Salivary Osteopontin as a Potential Biomarker for Oral Mucositis
Abstract
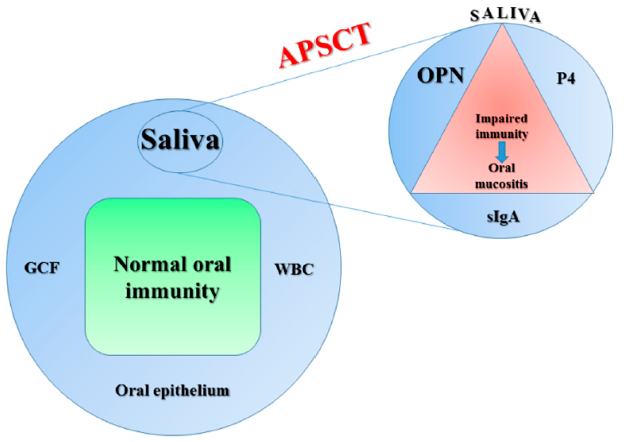
1. Introduction
2. Results
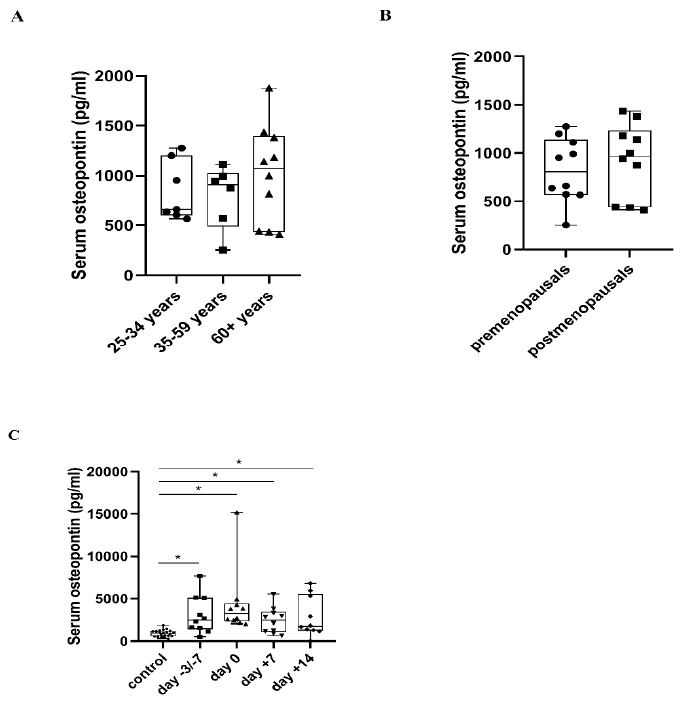
2.1. Serum OPN Levels
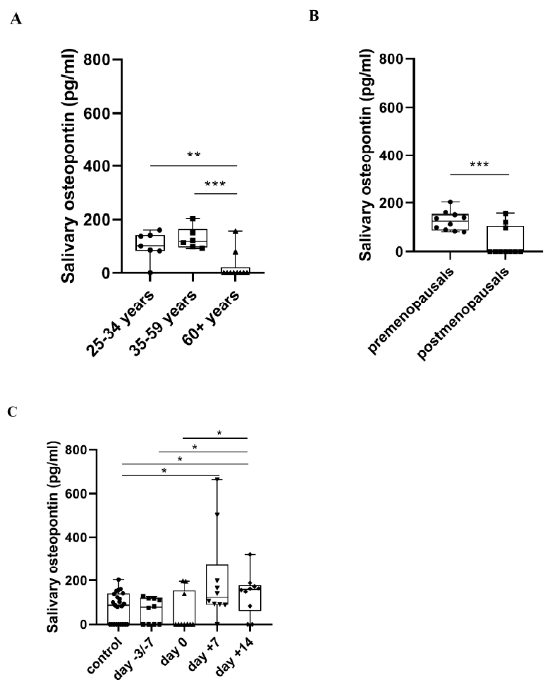
2.2. Salivary OPN Levels
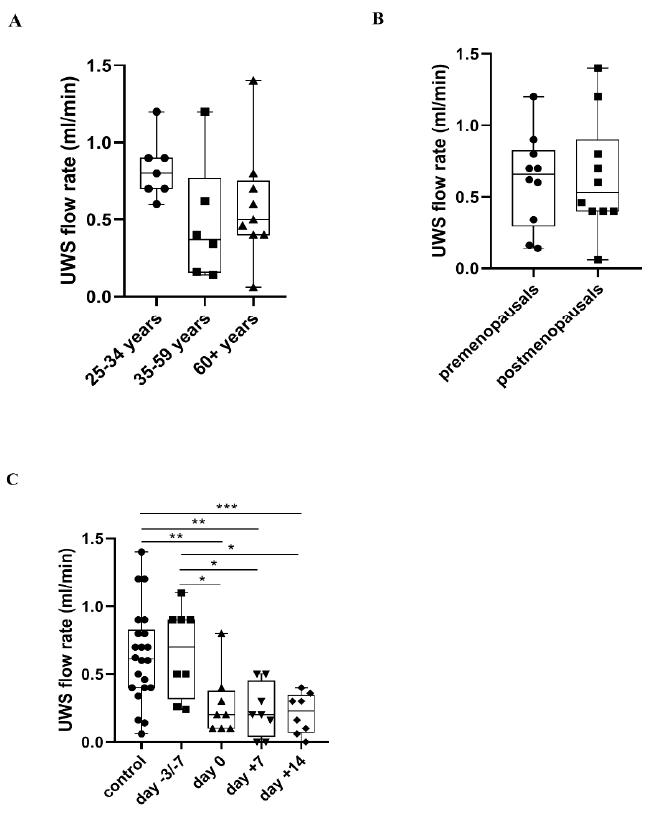
2.3. UWS Flow Rate
2.4. Serum CRP Levels
2.5. Correlation Analyses
3. Discussion
3.1. Serum OPN Levels in Healthy Controls and APSCT Patients
3.2. Salivary OPN Levels in Healthy Controls and APSCT Patients
3.3. Changes of UWS Flow Rate during APSCT
3.4. Correlations
4. Materials and Methods
4.1. Study Population, Ethics and Patient Characteristics
4.2. Serum and Unstimulated Whole Saliva (UWS) Sample Collection
4.3. Detection of Serum and Salivary OPN Levels
4.4. Detection of Serum C-Reactive Protein (CRP) and Lactate Dehydrogenase (LDH) Levels
4.5. Detection of Salivary Progesterone (P4) Levels
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| APSCT | autologous peripheral stem-cell transplantation |
| BEAM | BCNU, etoposide, cytosine arabinoside, melphalan |
| BMD | bone mineral density |
| BMT | bone marrow transplantation |
| CML | chronic myeloid leukemia |
| CMR | complete morphologic remission |
| CRP | c-reactive protein |
| EDTA | ethylenediaminetetraacetic acid |
| EBMT | European Society for Blood and Marrow Transplantation |
| ELISA | enzyme linked immunosorbent assays |
| ER-α | estrogen receptor-α |
| ER-ß | estrogen receptor-ß |
| GCF | gingival crevicular fluid |
| GVHD | graft-versus-host disease |
| HSC | hematopoietic stem cell |
| HSCT | hematopoietic stem cell transplantation |
| HL | Hodgkin lymphoma |
| IgA | immunoglobulin A |
| sIgA | secretory immunoglobulin A |
| LDH | lactate dehydrogenase |
| MBI | mucosal barrier injury |
| MNC | mononuclear cell |
| MM | multiple myeloma |
| NHL | non-Hodgkin lymphoma |
| OM | oral mucositis |
| OPN | osteopontin |
| OSCC | oral squamous cell carcinoma |
| P4 | progesterone |
| PR | partial remission |
| SG | salivary gland |
| SIBLING | Small Integrin-Binding Ligand N-linked Glycoprotein |
| TBI | total body irradiation |
| UWS | unstimulated whole saliva |
| WBC | white blood cells |
| WHO | World Health Organization |
References
- Feller, L.; Altini, M.; Khammissa, R.A.G.; Chadran, R.; Bouckaert, M.; Lemmer, J. Oral mucosal immunity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Gebri, E.; Kovács, Z.; Mészáros, B.; Tóth, F.; Simon, Á.; Jankovics, H.; Vonderviszt, F.; Kiss, A.; Guttman, A.; Hortobágyi, T. N-Glycosylation Alteration of Serum and Salivary Immunoglobulin A Is a Possible Biomarker in Oral Mucositis. J. Clin. Med. 2020, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Gebri, E.; Kiss, A.; Tóth, F.; Hortobágyi, T. Female sex as an independent prognostic factor in the development of oral mucositis during autologous peripheral stem cell transplantation. