Ac, acetyl group; Ac-CoA, acetyl-CoA; bp, base pair; ChIP-seq, chromatin immunoprecipitation sequencing; DMT1, divalent metal transporter 1; FLT, ferritin light chain; FTH1, ferritin heavy chain; FoxO, forkhead box O; HDAC, histone deacetylase; K, lysine residue; KD, knockdown; Me, methyl group; mTORC2, mechanistic target of rapamycin complex 2; PDH, pyruvate dehydrogenase; TFR, transferrin receptor; TSS, transcription start site.
Another intriguing example of cytoplasmic protein acetylation in cancer cell phenotypes is that protein acetylation, including the acetylation of Rictor (a core component of mTORC2), can be controlled through the balance between HAT and HDAC activities [118]. We recently demonstrated that mTORC2 suppresses the activity of class IIa HDACs in EGFR-mutant GBMs through a signal cascade that results in their inactivating phosphorylation [46]. Thus, if class IIa HDACs negatively regulate mTORC2 via deacetylation of Rictor, mTORC2 can establish a feedforward auto-activation loop through inactivation of class IIa HDACs to keep Rictor in an acetylated state, maintaining downstream signaling. We demonstrated that PKCα (protein kinase C alpha) phosphorylates and inactivates class IIa HDACs downstream of mTORC2 signaling, and Rictor is in turn physically associated with class IIa HDACs and deacetylated by them [81]. This signaling cascade forms an auto-activation loop of mTORC2 and promotes the activity of mTORC2 (Figure 3). Importantly, the circuitry of mTORC2 signaling, inactivating phosphorylation of class IIa HDACs, and Rictor acetylation contributes to the resistance of cancer cells to molecular-targeting therapies [81]. Together, these results indicate that mTORC2 forms an autoactivation loop through acetyl-CoA and HDAC-mediated Rictor acetylation, which underlies the mechanism of mTORC2′s response to nutrient availability and metabolic reprogramming in EGFR-mutant GBMs (Figure 3) [11].
mTORC2 was also reported to regulate cancer epigenetics via histone acetylation, a dynamic chromatin mark with various important roles in gene regulation. Histone acetylation including H3K9ac (H3 lysine 9 acetylation) and H3K14ac (H3 lysine 14 acetylation) are controlled by mTORC2-sensitive Akt-dependent regulation of acetyl-CoA-producing enzyme ACLY [119]. In yeast, TORC2 contributes to the regulation of several histone modifications [H3K9me2 (H3 lysine 9 di-methylation), H3K4me3 (H3 lysine 4 tri-methylation) and H4K16ac (H4 lysine 16 acetylation)] for its epigenetic stability [120]. Correspondingly, our recent study demonstrates that mTORC2 promotes histone acetylation (H3K9ac, H3K18ac, H3K27ac) in the actively transcribed promoters of GBM cells through metabolic reprogramming/Warburg effect (hence the production of nuclear acetyl-CoA) and dysregulation of histone modifying enzymes including PDH and HDACs [77]. Other types of HATs (GCN5/PCAF and CBP/p300) and HDACs (HDAC3, class IIa HDACs) could contribute to the acetylation of H3K9 [117], and future studies are needed to examine whether these acetylating/deacetylating enzymes could also be regulated by mTORC2. Intriguingly, mTORC2-dependent increase in H3K9ac peaks was uniquely induced at the promoter regions of genes related to mineral metabolism including iron (Figure 3). Iron metabolism-related enzymes including ferritin light chain (FTL), ferritin heavy chain (FTH1), transferrin receptor (TFR) and divalent metal transporter 1 (DMT1) were epigenetically upregulated through histone acetylation at the promoter regions (Figure 3). Eventually, GBM cells with activated mTORC2 signaling are addicted to iron metabolism for survival, which could be therapeutically exploitable [77]. In addition to mineral metabolism, mTORC2-dependent regulation of histone H3 lysine 56 acetylation (H3K56ac) epigenetically controls the expression of glycolytic genes via regulation of sirtuin 6 (SIRT6) [121]. Together, the findings suggest that mTORC2 plays a role in the integration of cancer metabolism and PTM of the protein in an intricate, multi-directional manner, and mutual dependency of metabolism and epigenetics could be the driving force for the progression of various types of cancer, including GBM.
5. Novel Therapeutic Strategies to Target Protein Acetylation Systems in Cancer
A series of novel epigenetic drug targets have been identified through the elucidation of protein acetylation mechanisms. Many KATs are not fully active unless associated with their partner proteins in KAT complexes, and integration into the complexes can affect not only the level of enzymatic activity but also substrate specificity [15]. Further, KATs themselves are subject to PTMs including acetylation that affect the activity, stability and subcellular localization [122]. Multi-layered regulation of KATs enable the control of KAT activities in an appropriate, spatio-temporal manner in the cell, but could provide specific vulnerabilities for development of small-molecule inhibitors that interfere with acetyl-CoA utilization and substrate binding (Figure 4). Such inhibition would affect acetylation of histones as well as associated transcription of oncogenes, and also have an impact on non-histone substrates, such as p53, FoxO, and c-Myc, thereby affecting the stability and activity of these transcription factors. Alternatively, small-molecule inhibitors might be designed to forestall interactions between KATs and other proteins, such as β-catenin and HIF (hypoxia-inducible factor), which would affect transcription of downstream oncogenic genes (Figure 4) [14]. However, the identification of KAT inhibitors (KATi) is not as well developed as that for HDAC inhibitors (HDACi) (Table 3).
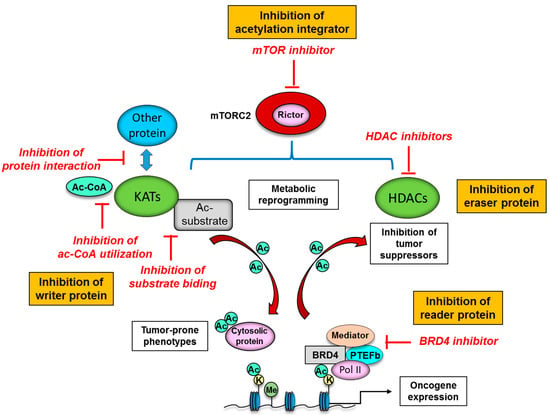
Figure 4.
