Discovery of Volatile Biomarkers for Bladder Cancer Detection and Staging through Urine Metabolomics
Abstract
1. Introduction
2. Results
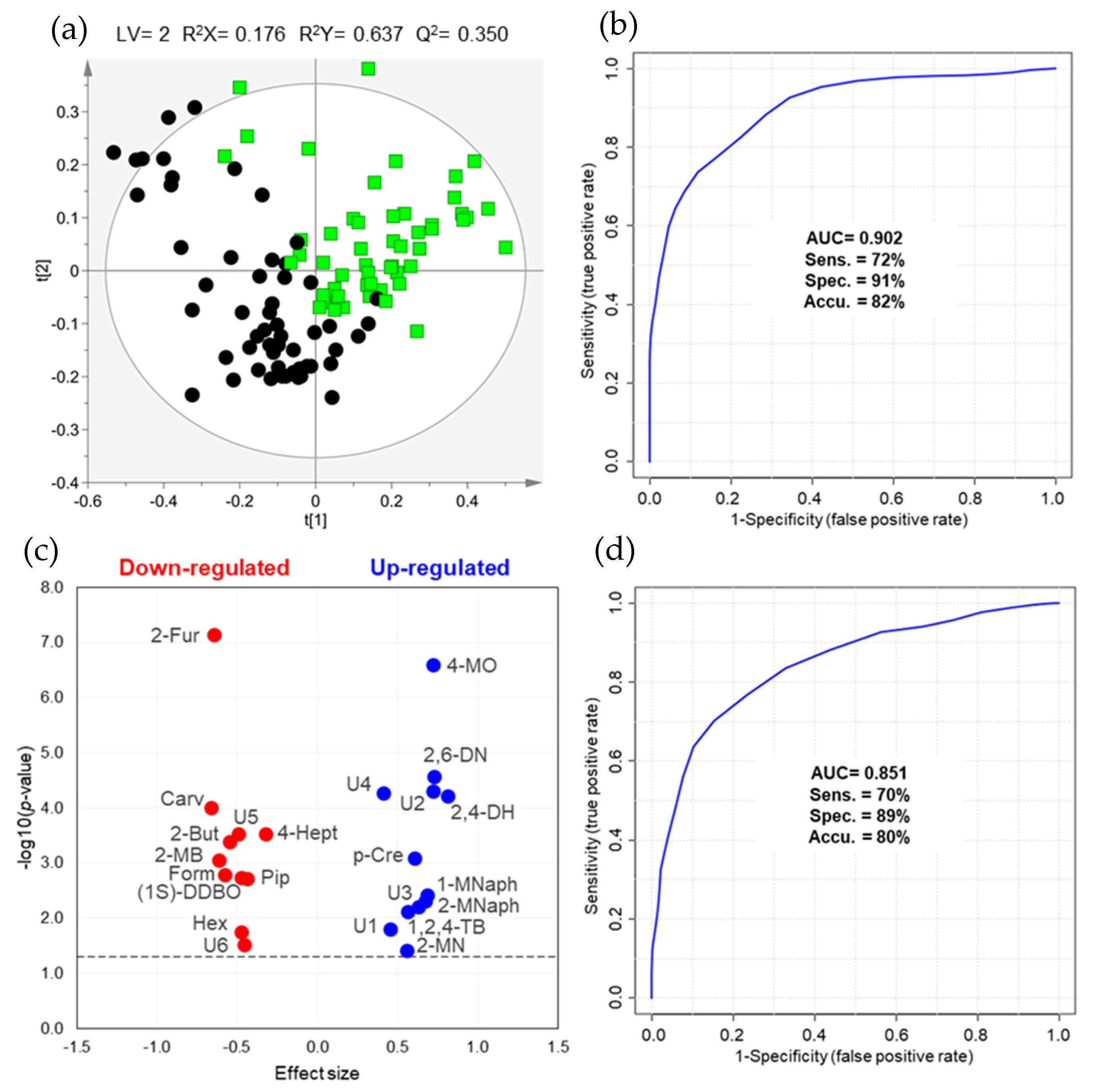
2.1. Differences in Urinary Volatile Profile of BC Patients vs. Controls
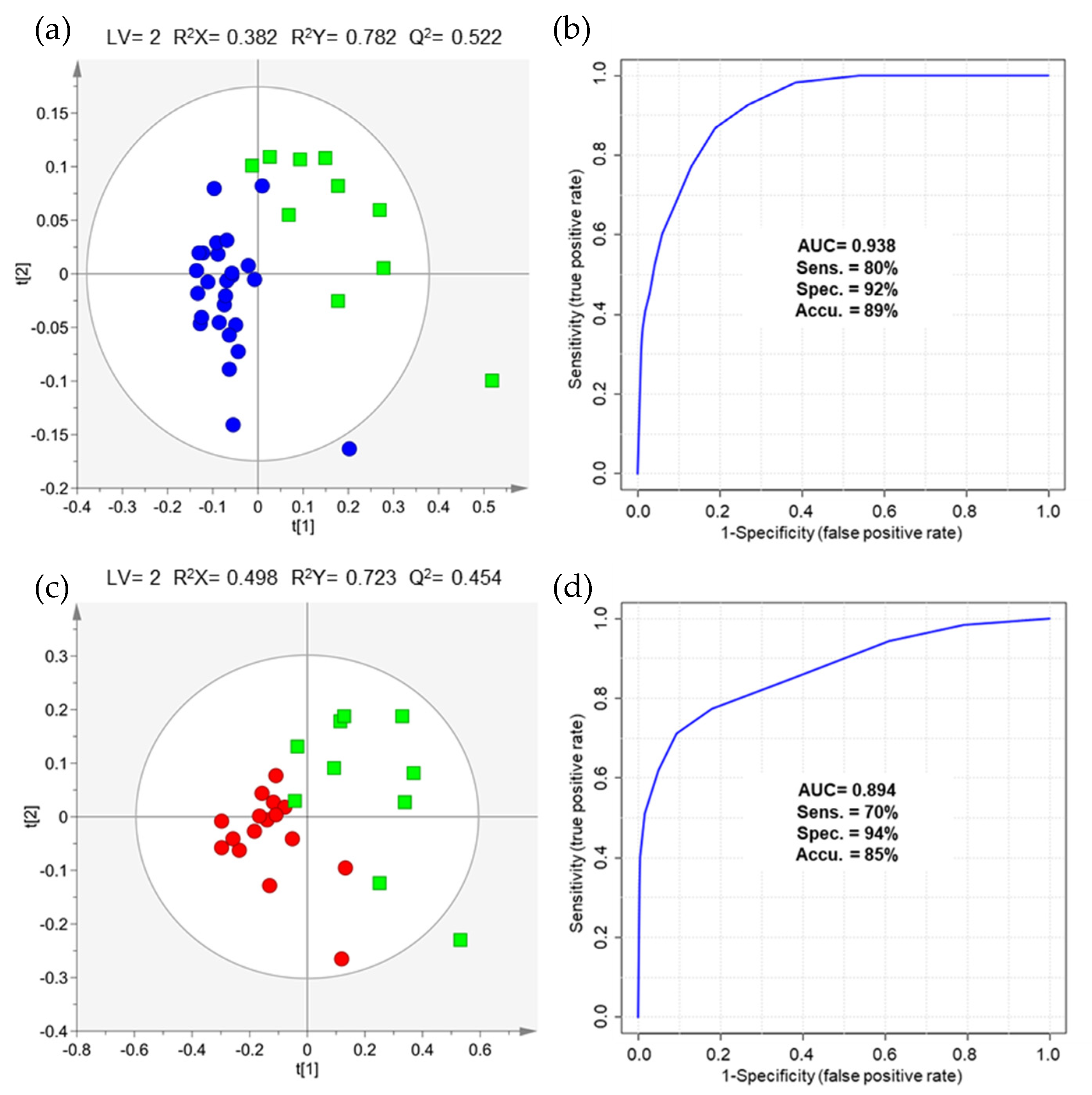
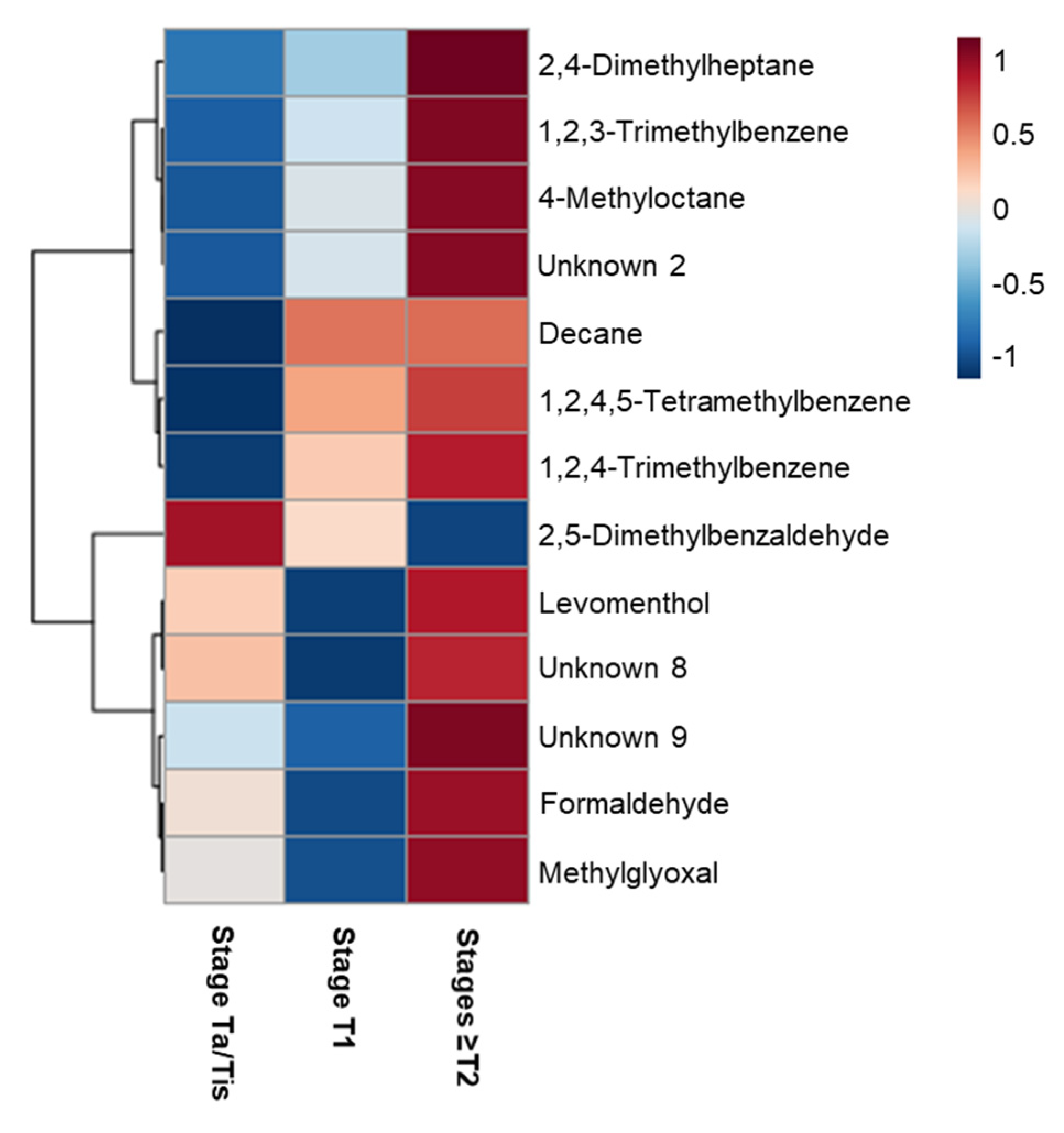
2.2. The Impact of NMIBC and MIBC on Urinary Volatile Profile
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Patients and Sample Collection
4.3. Sample Preparation
4.4. GC-MS Analysis and Metabolite Identification
4.5. Data Pre-Processing and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer a review. JAMA J. Am. Med. Assoc. 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Pashos, C.L.; Botteman, M.F.; Laskin, B.L.; Redaelli, A. Bladder cancer: Epidemiology, diagnosis, and management. Cancer Pract. 2002, 10, 311–322. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. EAU Guidelines: Non-muscle-invasive Bladder Cancer|Uroweb. Available online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/ (accessed on 4 February 2021).