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, B.; Kovacs, Z.; Gebri, E.; Jankovics, H.; Vonderviszt, F.; Kiss, A.; Simon, A.; Botka, S.; Hortobagyi, T.; Guttman, A. N-glycomic analysis of Z(IgA1) partitioned serum and salivary immunoglobulin A by capillary electrophoresis. Curr. Mol. Med. 2020, 20. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. The function of salivary proteins and the regulation of their secretion by salivary glands. Biomed. Rev. 1998, 9, 3–15. [Google Scholar] [CrossRef]
- López, J.P. Special Issue “Saliva and Oral Diseases”. J. Clin. Med. 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, D.; Søland, T.M.; Galtung, H.K.; Sand, L.P.; Giannecchini, S.; To, K.K.W.; Mendes-Correa, M.C.; Giglio, D.; Hasseus, B.; Braz-Silva, P.H. COVID-19 salivary signature: Diagnostic and research opportunities. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Khurshid, Z.; Akhbar, S.; Moin, S.F. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: An update. Proteomes 2016, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin-a promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef] [PubMed]
- Devoll, R.E.; Li, W.; Woods, K.V.; Pinero, G.J.; Butler, W.T.; Farach-Carson, M.C.; Happonen, R.P. Osteopontin (OPN) distribution in premalignant and malignant lesions of oral epithelium and expression in cell lines derived from squamous cell carcinoma of the oral cavity. J. Oral Pathol. Med. 2007, 28, 97–101. [Google Scholar] [CrossRef]
- Aravind, T.; Janardhanan, M.; Rakesh, S.; Savithri, V.; Unnikrishnan, U.G. Immunolocalization of osteopontin in dysplasias and squamous cell carcinomas arising from oral epithelium. J. Oral Maxillofac. Pathol. 2017, 21, 18–23. [Google Scholar]
- Haylock, D.N.; Nilsson, S.K. Osteopontin: A bridge between bone and blood. Br. J. Haematol. 2006, 134, 467–474. [Google Scholar] [CrossRef]
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014, 37, 131–141. [Google Scholar] [CrossRef]
- Sampayo-Escobar, V.; Green, R.; Cheung, M.B.; Bedi, R.; Mohapatra, S.; Mohapatra, S.S. Osteopontin plays a pivotal role in increasing severity of respiratory syncytial virus infection. PLoS ONE 2018, 13, e0192709. [Google Scholar] [CrossRef]
- Sodek, J.; Da Silva, A.P.B.; Zohar, R. Osteopontin and mucosal protection. J. Dent. Res. 2006, 85, 404–415. [Google Scholar] [CrossRef]