Potential therapeutic strategies to target protein acetylation systems in cancer. The interaction of KATs (writer protein) with other protein could provide specific vulnerabilities for development of small-molecule inhibitors to interfere with acetyl-CoA utilization, substrate binding and interaction with other protein such as β-catenin and HIF. Inhibition of eraser protein or HDAC can eventually reactivate the expression of tumor suppressors, resulting in cell cycle arrest, apoptosis, differentiation, and inhibition of angiogenesis and metastasis in cancer cells. BET (reader protein) inhibitors may target tumor cells without affecting normal tissues with the inhibitors’ preferential binding to super-enhancers, which are non-coding regions of DNA to bind multiple transcription factors and are critical to the expression of oncogenes. mTORC2 is an integrator of protein acetylation systems, and targeted therapies against mTORC2 could be the next-generation therapeutic strategies to interfere with cancer-specific, acetylation-dependent metabolism and epigenetics. Ac, acetyl group; Ac-CoA, acetyl-CoA; BRD4, bromodomain-containing protein 4; K, lysine residue; KAT, lysine deacetylase; HDAC, histone deacetylase; Me, methyl group; mTORC2, mechanistic target of rapamycin complex 2; PTEFb, positive transcription elongation factor b; Pol II, RNA polymerase II.
The reversible nature of lysine acetylation by HDACs makes them promising candidates for cancer treatment targets. Indeed, the development and availability of HDACi have not only accelerated our understanding of HDAC functions and action mechanisms, but provided a promising new class of compounds for cancer treatment (Figure 4) [123,124]. HDACi has already entered into clinical trials and practical usage, and the drugs have demonstrated some effects, especially in combination with other epigenetic inhibitors [125] or chemotherapy [126]. A series of synthetic compounds and natural molecules to target class I, II, and IV HDAC enzymes have been developed and classified into four groups, including hydroxamates, benzamides, short-chain fatty acids, and cyclic peptides based on their chemical structures (Table 3) [127]. As for the actionable mechanism, the rationale for targeting HDACs in cancer is based upon the findings that altered HDAC expression and function is frequently observed in a variety of cancer types. HDACs reversibly and dynamically modify the acetylation of histone and non-histone protein, and HDACi can restore the acetylation homeostasis in cancer cells, which can eventually reactivate the expression of tumor suppressors, resulting in cell cycle arrest, apoptosis, differentiation, and inhibition of angiogenesis and metastasis (Figure 4) [128]. Of interest, cancer cells are more sensitive to HDACi-induced apoptosis than normal cells, demonstrating additional therapeutic potential of HDACi [129]. Still, the precise mechanisms by which HDACi are effective in cancer treatment await further investigation in order to select the patient who will most benefit from the treatment, reduce the side effects and induce much more potent cytotoxic effects on cancer cells.
Considering their role as readers of lysine acetylation, BET proteins are a promising target for emerging cancer therapeutics. For instance, the BET inhibitor JQ1 could displace BRD4 from chromatin and induce cell differentiation, G1 cell cycle arrest, and apoptosis in vitro as well as patient-derived xenograft models (Figure 4) [130]. In spite of peculiar pharmacologic features such as short half-life, further development of new BET inhibitors is ongoing, and the BET inhibitors were reported to downregulate the spindle checkpoint kinase [131], and cause downregulation of critical cell cycle genes as well as upregulation of cyclin dependent kinase inhibitors [132]. Even more promising, in preclinical models, BET inhibitors may target tumor cells without affecting normal tissues due to the inhibitors’ preferential binding to super-enhancers, which are non-coding regions of DNA that bind multiple transcription factors and are critical to the expression of genes that determine cellular identity [133]. BET inhibitors thus could represent potential candidates for achieving precision treatment of each cancer patient.
As for the specific role of mTORC2 in metabolic and epigenetic reprogramming, targeted therapies against mTORC2 could exemplify next-generation therapeutic strategies to interfere with cancer-specific, acetylation-dependent metabolism and epigenetics (Figure 4). However, an mTORC2-specific inhibitor is not clinically available, and it is exceptionally difficult to develop a potent and selective small-molecule inhibitor to target mTORC2 due to the intricate, multifaceted protein–protein interactions of the mTORC2 complex [134]. Novel approaches to selectively inhibit mTORC2 are emerging, which potentially provide more specific anti-cancer effects, including the targeting of complex-specific protein-protein interactions [135], and the disruption of mTORC2 substrate recruitment [136]. The demonstration that mTOR-targeting therapies could be effective cancer therapeutics through the modulation of cancer metabolism and epigenetics [137], and the future development of specific and accurate ways to inhibit mTORC2 activity represent promising strategies to target cancer metabolism and protein PTM networks.

Table 3.
Selected KAT inhibitors and HDAC inhibitors.
Table 3.
Selected KAT inhibitors and HDAC inhibitors.
| KAT Inhibitors | ||
|---|---|---|
| Mechanisms | Status | Inhibitors |
| Compete with substrates | Preclinical | CPTH2, CPTH6, BF1 [138,139] |
| Inhibit Ac-CoA utilization | Preclinical | Garcinol, C646, TH1834, Lys-CoA [140,141,142] |
| Block interaction with other protein | Preclinical | Chetomin (HIF), KCN1 (HIF), ICG-001 (β-catenin), Windorphen (β-catenin) [143,144,145,146] |
| HDAC Inhibitors | ||
| Class | Status | Inhibitors (targeted HDAC) |
| Hydroxamates | FDA-approved Preclinical | * Vorinostat (SAHA) (pan-class), Belinostat (pan-class), Panobinostat (pan-class) [147,148,149] Trichostatin A (pan-class) [150] |
| Benzamides | Clinical trials | Entinostat (class I), Mocetinostat (class I, IV), Tacedinaline (class I) [151,152,153] |
| Short-chain fatty acids | Clinical trials | Valproic acid (class I, IIa), Butyric acid (class I, II), Phenylbutyrate (class I, II) [154,155,156] |
| Cyclic peptides | FDA-approved | Romidepsin (class I) [157] |
* Combination with other epigenetic or chemotherapeutic agents could be experimentally effective in cancer models [125,126]. Ac-CoA, acetyl-CoA; HIF, hypoxia-inducible factor; KAT, lysine acetyltransferase. FDA, Food and Drug Administration; HDAC, histone deacetylase; SAHA, suberoylanilide hydroxamic acid.