- Zhu, C.Z.; Ting, H.N.; Ng, K.H.; Ong, T.A. A review on the accuracy of bladder cancer detection methods. J. Cancer 2019, 10, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Galgano, S.J.; Porter, K.K.; Burgan, C.; Rais-Bahrami, S. The Role of Imaging in Bladder Cancer Diagnosis and Staging. Diagnostics 2020, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Konety, B.R. Molecular markers in bladder cancer: A critical appraisal. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Oeyen, E.; Hoekx, L.; De Wachter, S.; Baldewijns, M.; Ameye, F.; Mertens, I. Bladder cancer diagnosis and follow-up: The current status and possible role of extracellular vesicles. Int. J. Mol. Sci. 2019, 20, 821. [Google Scholar] [CrossRef]
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary volatile organic compound analysis for the diagnosis of cancer: A systematic literature review and quality assessment. Metabolites 2021, 11, 17. [Google Scholar] [CrossRef]
- Cauchi, M.; Weber, C.M.; Bolt, B.J.; Spratt, P.B.; Bessant, C.; Turner, D.C.; Willis, C.M.; Britton, L.E.; Turner, C.; Morgan, G. Evaluation of gas chromatography mass spectrometry and pattern recognition for the identification of bladder cancer from urine headspace. Anal. Methods 2016, 8, 4037–4046. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4. [Google Scholar] [CrossRef]
- Reingruber, H.; Pontel, L.B. Formaldehyde metabolism and its impact on human health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Lameire, N.; Vanholder, R.; De Smet, R. Uremic toxins and peritoneal dialysis. Kidney Int. 2001, 59, S292–S297. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takahashi, N.; Hirai, S.; Kawada, T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 483958. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Berben, L.; Sereika, S.M.; Engberg, S. Effect size estimation: Methods and examples. Int. J. Nurs. Stud. 2012, 49, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef]
- Willis, C.M.; Britton, L.E.; Harris, R.; Wallace, J.; Guest, C.M. Volatile organic compounds as biomarkers of bladder cancer: Sensitivity and specificity using trained sniffer dogs. Cancer Biomark. 2010, 8, 145–153. [Google Scholar] [CrossRef]
- Baldini, C.; Billeci, L.; Sansone, F.; Conte, R.; Domenici, C.; Tonacci, A. Electronic Nose as a Novel Method for Diagnosing Cancer: A Systematic Review. Biosensors 2020, 10, 84. [Google Scholar] [CrossRef]
- Weber, C.M.; Cauchi, M.; Patel, M.; Bessant, C.; Turner, C.; Britton, L.E.; Willis, C.M. Evaluation of a gas sensor array and pattern recognition for the identification of bladder cancer from urine headspace. Analyst 2011, 136, 359–364. [Google Scholar] [CrossRef]
- Gut, J.M.; Hegele, A.; Hofmann, R.; Boeselt, T.; Hattesohl, A.; Koczulla, A.R.; Heers, H. Non-invasive detection of bladder tumors through volatile organic compounds: A pilot study with an electronic nose. Anticancer Res. 2018, 38, 833–837. [Google Scholar] [CrossRef]
- Horstmann, M.; Steinbach, D.; Fischer, C.; Enkelmann, A.; Grimm, M.-O.; Voss, A. PD25-03 An electronic nose system detects bladder cancer in urine specimen: First results of a pilot study. J. Urol. 2015, 193, e560–e561. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary biomarkers for diagnosis of bladder cancer: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Greenberg, J.; Grodman, R.; Gunawardena, R.; Naidu, A. Effect of oxygen on breath markers of oxidative stress. Eur. Respir. J. 2003, 21, 48–51. [Google Scholar] [CrossRef]