- McCann, S.; Schwenkglenks, M.; Bacon, P.; Einsele, H.; D’Addio, A.; Maertens, J.; Niederwiser, D.; Rabitsch, W.; Roosaar, A.; Ruutu, T.; et al. The Prospective oral mucositis audit: Relationship of severe oral mucositis with clinical and medical resource use outcomes in patients receiving high-dose melphalan or BEAM-conditioning chemotherapy and autologous SCT. Bone Marrow Transplant. 2009, 43, 141–147. [Google Scholar] [CrossRef]
- Blijlevens, N.M.A.; Donnelly, J.P.; De Pauw, B.E. Mucosal barrier injury: Biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: An overview. Bone Marrow Transplant. 2000, 25, 1269–1278. [Google Scholar] [CrossRef]
- Abram, T.J.; Pickering, C.R.; Lang, A.K.; Bass, N.E.; Raja, R.; Meena, C.; Alousi, A.M.; Myers, J.N.; McDevitt, J.T.; Gillenwater, A.M.; et al. Risk Stratification of Oral Potentially Malignant Disorders in Fanconi Anemia Patients Using Autofluorescence Imaging and Cytology-On-A Chip Assay. Transl. Oncol. 2018, 11, 477–486. [Google Scholar] [CrossRef]
- Grubbs, J.B.; Exline, J.J.; McCain, J.; Campbell, W.K.; Twenge, J.M. Emerging adult reactions to labeling regarding age-group differences in narcissism and entitlement. PLoS ONE 2019, 14, e0215637. [Google Scholar] [CrossRef]
- Auner, H.W.; Iacobelli, S.; Sbianchi, G.; Knol-Bout, C.; Blaise, D.; Russel, N.H.; Apperley, J.F.; Pohlreich, D.; Browne, P.V.; Kobbe, G.; et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: Results from the collaboration to collect autologous transplant outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT chronic malignancies working party. Haematologica 2018, 103, 514–521. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Bravo, S.B.; Lopez-Jornet, P.; García-Vence, M.; Alonso-Sampedro, M.; Carballo, J.; García-García, A. Protein-Based salivary profiles as novel biomarkers for oral diseases. Dis. Markers 2018, 2018, 6141845. [Google Scholar] [CrossRef]
- Cristaudo, A.; Foddis, R.; Bonotti, A.; Simonini, S.; Vivaldi, A.; Guglielmi, G.; Ambrosino, N.; Canessa, P.A.; Chella, A.; Lucchi, M.; et al. Comparison between plasma and serum osteopontin levels: Usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int. J. Biol. Markers 2010, 25, 164–170. [Google Scholar] [CrossRef]
- Miyajima, J.; Hayashi, T.; Saito, K.; Iida, S.; Matsuoka, K. The Interaction between Female Sex Hormone Receptors and Osteopontin in a Rat Hyperoxaluric Model. Kurume Med. J. 2010, 57, 73–80. [Google Scholar] [CrossRef]
- Chang, I.C.; Chiang, T.I.; Yeh, K.T.; Lee, H.; Cheng, Y.-W. Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporos Int. 2010, 21, 1401–1409. [Google Scholar] [CrossRef]
- Cho, E.H.; Cho, K.H.; Lee, H.A.; Kim, S.W. High Serum Osteopontin Levels Are Associated with Low Bone Mineral Density in Postmenopausal Women. J. Korean Med. Sci. 2013, 28, 1496–1499. [Google Scholar] [CrossRef]