6. Conclusions and Future Perspectives
Metabolic reprogramming is an emerging hallmark of cancer [158], and tumor development, progression and therapy response are profoundly influenced by the intracellular metabolism and the exogenous microenvironment of tumor cells, where metabolic shifts are driven by the aberration of oncogenes and tumor suppressors. Importantly, metabolic reprogramming potentially shifts the landscape of protein PTM and protein lysine acetylation lies at the interface of genetics, epigenetics and the microenvironment. In addition to the genetic aberrations themselves, the components involved in protein acetylation (i.e., writer, eraser and reader of lysine acetylation) contribute to tumorigenesis in a multifaceted fashion, implicating their importance as both regulators and effectors of aggressive cancer cell phenotypes. Recent reports suggest that the axis of metabolic reprogramming and protein modification is not unidirectional, but comprises inherently co-dependent relationships that enable tumor cells to appropriately respond to their microenvironment and ensure cell survival [4,91]. These networks enable cells to rapidly adapt to a shift in environmental nutrient condition through acetylation-dependent interaction between the promoter and enhancer regions of the survival genes. Such phenomena are well recognized in the early developmental stage of organisms, and the regulatory mechanisms could also be harnessed by cancer cells. At the same time, a slight tip in the balance of this regulation is sufficient to result in a tumor cell catastrophe. Considering that tissue context-based cues can shape metabolic dependencies [159], the “metabolic and epigenetic vulnerability” of certain cancer cells is reminiscent of the notion of “oncogene addiction” [160], the knowledge of which will lead to rational combination of cytotoxic and molecular targeted therapies [161], in order to effectively target the metabolic and epigenetic networks upon which cancer cells heavily depend. Future studies are needed to determine precisely how the primary genetic mutations specific for each tumor entity facilitate cancer metabolic reprogramming and protein modification and how, at the same time, extracellular nutrients modulate oncogenic signaling in order to translate these insights into more effective treatments for cancer patients.
Funding
This work is supported by a Grant- in-Aid from Takeda Science Foundation (K.M.), Japan Society for the Promotion of Science KAKENHI Grant 19K07649 (K.M.), and National Institutes of Health NS73831 (P.S.M.).
Acknowledgments
We thank Department of Neurosurgery, Tokyo Women’s Medical University for biospecimen and biorepository support.
Conflicts of Interest
P.S.M. is a co-founder of Boundless Bio, Inc. He has equity interest and serves as the chair of its scientific advisory board. W.K.C. is a co-founder of InVaMet, Inc and Interleukin Combinatorial Therapies, Inc. None of these companies contributed financial support to the studies described in the present paper.
References
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y.; Van Der Heiden, M.G. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 2016, 16, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, L.K.; Deberardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Chowdhry, S.; Wu, S.; Zhang, W.; Masui, K.; Mischel, P.S. Altered cellular metabolism in gliomas—An emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer 2020, 20, 57–70. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal. Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dahan, P.; Lu, V.; Nguyen, R.M.T.; Kennedy, S.A.L.; Teitell, M.A. Metabolism in pluripotency: Both driver and passenger? J. Biol. Chem. 2019, 294, 5420–5429. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Kori, Y.; Sidoli, S.; Yuan, Z.F.; Lund, P.J.; Zhao, X.; Garcia, B.A. Proteome-wide acetylation dynamics in human cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Di Martile, M.; Del Bufalo, D.; Trisciuoglio, D. The multifaceted role of lysine acetylation in cancer: Prognostic biomarker and therapeutic target. Oncotarget 2016, 7, 55789–55810. [Google Scholar] [CrossRef]
- Masui, K.; Shibata, N.; Cavenee, W.K.; Mischel, P.S. mTORC2 activity in brain cancer: Extracellular nutrients are required to maintain oncogenic signaling. BioEssays 2016, 38, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, T.; Zhang, N.; Chen, J.; Zhang, P.; Li, S.; Luo, L.; Cui, Z.; Qin, Y.; Liu, F. Nuclear e-cadherin acetylation promotes colorectal tumorigenesis via enhancing β-catenin activity. Mol. Cancer Res. 2019, 17, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Cui, K.; Schones, D.E.; Barski, A.; Peng, W.; Zhao, K. Genome-wide Mapping of HATs and HDACs Reveals Distinct Functions in Active and Inactive Genes. Cell 2009, 138, 1019–1031. [Google Scholar] [CrossRef]
- Farria, A.; Li, W.; Dent, S.Y.R. KATs in cancer: Functions and therapies. Oncogene 2015, 34, 4901–4913. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, B.N.; Akhtar, A. The many lives of KATs—Detectors, integrators and modulators of the cellular environment. Nat. Rev. Genet. 2019, 20, 7–23. [Google Scholar] [CrossRef]
- McCullough, C.E.; Marmorstein, R. Molecular Basis for Histone Acetyltransferase Regulation by Binding Partners, Associated Domains, and Autoacetylation. ACS Chem. Biol. 2016, 11, 632–642. [Google Scholar] [CrossRef]
- Butler, J.S.; Koutelou, E.; Schibler, A.C.; Dent, S.Y.R. Histone-modifying enzymes: Regulators of developmental decisions and drivers of human disease. Epigenomics 2012, 4, 163–177. [Google Scholar] [CrossRef]
- Nagy, Z.; Tora, L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 2007, 26, 5341–5357. [Google Scholar] [CrossRef]