- Mitsui, T.; Kondo, T.; Phillips, M.; Cataneo, R.N.; Greenberg, J. Inadequacy of theoretical basis of breath methylated alkane contour for assessing oxidative stress [1] (multiple letters). Clin. Chim. Acta 2003, 333, 91–94. [Google Scholar] [CrossRef]
- Filipiak, W.; Sponring, A.; Mikoviny, T.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro. Cancer Cell Int. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Pinto, J.; Azevedo, A.I.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; de Pinho, P.G.; Carvalho, M. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br. J. Cancer 2019, 121, 857–868. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; de Bastos, M.L.; Carvalho, M.; de Pinho, P.G. A Panel of Urinary Volatile Biomarkers for Differential Diagnosis of Prostate Cancer from Other Urological Cancers. Cancers 2020, 12, 2017. [Google Scholar] [CrossRef]
- Guadagni, R.; Miraglia, N.; Simonelli, A.; Silvestre, A.; Lamberti, M.; Feola, D.; Acampora, A.; Sannolo, N. Solid-phase microextraction–gas chromatography–mass spectrometry method validation for the determination of endogenous substances: Urinary hexanal and heptanal as lung tumor biomarkers. Anal. Chim. Acta 2011, 701, 29–36. [Google Scholar] [CrossRef]
- Liu, J.F.; Yuan, B.F.; Feng, Y.Q. Determination of hexanal and heptanal in human urine using magnetic solid phase extraction coupled with in-situ derivatization by high performance liquid chromatography. Talanta 2015, 136, 54–59. [Google Scholar] [CrossRef]
- Song, D.; Gu, Y.; Liang, L.; Ai, Z.; Zhang, L.; Xu, H. Magnetic solid-phase extraction followed by high performance liquid chromatography for determination of hexanal and heptanal in human urine. Anal. Methods 2011, 3, 1418–1423. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Pereira, J.; Miekisch, W.; Câmara, J.S. Exploring the potential of NTME/GC-MS, in the establishment of urinary volatomic profiles. Lung cancer patients as case study. Sci. Rep. 2018, 8, 13113. [Google Scholar] [CrossRef] [PubMed]
- Opitz, P.; Herbarth, O. The volatilome—Investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC). J. Otolaryngol. Head Neck Surg. 2018, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: A first approach for breast cancer. Metabolomics 2019, 15, 64. [Google Scholar] [CrossRef]
- Spaněl, P.; Smith, D.; Holland, T.A.; Al Singary, W.; Elder, J.B. Analysis of formaldehyde in the headspace of urine from bladder and prostate cancer patients using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 1354–1359. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wheelock, Å.M.; Morin, D.; Baldwin, R.M.; Lee, M.G.; Taff, A.; Plopper, C.; Buckpitt, A.; Rohde, A. Toxicity and metabolism of methylnaphthalenes: Comparison with naphthalene and 1-nitronaphthalene. Toxicology 2009, 260, 16–27. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. Exploring the potential of needle trap microextraction combined with chromatographic and statistical data to discriminate different types of cancer based on urinary volatomic biosignature. Anal. Chim. Acta 2018, 1023, 53–63. [Google Scholar] [CrossRef]
- Jiménez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; López-Luque, A.; Miján-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 243.e21–243.e27. [Google Scholar] [CrossRef]