- Yoshitake, H.; Rittling, S.R.; Denhardt, D.T.; Noda, M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc. Natl. Acad. Sci. USA 1999, 96, 8156–8160. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.S.; Turki, K.M.; Munshed, M.H. Serum Osteocalcin and Serum Osteopontin Levels in Osteoporotic Postmenopausal Women with and without Vertebral Fractures. J. Fac. Med. Baghdad 2015, 57, 257–262. [Google Scholar] [CrossRef]
- Nilsson, S.K.; Johnston, H.M.; Whitty, G.A.; Williams, B.; Webb, R.J.; Denhardt, D.T.; Bertoncello, I.; Bendall, L.J.; Simmons, P.J.; Haylock, D.N. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005, 106, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Ter Huurne, M.; Figdor, C.G.; Torensma, R. Hematopoietic stem cells are coordinated by the molecular cues of the endosteal niche. Stem Cells Dev. 2010, 19, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; García, C.C.; Riedt, T.; Brandes, M.; Szczepanski, S.; Brossart, P.; Wagner, W.; Janzen, V. Murine hematopoietic stem cell reconstitution potential is maintained by osteopontin during aging. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Mima, T.; Ishii, T.; Ogata, A.; Kobayashi, H.; Oshima, S.; Ishida, T.; Tabunoki, Y.; Kitayama, H.; Mizuki, M.; et al. Enhanced production of osteopontin in multiple myeloma: Clinical and pathogenic implications. Br. J. Haematol. 2003, 123, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Standal, T.; Hjorth-Hansen, H.; Rasmussen, T.; Dahl, I.M.S.; Lehoff, S.; Brenne, A.T.; Seidel, C.; Baykov, V.; Waage, A.; Borset, M.; et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica 2004, 89, 174–182. [Google Scholar]
- Flamant, S.; Kortulewski, T.; Dugray, A.; Bonnet, M.-L.; Guillier, M.; Guilhot, F.; Bourhis, J.-H.; Vainchenker, W.; Tronik-Le Roux, D.; Turhan, A.G. Osteopontin is upregulated by BCR-ABL. Biochem. Biophys. Res. Commun. 2005, 333, 1378–1384. [Google Scholar] [CrossRef]
- Barranco, G.; Fernández, E.; Rivas, S.; Quezada, R.; Nava, D.; Aguilar, J.; Garcia, A.; Astudillo, H.; Lome, C.; Ruiz, E. Osteopontin expression and its relationship with prognostic factors in diffuse large b-cell lymphoma. Hematol Rep. 2019, 11, 7964. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, S.; Li, S.; Du, Z. Prognostic significance of osteopontin in acute myeloid leukemia: A meta-analysis. Mol. Clin. Oncol. 2017, 7, 275. [Google Scholar] [CrossRef]
- Boyerinas, B.; Zafrir, M.; Yesilkanal, A.E.; Price, T.T.; Hyjek, E.M.; Sipkins, D.A. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 2013, 121, 4821–4831. [Google Scholar] [CrossRef]