- Özdaǧ, H.; Batley, S.J.; Försti, A.; Iyer, N.G.; Daigo, Y.; Boutell, J.; Arends, M.J.; Ponder, B.A.J.; Kouzarides, T.; Caldas, C. Mutation analysis of CBP and PCAF reveals rare inactivating mutations in cancer cell lines but not in primary tumours. Br. J. Cancer 2002, 87, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Ladang, A.; Rapino, F.; Heukamp, L.C.; Tharun, L.; Shostak, K.; Hermand, D.; Delaunay, S.; Klevernic, I.; Jiang, Z.; Jacques, N.; et al. Elp3 drives Wnt-dependent tumor initiation and regeneration in the intestine. J. Exp. Med. 2015, 212, 2057–2075. [Google Scholar] [CrossRef]
- Peng, Y.; Li, M.; Clarkson, B.D.; Pehar, M.; Lao, P.J.; Hillmer, A.T.; Barnhart, T.E.; Christian, B.T.; Mitchell, H.A.; Bendlin, B.B.; et al. Deficient import of Acetyl-CoA into the ER lumen causes neurodegeneration and propensity to infections, inflammation, and cancer. J. Neurosci. 2014, 34, 6772–6789. [Google Scholar] [CrossRef]
- Ward, R.; Johnson, M.; Shridhar, V.; Van Deursen, J.; Couch, F.J. CBP truncating mutations in ovarian cancer. J. Med Genet. 2005, 42, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Kohno, T.; Okudela, K.; Otsuka, A.; Sasaki, H.; Tanabe, C.; Sakiyama, T.; Hirama, C.; Kitabayashi, I.; Minna, J.D.; et al. Mutations and Deletions of the CBP Gene in Human Lung Cancer. Clin. Cancer Res. 2005, 11, 512–519. [Google Scholar] [PubMed]
- Iyer, N.G.; Özdag, H.; Caldas, C. p300/CBP and cancer. Oncogene 2004, 23, 4225–4231. [Google Scholar] [CrossRef]
- Attar, N.; Kurdistani, S.K. Exploitation of EP300 and CREBBP lysine acetyltransferases by cancer. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Molkentine, D.; Molkentine, J.; Bridges, K.; Xie, T.; Yang, L.; Hefner, A.; Gao, M.; Frederick, M.; Seth, S.; et al. Inhibition of histone acetyltranserase function radiosensitizes CREBBP/EP300 mutants via repression of homologous recombination, potentially targeting a novel gain of function. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gorrini, C.; Squatrito, M.; Luise, C.; Syed, N.; Perna, D.; Wark, L.; Martinato, F.; Sardella, D.; Verrecchia, A.; Bennett, S.; et al. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature 2007, 448, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Chevillard-briet, M.; Quaranta, M.; Grézy, A.; Mattera, L.; Courilleau, C.; Philippe, M.; Mercier, P.; Corpet, D.; Lough, J.; Ueda, T.; et al. Interplay between chromatin-modifying enzymes controls colon cancer progression through Wnt signaling. Hum. Mol. Genet. 2014, 23, 2120–2131. [Google Scholar] [CrossRef]
- Zhu, J.; Sammons, M.A.; Donahue, G.; Dou, Z.; Vedadi, M.; Getlik, M.; Barsyte-Lovejoy, D.; Al-Awar, R.; Katona, B.W.; Shilatifard, A.; et al. Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature 2015, 525, 206–211. [Google Scholar] [CrossRef]
- Borrow, J.; Stanton, V.P.; Andresen, J.M.; Becher, R.; Behm, F.G.; Chaganti, R.S.K.; Civin, C.I.; Disteche, C.; Dubé, I.; Frischauf, A.M.; et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat. Genet. 1996, 14, 33–41. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Fioretos, T.; Isaksson, M.; Samuelsson, U.; Billström, R.; Strömbeck, B.; Mitelman, F.; Johansson, B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13). Hum. Mol. Genet. 2001, 10, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.D.P.; Herrick, S.R.; Ince, T.A.; Kleinman, M.S.; Dal Cin, P.; Morton, C.C.; Quade, B.J. Uterine leiomyomata with t(10;17) disrupt the histone acetyltrasferase MORF. Cancer Res. 2004, 64, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.L.T.; Akli, S.; Macalou, S.; Biernacka, A.; Debeb, B.G.; Yi, M.; Hunt, K.K.; Keyomarsi, K. Hbo1 is a cyclin E/CDK2 substrate that enriches breast cancer stem-like cells. Cancer Res. 2013, 73, 5556–5568. [Google Scholar] [CrossRef]
- Dong, Z.; Zou, J.; Li, J.; Pang, Y.; Liu, Y.; Deng, C.; Chen, F.; Cui, H. MYST1/KAT8 contributes to tumor progression by activating EGFR signaling in glioblastoma cells. Cancer Med. 2019, 8, 7793–7808. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.K.; Pearson, D.; Lee, H.W.; Kim, S. Nonenzymatic acetylation of histones with acetyl-CoA. Bba Sect. Nucleic Acids Protein Synth. 1970, 213, 513–522. [Google Scholar] [CrossRef]
- James, A.M.; Hoogewijs, K.; Logan, A.; Hall, A.R.; Ding, S.; Fearnley, I.M.; Murphy, M.P. Non-enzymatic N-acetylation of Lysine Residues by AcetylCoA Often Occurs via a Proximal S-acetylated Thiol Intermediate Sensitive to Glyoxalase II. Cell Rep. 2017, 18, 2105–2112. [Google Scholar] [CrossRef]
- Hong, S.Y.; Ng, L.T.; Ng, L.F.; Inoue, T.; Tolwinski, N.S.; Hagen, T.; Gruber, J. The role of mitochondrial non-enzymatic protein acylation in ageing. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J.; et al. Calorie Restriction and SIRT3 Trigger Global Reprogramming of the Mitochondrial Protein Acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef]
- Gil, J.; Ramírez-Torres, A.; Encarnación-Guevara, S. Lysine acetylation and cancer: A proteomics perspective. J. Proteom. 2017, 150, 297–309. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Micelli, C.; Rastelli, G. Histone deacetylases: Structural determinants of inhibitor selectivity. Drug Discov. Today 2015, 20, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 2015, 15, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Seto, E. Histone deacetylases and cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Vasquez, D.S.; Ravnskjaer, K.; Denechaud, P.D.; Yu, R.T.; Alvarez, J.G.; Downes, M.; Evans, R.M.; Montminy, M.; Shaw, R.J. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 2011, 145, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Tanaka, K.; Akhavan, D.; Babic, I.; Gini, B.; Matsutani, T.; Iwanami, A.; Liu, F.; Villa, G.R.; Gu, Y.; et al. MTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013, 18, 726–739. [Google Scholar] [CrossRef]