- Al-Asadi, M.G.; Brindle, G.; Castellanos, M.; May, S.T.; Mills, K.I.; Russel, N.H.; Seedhouse, C.H.; Pallis, M. A Molecular Signature of Dormancy in CD34 + CD38-Acute Myeloid Leukaemia Cells. Oncotarget 2017, 8, 111405–111418. [Google Scholar] [CrossRef]
- Lee, C.Y.; Tien, H.F.; Hou, H.A.; Chou, W.C.; Lin, L.I. Marrow osteopontin level as a prognostic factor in acute myeloid leukaemia. Br. J. Haematol. 2008, 141, 736–739. [Google Scholar] [CrossRef]
- Asaka, M.; Ohta, K.; Muramatsu, T.; Kurokawa, M.; Kizaki, H.; Hashimoto, S.; Shimono, M. The expression and localization of osteopontin in the mouse major salivary glands. Arch. Histol. Cytol. 2006, 69, 181–188. [Google Scholar] [CrossRef][Green Version]
- Obermüller, N.; Gassler, N.; Gretz, N.; Kränzlin, B.; Hoffman, S.; Geiger, H.; Gauer, S. Distinct immunohistochemical expression of osteopontin in the adult rat major salivary glands. J. Mol. Histol. 2006, 37, 53–60. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Peruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell. 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral. Biol. 2005, 50, 7–16. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Liukkonen, J.; Jula, A.; Huumonen, S.; Suominen, A.L.; Puukka, P.; Könönen, E. Associations Between Salivary Bone Metabolism Markers and Periodontal Breakdown. J. Periodontol. 2016, 87, 367–375. [Google Scholar] [CrossRef]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.-A.; Laine, M. Estrogen receptor-β is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef]
- Johnson, G.A.; Spencer, T.E.; Burghardt, R.C.; Taylor, K.M.; Gray, C.A.; Bazer, F.W. Progesterone Modulation of Osteopontin Gene Expression in the Ovine Uterus1. Biol. Reprod. 2000, 62, 1315–1321. [Google Scholar] [CrossRef]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Spencer, T.E. Osteopontin: Roles in Implantation and Placentation. Biol. Reprod. 2003, 69, 1458–1471. [Google Scholar] [CrossRef]
- Gao, N.; Zhang-Brotzge, X.; Wali, B.; Sayeed, I.; Chern, J.J.; Blackwell, L.S.; Kuan, C.-Y.; Reisner, A. Plasma osteopontin may predict neuroinflammation and the severity of pediatric traumatic brain injury. J. Cereb. Blood Flow Metab. 2020, 40, 35–43. [Google Scholar] [CrossRef]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heingård, D. Osteopontin–A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Vaalamo, M.; Karjalainen-Lindsberg, M.L.; Puolakkainen, P.; Kere, J.; Saarialho-Kere, U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am. J. Pathol. 1998, 152, 1005–1014. [Google Scholar]
- Wolff, D.; Greinix, H.; Lee, S.J.; Gooley, T.; Paczesny, S.; Pavletic, S.; Hakim, F.; Malard, F.; Jagasia, M.; Lawitschka, A.; et al. Biomarkers in chronic graft-versus-host disease: Quo vadis? Bone Marrow Transplant. 2018, 53, 832–837. [Google Scholar] [CrossRef]