- Ali, M.N.; Choijookhuu, N.; Takagi, H.; Srisowanna, N.; Nguyen Nhat Huynh, M.; Yamaguchi, Y.; Synn Oo, P.; Tin Htwe Kyaw, M.; Sato, K.; Yamaguchi, R.; et al. The HDAC Inhibitor, SAHA, Prevents Colonic Inflammation by Suppressing Pro-inflammatory Cytokines and Chemokines in DSS-induced Colitis. Acta Histochem. Et Cytochem. 2018, 51, 33–40. [Google Scholar] [CrossRef]
- Hanigan, C.L.; van Engeland, M.; De Bruine, A.P.; Wouters, K.A.; Weijenberg, M.P.; Eshleman, J.R.; Herman, J.G. An Inactivating Mutation in HDAC2 Leads to Dysregulation of Apoptosis Mediated by APAF. Gastroenterology 2008, 135. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, Y.; Zhuang, X.; Zhu, Y.; Wu, Z.; Lu, Y.; Li, S.; Zeng, Y.; Lu, Q.R.; Huo, Y.; et al. HDAC3 deficiency promotes liver cancer through a defect in H3K9ac/H3K9me3 transition. Cancer Res. 2019, 79, 3676–3688. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, H.; Zhang, M.; Xu, J. Histone deacetylase 3 is associated with gastric cancer cell growth via the miR-454-mediated targeting of Chdinternational. J. Mol. Med. 2018, 41, 155–163. [Google Scholar] [CrossRef]
- Durst, K.L.; Lutterbach, B.; Kummalue, T.; Friedman, A.D.; Hiebert, S.W. The inv(16) Fusion Protein Associates with Corepressors via a Smooth Muscle Myosin Heavy-Chain Domain. Mol. Cell. Biol. 2003, 23, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lachenmayer, A.; Toffanin, S.; Cabellos, L.; Alsinet, C.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Tsai, H.W.; Ward, S.C.; Thung, S.; et al. Combination therapy for hepatocellular carcinoma: Additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol. 2012, 56, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, L.; Zhang, S.; Guo, S.; Li, X.; Wang, J.; Su, B.; Fang, Y.; Chen, X.; Ke, H.; et al. Hdac7 promotes lung tumorigenesis by inhibiting Stat3 activation. Mol. Cancer 2017, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Milde, T.; Oehme, I.; Korshunov, A.; Kopp-Schneider, A.; Remke, M.; Northcott, P.; Deubzer, H.E.; Lodrini, M.; Taylor, M.D.; Von Deimling, A.; et al. HDAC5 and HDAC9 in medulloblastoma: Novel markers for risk stratification and role in tumor cell growth. Clin. Cancer Res. 2010, 16, 3240–3252. [Google Scholar] [CrossRef]
- Bitler, B.G.; Wu, S.; Park, P.H.; Hai, Y.; Aird, K.M.; Wang, Y.; Zhai, Y.; Kossenkov, A.V.; Vara-Ailor, A.; Rauscher, F.J.; et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat. Cell Biol. 2017, 19, 962–973. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhu, S.; Dejene, E.A.; Peng, W.; Sepulveda, A.; Seto, E. HDAC10 regulates cancer stem-like cell properties in KRAS-driven lung adenocarcinoma. Cancer Res. 2020, 80, 3265–3278. [Google Scholar] [CrossRef]
- Deng, C.X. SIRT1, is it a tumor promoter or tumor suppressor? Int. J. Biol. Sci. 2009, 5, 147–152. [Google Scholar] [CrossRef]
- Taniguchi, R.; Utani, K.; Thakur, B.; Ishine, K.; Aladjem, M.I.; Shimizu, N. SIRT1 stabilizes extrachromosomal gene amplification and contributes to repeat-induced gene silencing. J. Biol. Chem. 2021, 296, 100356. [Google Scholar] [CrossRef]
- Head, P.E.; Zhang, H.; Bastien, A.J.; Koyen, A.E.; Withers, A.E.; Daddacha, W.B.; Cheng, X.; Yu, D.S. Sirtuin 2 mutations in human cancers impair its function in genome maintenance. J. Biol. Chem. 2017, 292, 9919–9931. [Google Scholar] [CrossRef]
- Bhalla, K.; Jaber, S.; Reagan, K.; Hamburg, A.; Underwood, K.F.; Jhajharia, A.; Singh, M.; Bhandary, B.; Bhat, S.; Nanaji, N.M.; et al. SIRT3, a metabolic target linked to ataxia-telangiectasia mutated (ATM) gene deficiency in diffuse large B-cell lymphoma. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Finley, L.W.S.; Carracedo, A.; Lee, J.; Souza, A.; Egia, A.; Zhang, J.; Teruya-Feldstein, J.; Moreira, P.I.; Cardoso, S.M.; Clish, C.B.; et al. SIRT3 Opposes Reprogramming of Cancer Cell Metabolism through HIF1α Destabilization. Cancer Cell 2011, 19, 416–428. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Principe, D.R.; Zou, X.; Vassilopoulos, A.; Gius, D. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab. 2014, 2, 15. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, H.L.; Xu, J.; Tan, J.; Fu, L.N.; Wang, J.L.; Zou, T.H.; Sun, D.F.; Gao, Q.Y.; Chen, Y.X.; et al. Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Lerrer, B.; Gertler, A.A.; Cohen, H.Y. The complex role of SIRT6 in carcinogenesis. Carcinogenesis 2015, 37, 108–118. [Google Scholar] [CrossRef]
- Malik, S.; Villanova, L.; Tanaka, S.; Aonuma, M.; Roy, N.; Berber, E.; Pollack, J.R.; Michishita-Kioi, E.; Chua, K.F. SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Yue, L.; Sharma, V.; Horvat, N.P.; Akuffo, A.A.; Beatty, M.S.; Murdun, C.; Colin, C.; Billington, J.M.R.; Goodheart, W.E.; Sahakian, E.; et al. HDAC11 deficiency disrupts oncogene-induced hematopoiesis in myeloproliferative neoplasms. Blood 2020, 135, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Vakoc, C.R. The Mechanisms behind the Therapeutic Activity of BET Bromodomain Inhibition. Mol. Cell 2014, 54, 728–736. [Google Scholar] [CrossRef]
- Moon, K.J.; Mochizuki, K.; Zhou, M.; Jeong, H.S.; Brady, J.N.; Ozato, K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 2005, 19, 523–534. [Google Scholar] [CrossRef]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Rahnamoun, H.; Lee, J.; Sun, Z.; Lu, H.; Ramsey, K.M.; Komives, E.A.; Lauberth, S.M. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat. Struct. Mol. Biol. 2018, 25, 687–697. [Google Scholar] [CrossRef]
- Kaelin, W.G.; McKnight, S.L. Influence of metabolism on epigenetics and disease. Cell 2013, 153, 56–69. [Google Scholar] [CrossRef]