- Williams, K.M.; Hakim, F.T.; Rosenberg, A.; Kleiner, D.; Mitchell, S.A.; Tamm, M.; Avila, D.; Lee, S.J.; Halter, J.; Prince, S.S.; et al. Plasma osteopontin is a biomarker specifically associated with bronchiolitis obliterans syndrome after HCT. Biol. Blood Marrow Transplant. 2016, 22, S417–S418. [Google Scholar] [CrossRef]
- Da Silva Santos, P.S.; Coracin, F.L.; José Carlos de Almeida, B.; Gallottini, M.H.C. Histopathologic diagnosis of chronic graft-versus-host disease of the oral mucosa according to the National Institutes of Health Consensus. Einstein 2014, 12, 204–210. [Google Scholar] [CrossRef]
- Van Leeuwen, S.J.M.; Proctor, G.B.; Potting, C.M.J.; Hoopen, S.T.; van Groningen, L.F.J.; Bronkhorst, E.M.; Blijlevens, N.M.A.; Huysmans, M. Early salivary changes in multiple myeloma patients undergoing autologous HSCT. Oral Dis. 2018, 24, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Rafid, M.; Saka, S.; Abdullah, H.I. Assessment of salivary flow rate and secretory immunoglobulin A and oral mucosal changes in acute myeloid leukemia before and after the induction phase of chemotherapy. J. Baghdad Coll. Dent. 2009, 21, 82–89. [Google Scholar]
- Degirmencioglu, S. The role of osteopontin expression in the prognosis of Malignant Melanoma. Nov. Approaches Cancer Study 2018. [Google Scholar] [CrossRef]
- Hotte, S.J.; Winquist, E.W.; Stitt, L.; Wilson, S.M.; Chambers, A.F. Plasma osteopontin. Cancer 2002, 95, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Bratman, S.; Su, J.; Tong, L.; Kim, J.H.; Waldron, J.N.; Hansen, A.R.; Goldstein, D.; Bayley, A.; Cho, J. Independent Adverse Prognosis of Elevated Serum LDH in Human Papillomavirus–Related Oropharyngeal Cancer. Int. J. Radiat. Oncol. 2016, 96, S83. [Google Scholar] [CrossRef][Green Version]
- van leeuwen, S.J.M.; Proctor, G.B.; Laheij, A.M.G.A.; Potting, C.J.M.; Smits, O.; Bronkhorst, E.M.; Hazenberg, M.D.; Haverman, T.M.; Brennan, M.T.; von Bültzingslöwen, I.; et al. Significant salivary changes in relation to oral mucositis following autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2021. [Google Scholar] [CrossRef]
- Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal injury during Anti-Cancer treatment: From pathobiology to bedside. Cancers 2019, 11, 857. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef]