- Tran, T.Q.; Lowman, X.H.; Kong, M. Molecular pathways: Metabolic control of histone methylation and gene expression in cancer. Clin. Cancer Res. 2017, 23, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Saha, N.; Gajan, A.; Saadat, N.; Gupta, S.V.; Pile, L.A. A complex interplay between SAM synthetase and the epigenetic regulator SIN3 controls metabolism and transcription. J. Biol. Chem. 2020, 295, 375–389. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Sutendra, G.; Kinnaird, A.; Dromparis, P.; Paulin, R.; Stenson, T.H.; Haromy, A.; Hashimoto, K.; Zhang, N.; Flaim, E.; Michelakis, E.D. A nuclear pyruvate dehydrogenase complex is important for the generation of Acetyl-CoA and histone acetylation. Cell 2014, 158, 84–97. [Google Scholar] [CrossRef]
- Masui, K.; Harachi, M.; Ikegami, S.; Yang, H.; Onizuka, H.; Yong, W.H.; Cloughesy, T.F.; Muragaki, Y.; Kawamata, T.; Arai, N.; et al. MTORC2 links growth factor signaling with epigenetic regulation of iron metabolism in glioblastoma. J. Biol. Chem. 2019, 294, 19740–19751. [Google Scholar] [CrossRef]
- Eguchi, K.; Nakayama, K. Prolonged hypoxia decreases nuclear pyruvate dehydrogenase complex and regulates the gene expression. Biochem. Biophys. Res. Commun. 2019, 520, 128–135. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y.; et al. ATP citrate lyase: Activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef]
- Masui, K.; Tanaka, K.; Ikegami, S.; Villa, G.R.; Yang, H.; Yong, W.H.; Cloughesy, T.F.; Yamagata, K.; Arai, N.; Cavenee, W.K.; et al. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 9406–9411. [Google Scholar] [CrossRef]
- Schug, Z.T.; Vande Voorde, J.; Gottlieb, E. The metabolic fate of acetate in cancer. Nat. Rev. Cancer 2016, 16, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Schug, Z.T.; Peck, B.; Jones, D.T.; Zhang, Q.; Grosskurth, S.; Alam, I.S.; Goodwin, L.M.; Smethurst, E.; Mason, S.; Blyth, K.; et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015, 27, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Arfuso, F.; Arumugam, S.; Chinnathambi, A.; Jinsong, B.; Warrier, S.; Wang, L.Z.; Kumar, A.P.; Ahn, K.S.; Sethi, G.; et al. Role of novel histone modifications in cancer. Oncotarget 2018, 9, 11414–11426. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.; Peserico, A. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. J. Biomed. Biotechnol. 2010, 2011. [Google Scholar] [CrossRef]
- Dekker, J.; Mirny, L. The 3D Genome as Moderator of Chromosomal Communication. Cell 2016, 164, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef]
- Hall, A.W.; Battenhouse, A.M.; Shivram, H.; Morris, A.R.; Cowperthwaite, M.C.; Shpak, M.; Iyer, V.R. Bivalent chromatin domains in glioblastoma reveal a subtype-specific signature of glioma stem cells. Cancer Res. 2018, 78, 2463–2474. [Google Scholar] [CrossRef]
- Rice, J.C.; Allis, C.D. Histone methylation versus histone acetylation: New insights into epigenetic regulation. Curr. Opin. Cell Biol. 2001, 13, 263–273. [Google Scholar] [CrossRef]
- Masui, K.; Harachi, M.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. Codependency of metabolism and epigenetics drives cancer progression: A review. Acta Histochem. Et Cytochem. 2020, 53, 1–10. [Google Scholar] [CrossRef]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.A.; Jones, D.T.W.; Konermann, C.; Pfaff, E.; Tönjes, M.; Sill, M.; Bender, S.; et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Song, J.; Wang, Y.; Zhao, Y.; Guda, K.; Yang, S.; Kao, H.Y.; Xu, Y.; Willis, J.; Markowitz, S.D.; et al. DNMT1 stability is regulated by proteins coordinating deubiquitination and acetylation-driven ubiquitination. Sci. Signal. 2010, 3, ra80. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Liu, L.; Chehab, N.H.; Mansfield, K.; Harris, K.G.; Halazonetis, T.D.; Berger, S.L. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 2001, 8, 1243–1254. [Google Scholar] [CrossRef]
- Rg Vervoorts, J.; Lü Scher-Firzlaff, J.; Lü Scher, B. The Ins and Outs of MYC Regulation by Posttranslational Mechanisms. J. Biol. Chem. 2006. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Jo, S.H.; Kim, M.Y.; Kim, T.H.; Ahn, Y.H. Role of transcription factor acetylation in the regulation of metabolic homeostasis. Protein Cell 2015, 6, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Aoyama, H.; Yoshimochi, K.; Fukamizu, A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, Y.; Roy, K.; Price, B.D. DNA Damage-Induced Acetylation of Lysine 3016 of ATM Activates ATM Kinase Activity. Mol. Cell. Biol. 2007, 27, 8502–8509. [Google Scholar] [CrossRef]
- Spange, S.; Wagner, T.; Heinzel, T.; Krämer, O.H. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 2009, 41, 185–198. [Google Scholar]
- Sadoul, K.; Wang, J.; Diagouraga, B.; Khochbin, S. The tale of protein lysine acetylation in the cytoplasm. J. Biomed. Biotechnol. 2010, 2011. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The major α-tubulin K40 acetyltransferase αTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Aldana-Masangkay, G.I. The role of HDAC6 in cancer. J. Biomed. Biotechnol. 2010, 2011. [Google Scholar] [CrossRef]
- Zhang, Z.; Yamashita, H.; Toyama, T.; Sugiura, H.; Omoto, Y.; Ando, Y.; Mita, K.; Hamaguchi, M.; Hayashi, S.-I.; Iwase, H. HDAC6 Expression Is Correlated with Better Survival in Breast Cancer. Clin. Cancer Res. 2004, 10, 6962–6968. [Google Scholar] [CrossRef]
- Kim, S.C.; Sprung, R.; Chen, Y.; Xu, Y.; Ball, H.; Pei, J.; Cheng, T.; Kho, Y.; Xiao, H.; Xiao, L.; et al. Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Mol. Cell 2006, 23, 607–618. [Google Scholar] [CrossRef]
- Anderson, K.A.; Hirschey, M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012, 52, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Koves, T.R.; Davidson, M.T.; Crown, S.B.; Fisher-Wellman, K.H.; Torres, M.J.; Draper, J.A.; Narowski, T.M.; Slentz, D.H.; Lantier, L.; et al. Disruption of Acetyl-Lysine Turnover in Muscle Mitochondria Promotes Insulin Resistance and Redox Stress without Overt Respiratory Dysfunction. Cell Metab. 2020, 31, 131–147.e11. [Google Scholar] [CrossRef]
- Wagner, G.R.; Payne, R.M. Mitochondrial acetylation and diseases of aging. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Pehar, M.; Lehnus, M.; Karst, A.; Puglielli, L. Proteomic Assessment Shows That Many Endoplasmic Reticulum (ER)-resident Proteins Are Targeted by N-Lysine Acetylation in the Lumen of the Organelle and Predicts Broad Biological Impact. J. Biol. Chem. 2012, 287, 22436–22440. [Google Scholar] [CrossRef]
- Mi, H.K.; Puglielli, L. Two Endoplasmic Reticulum (ER)/ER golgi intermediate compartment-based lysine acetyltransferases post-translationally regulate BACE1 levels. J. Biol. Chem. 2009, 284, 2482–2492. [Google Scholar] [CrossRef]
- Kitabayashi, I.; Aikawa, Y.; Yokoyama, A.; Hosoda, F.; Nagai, M.; Kakazu, N.; Abe, T.; Ohki, M. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with a t(8;22)(p11;q13) chromosome translocation. Leukemia 2001, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Lorenzini, E.; Ciarrocchi, A. BRD4 and Cancer: Going beyond transcriptional regulation. Mol. Cancer 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, A.A.; Walsh, E.M.; Wang, X.; Grayson, A.R.; Hsi, P.T.; Kharchenko, P.V.; Kuroda, M.I.; French, C.A. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rhyasen, G.W.; Yao, Y.; Zhang, J.; Dulak, A.; Castriotta, L.; Jacques, K.; Zhao, W.; Gharahdaghi, F.; Hattersley, M.M.; Lyne, P.D.; et al. BRD4 amplification facilitates an oncogenic gene expression program in high-grade serous ovarian cancer and confers sensitivity to BET inhibitors. PLoS ONE 2018, 13, e0200826. [Google Scholar] [CrossRef]
- Sun, C.; Yin, J.; Fang, Y.; Chen, J.; Jeong, K.J.; Chen, X.; Vellano, C.P.; Ju, Z.; Zhao, W.; Zhang, D.; et al. BRD4 Inhibition Is Synthetic Lethal with PARP Inhibitors through the Induction of Homologous Recombination Deficiency. Cancer Cell 2018, 33, 401–416.e8. [Google Scholar] [CrossRef]
- Harachi, M.; Masui, K.; Okamura, Y.; Tsukui, R.; Mischel, P.S.; Shibata, N. mTOR complexes as a nutrient sensor for driving cancer progression. Int. J. Mol. Sci. 2018, 19, 3267. [Google Scholar] [CrossRef]
- Masui, K.; Harachi, M.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. mTOR complex 2 is an integrator of cancer metabolism and epigenetics. Cancer Lett. 2020, 478, 1–7. [Google Scholar] [CrossRef]
- Glidden, E.J.; Gray, L.G.; Vemuru, S.; Li, D.; Harris, T.E.; Mayo, M.W. Multiple site acetylation of rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J. Biol. Chem. 2012, 287, 581–588. [Google Scholar] [CrossRef]
- Martinez Calejman, C.; Trefely, S.; Entwisle, S.W.; Luciano, A.; Jung, S.M.; Hsiao, W.; Torres, A.; Hung, C.M.; Li, H.; Snyder, N.W.; et al. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Cohen, A.; Habib, A.; Laor, D.; Yadav, S.; Kupiec, M.; Weisman, R. TOR complex 2 in fission yeast is required for chromatin-mediated gene silencing and assembly of heterochromatic domains at subtelomeres. J. Biol. Chem. 2018, 293, 8138–8150. [Google Scholar] [CrossRef]
- Vadla, R.; Haldar, D. Mammalian target of rapamycin complex 2 (mTORC2) controls glycolytic gene expression by regulating Histone H3 Lysine 56 acetylation. Cell Cycle 2018, 17, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosa, H.; Valls, E.; Kouzarides, T.; Martínez-Balbás, M. Mechanisms of P/CAF auto-acetylation. Nucleic Acids Res. 2003, 31, 4285–4292. [Google Scholar] [CrossRef]
- Schrump, D.S. Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: Mechanisms and potential clinical implications. Clin. Cancer Res. 2009, 15, 3947–3957. [Google Scholar] [CrossRef]
- Kuznetsoff, J.N.; Owens, D.A.; Lopez, A.; Rodriguez, D.A.; Chee, N.T.; Kurtenbach, S.; Bilbao, D.; Roberts, E.R.; Volmar, C.H.; Wahlestedt, C.; et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol. Cancer Res. 2021, 19, 215–222. [Google Scholar] [CrossRef]
- Kalac, M.; Scotto, L.; Marchi, E.; Amengual, J.; Seshan, V.E.; Bhagat, G.; Ulahannan, N.; Leshchenko, V.V.; Temkin, A.M.; Parekh, S.; et al. HDAC inhibitors and decitabine are highly synergistic and associated with unique gene-expression and epigenetic profiles in models of DLBCL. Blood 2011, 118, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.T.H.; Yamaguchi, Y.; Choijookhuu, N.; Yano, K.; Takagi, H.; Takahashi, N.; Oo, P.S.; Sato, K.; Hishikawa, Y. The HDAC inhibitor, SAHA, combined with cisplatin synergistically induces apoptosis in alpha-fetoprotein-producing hepatoid adenocarcinoma cells. Acta Histochem. Et Cytochem. 2019, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mwakwari, S.C.; Patil, V.; Guerrant, W.; Oyelere, A.K. Macrocyclic histone deacetylase inhibitors. Curr. Top. Med. Chem. 2010, 10, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Sowa, Y.; Xu, W.S.; Shao, Y.; Dokmanovic, M.; Perez, G.; Ngo, L.; Holmgren, A.; Jiang, X.; Marks, P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 673–678. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef]
- Picaud, S.; Da Costa, D.; Thanasopoulou, A.; Filippakopoulos, P.; Fish, P.V.; Philpott, M.; Fedorov, O.; Brennan, P.; Bunnage, M.E.; Owen, D.R.; et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET bromodomains. Cancer Res. 2013, 73, 3336–3346. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.F.; Fontanals-Cirera, B.; Gaziel-Sovran, A.; Guijarro, M.V.; Hanniford, D.; Zhang, G.; González-Gomez, P.; Morante, M.; Jubierre, L.; Zhang, W.; et al. BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 2013, 73, 6264–6276. [Google Scholar] [CrossRef]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Kowalsky, A.H.; Namkoong, S.; Mettetal, E.; Park, H.W.; Kazyken, D.; Fingar, D.C.; Lee, J.H. The GATOR2-mTORC2 axis mediates Sestrin2-induced AKT Ser/Thr kinase activation. J. Biol. Chem. 2020, 295, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Serrato, A.; Lee, J.; Holmes, B.; Landon, K.A.; Bashir, T.; Jung, M.E.; Lichtenstein, A.; Gera, J. Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma. PLoS ONE 2017, 12, e0176599. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Proud, C.G. Crosstalk between mTor Complexes. Nat. Cell Biol. 2013, 15, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Harachi, M.; Masui, K.; Honda, H.; Muragaki, Y.; Kawamata, T.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. Dual Regulation of Histone Methylation by mTOR Complexes Controls Glioblastoma Tumor Cell Growth via EZH2 and SAM. Mol. Cancer Res. Mcr. 2020, 18, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Fioravanti, R.; Granese, A.; Carradori, S.; Tosi, F.; et al. A novel histone acetyltransferase inhibitor modulating Gcn5 network: Cyclopentylidene-[4-(4′-chlorophenyl)thiazol-2-yl)hydrazone. J. Med. Chem. 2009, 52, 530–536. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Ragazzoni, Y.; Pelosi, A.; Desideri, M.; Carradori, S.; Gabellini, C.; Maresca, G.; Nescatelli, R.; Secci, D.; Bolasco, A.; et al. CPTH6, a thiazole derivative, induces histone hypoacetylation and apoptosis in human leukemia cells. Clin. Cancer Res. 2012, 18, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Bourke, E.; Scobie, M.; Famme, M.A.; Koolmeister, T.; Helleday, T.; Eriksson, L.A.; Lowndes, N.F.; Brown, J.A.L. Rational design and validation of a Tip60 histone acetyltransferase inhibitor. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Gao, X.N.; Lin, J.; Ning, Q.Y.; Gao, L.; Yao, Y.S.; Zhou, J.H.; Li, Y.H.; Wang, L.L.; Yu, L. A Histone Acetyltransferase p300 Inhibitor C646 Induces Cell Cycle Arrest and Apoptosis Selectively in AML1-ETO-Positive AML Cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, K.; Altaf, M.; Varier, R.A.; Swaminathan, V.; Ravindran, A.; Sadhale, P.P.; Kundu, T.K. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 2004, 279, 33716–33726. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ao, A.; Zhou, L.; Murphy, C.K.; Frist, A.Y.; Keel, J.J.; Thorne, C.A.; Kim, K.; Lee, E.; Hong, C.C. Selective small molecule targeting β-catenin function discovered by in vivo chemical genetic screen. Cell Rep. 2013, 4, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Y.; Shu, M.; Tang, J.; Huang, Y.; Zhou, Y.; Liang, Y.; Yan, G. Hypoxia-inducible factor-1α blocks differentiation of malignant gliomas. Febs J. 2009, 276, 7291–7304. [Google Scholar] [CrossRef]
- Yin, S.; Kaluz, S.; Devi, N.S.; Jabbar, A.A.; De Noronha, R.G.; Mun, J.; Zhang, Z.; Boreddy, P.R.; Wang, W.; Wang, Z.; et al. Arylsulfonamide KCN1 Inhibits In Vivo Glioma Growth and Interferes with HIF Signaling by Disrupting HIF-1a Interaction with Cofactors p300/CBP. Clin. Cancer Res. 2012. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Oh, S.W.; Kim, H.Y.; et al. A small molecule inhibitor of β-catenin/cyclic AMP response element-binding protein transcription. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.; Jimeno, A. Belinostat for the treatment of peripheral T-cell lymphomas. Drugs Today 2014, 50, 337–345. [Google Scholar] [CrossRef]
- Richardson, P.G.; Laubach, J.P.; Lonial, S.; Moreau, P.; Yoon, S.S.; Hungria, V.T.; Dimopoulos, M.A.; Beksac, M.; Alsina, M.; San-Miguel, J.F. Panobinostat: A novel pan-deacetylase inhibitor for the treatment of relapsed or relapsed and refractory multiple myeloma. Expert Rev. Anticancer. Ther. 2015, 15, 737–748. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Corner, G.A.; Augenlicht, L.H. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: Comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000, 60, 4561–4572. [Google Scholar]
- Knipstein, J.; Gore, L. Entinostat for treatment of solid tumors and hematologic malignancies. Expert Opin. Investig. Drugs 2011, 20, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Oki, Y.; Bociek, R.G.; Kuruvilla, J.; Fanale, M.; Neelapu, S.; Copeland, A.; Buglio, D.; Galal, A.; Besterman, J.; et al. Mocetinostat for relapsed classical Hodgkin’s lymphoma: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2011, 12, 1222–1228. [Google Scholar] [CrossRef]
- Pauer, L.R.; Olivares, J.; Cunningham, C.; Williams, A.; Grove, W.; Kraker, A.; Olson, S.; Nemunaitis, J. Phase I study of oral CI-994 in combination with carboplatin and paclitaxel in the treatment of patients with advanced solid tumors. Cancer Investig. 2004, 22, 886–896. [Google Scholar] [CrossRef]
- Bilen, M.A.; Fu, S.; Falchook, G.S.; Ng, C.S.; Wheler, J.J.; Abdelrahim, M.; Erguvan-Dogan, B.; Hong, D.S.; Tsimberidou, A.M.; Kurzrock, R.; et al. Phase i trial of valproic acid and lenalidomide in patients with advanced cancer. Cancer Chemother. Pharmacol. 2015, 75, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Rowinsky, E.K.; Villalona, M.A.; Hammond, L.A.; Britten, C.D.; Siu, L.L.; Goetz, A.; Felton, S.A.; Burton, S.; Valone, F.H.; et al. A Phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin. Cancer Res. 2002, 8, 2142–2148. [Google Scholar]
- Iannitti, T.; Palmieri, B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D 2011, 11, 227–249. [Google Scholar] [CrossRef]
- Frye, R.; Myers, M.; Axelrod, K.C.; Ness, E.A.; Piekarz, R.L.; Bates, S.E.; Booher, S. Romidepsin: A new drug for the treatment of cutaneous T-cell lymphoma. Clin. J. Oncol. Nurs. 2012, 16, 195–204. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zanca, C.; Rajkumar, U.; Koga, T.; Diao, Y.; Raviram, R.; Liu, F.; Turner, K.; Yang, H.; Brunk, E.; et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling. Nature 2019, 569, 570–575. [Google Scholar] [CrossRef]
- Weinstein, I.B. Cancer: Addiction to oncogenes—The Achilles heal of cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Gini, B.; Wykosky, J.; Zanca, C.; Mischel, P.S.; Furnari, F.B.; Cavenee, W.K. A tale of two approaches: Complementary mechanisms of cytotoxic and targeted therapy resistance may inform next-generation cancer treatments. Carcinogenesis 2013, 34, 725–738. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).