- Niscola, P.; Romani, C.; Cupelli, L.; Scaramucci, L.; Tendas, A.; Dentamaro, T.; Amadori, S.; de Fabritiis, P. Mucositis in patients with hematologic malignancies: An overview. Haematologica 2007, 92, 222–231. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Osman, T.A.; Costea, D.E.; Johannessen, A.C. The use of salivary cytokines as a screening tool for oral squamous cell carcinoma: A review of the literature. J. Oral Maxillofac. Pathol. 2012, 16, 256–261. [Google Scholar] [CrossRef]

| Patients | Controls | ||||
|---|---|---|---|---|---|
| (n = 10) | (n = 23) | ||||
| Average age (years) | 52.20 ± 13.78 | 52.20 | |||
| Male/female ratio | 3:7 | 3:20 | |||
| Pretreatment time (months) | 14.5 ± 6.74 | ||||
| Total patient group | 15 ± 4.9 | ||||
| Lymphoma group (HL/NHL) | 13.33 ± 11.37 | ||||
| MM group | |||||
| Diagnosis | |||||
| Hodgkin lymphoma | 1 | ||||
| Non-Hodgkin lymphoma | 6 | ||||
| Multiple myeloma | 3 | ||||
| Pretransplantational stage of the disease | |||||
| Complete remission | 7 | ||||
| Partial remission | 3 | ||||
| Conditioning regimen | |||||
| BEAM | 1 | ||||
| R-BEAM | 5 | ||||
| Adcetris-BEAM | 1 | ||||
| Melphalan (140 mg/m2) | 1 | ||||
| Melphalan (≥200 mg/m2) | 1 | ||||
| R-Bendamustin-Melphalan | 1 | ||||
| Pretransplantational LDH level (physiological range: 135–220 U/L) | 277.33 ± 66.97 | ||||
| Amount of stem cells (106/body mass kg) | 4.56 ± 1.87 | ||||
| Stem cell viability (%) | 87.17 ± 11.73 | ||||
| Viable cell count (106/body mass kg) | 3.95 ± 1.57 | ||||
| MNC (108/body mass kg) | 5.59 ± 6.11 | ||||
| Highest grade of OM (WHO) | |||||
| 0 | 0 | ||||
| 1 | 4 | ||||
| 2 | 3 | ||||
| 3 | 2 | ||||
| 4 | 1 | ||||
| Last status | |||||
| Alive | 8 | ||||
| Dead | 2 | ||||
| Hormonal status in female (n) | |||||
| Premenopausal | 1 | 10 | |||
| Postmenopausal | 6 | 10 | |||
| Salivary progesterone (P4) levels (µg/L) | |||||
| Premenopausal’s | Postmenopausals’ | 0.34 ± 0.10 | 0.21 ± 0.03 | ||
| day −3/−7 | 0.24 | 0.21 ± 0.02 | |||
| day 0 | 0.20 | 0.20 | |||
| day +7 | 1.86 | 0.56 ± 0.73 | |||
| day +14 | 0.87 | 0.84 ± 1.20 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebri, E.; Kiss, A.; Tóth, F.; Hortobágyi, T. Salivary Osteopontin as a Potential Biomarker for Oral Mucositis. Metabolites 2021, 11, 208. https://doi.org/10.3390/metabo11040208
Gebri E, Kiss A, Tóth F, Hortobágyi T. Salivary Osteopontin as a Potential Biomarker for Oral Mucositis. Metabolites. 2021; 11(4):208. https://doi.org/10.3390/metabo11040208
Chicago/Turabian StyleGebri, Enikő, Attila Kiss, Ferenc Tóth, and Tibor Hortobágyi. 2021. "Salivary Osteopontin as a Potential Biomarker for Oral Mucositis" Metabolites 11, no. 4: 208. https://doi.org/10.3390/metabo11040208
APA StyleGebri, E., Kiss, A., Tóth, F., & Hortobágyi, T. (2021). Salivary Osteopontin as a Potential Biomarker for Oral Mucositis. Metabolites, 11(4), 208. https://doi.org/10.3390/metabo11040208