- Pérez Antón, A.; Ramos, Á.G.; del Nogal Sánchez, M.; Pavón, J.L.P.; Cordero, B.M.; Pozas, Á.P.C. Headspace-programmed temperature vaporization-mass spectrometry for the rapid determination of possible volatile biomarkers of lung cancer in urine. Anal. Bioanal. Chem. 2016, 408, 5239–5246. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Pi, X.; Guo, L.; Wang, Y.; Li, M.; Feng, Y.; Lin, Z.; Hou, W.; Li, E. Urinary volatile organic compounds as potential biomarkers for renal cell carcinoma. Biomed. Rep. 2016, 5, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.T.; Castillo, S.; Villar-Briones, A.; Oresic, M.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 1–11. [Google Scholar] [CrossRef]
| Group | Number of Samples (F/M) | Age Range (years) | Mean Age ± SD (years) |
|---|---|---|---|
| Cancer-Free Controls | 56 (16/40) | 45–66 | 51.9 ± 5.2 |
| BC Patients | 53 (14/39) | 43–87 | 68.9 ± 10.6 |
| Ta/Tis | 26 (6/20) | 43–87 | 68.6 ± 10.8 |
| T1 | 17 (6/11) | 53–83 | 72.0 ± 8.9 |
| T2 | 4 (1/3) | 51–69 | 62.0 ± 7.5 |
| T3 | 4 (1/3) | 43–80 | 65.3 ± 15.0 |
| T4 | 2 (0/2) | 65–70 | 67.5 ± 2.5 |
| Metabolite a | Effect Size ± ESSE b | Variation ± Uncertainty (%) | p-Value Original | p-Value FDR c | AUC | Down- or Up-Regulated | HMDB ID | Potential Biochemical Pathway |
|---|---|---|---|---|---|---|---|---|
| Alkanes | ||||||||
| 2-Methylnonane d, L2 | 0.56 ± 0.38 | 55.6 ± 15.4 | 0.0390 | 0.0477 | 0.615 | ↑ | − | − |
| 2,4-Dimethylheptane d, L2 | 0.81 ± 0.39 | 183.2 ± 23.6 | <0.0001 | 0.0002 | 0.724 | ↑ | − | − |
| 2,6-Dimethylnonane d, L2 | 0.73 ± 0.39 | 122.1 ± 20.7 | <0.0001 | 0.0002 | 0.734 | ↑ | − | − |
| 4-Methyloctane d, L2 | 0.72 ± 0.39 | 420.9 ± 37.7 | <0.0001 | <0.0001 | 0.787 | ↑ | − | − |
| Aldehydes | ||||||||
| 2-Furaldehyde (furan-2-carbaldehyde) e, L1 | −0.65 ± 0.39 | −48.4 ± 18.8 | <0.0001 | <0.0001 | 0.800 | ↓ | HMDB0032914 | - |
| 2-Methylbutanal d, L1 | −0.61 ± 0.38 | −40.3 ± 15.4 | 0.0009 | 0.0021 | 0.685 | ↓ | HMDB0031526 | Aldehyde oxidation (ALDH) and lipid peroxidation [12] |
| Formaldehyde e, L1 | −0.58 ± 0.38 | −25.0 ± 9.4 | 0.0016 | 0.0035 | 0.676 | ↓ | HMDB0001426 | Folate derivatives breakdown, protein and nucleic acid demethylations, glycine and serine metabolisms [13,14] |
| Hexanal e, L1 | −0.47 ± 0.38 | −24.8 ± 11.3 | 0.0178 | 0.0245 | 0.632 | ↓ | HMDB0005994 | Aldehyde oxidation (ALDH) and lipid peroxidation [12] |
| Aromatic compounds | ||||||||
| 1-Methylnaphthalene d, L2 | 0.69 ± 0.39 | 46.6 ± 10.8 | 0.0038 | 0.0070 | 0.661 | ↑ | HMDB0032860 | − |
| 2-Methylnaphthalene d, L2 | 0.67 ± 0.39 | 43.5 ± 10.4 | 0.0048 | 0.0083 | 0.657 | ↑ | − | − |
| 1,2,4-Trimethylbenzene d, L2 | 0.57 ± 0.38 | 43.7 ± 12.3 | 0.0077 | 0.0121 | 0.648 | ↑ | HMDB0013733 | − |
| p-Cresol (4-methylphenol) d, L1 | 0.61 ± 0.38 | 136.3 ± 26.4 | 0.0008 | 0.0021 | 0.686 | ↑ | HMDB0001858 | Tyrosine and phenylalanine metabolism [15] |
| Heterocyclic compounds | ||||||||
| (1S,5R)-1,5-dimethyl-6,8-dioxabicyclo[3.2.1]octane d, L2 | −0.48 ± 0.38 | −34.4 ± 16.5 | 0.0018 | 0.0037 | 0.674 | ↓ | − | − |
| Ketones | ||||||||
| 2-Butanone (butan-2-one) e, L1 | −0.54 ± 0.38 | −23.8 ± 9.5 | 0.0004 | 0.0012 | 0.697 | ↓ | HMDB0000474 | Fatty acid metabolism (β-oxidation) [12] |
| 4-Heptanone (heptan-4-one) e, L1 | −0.33 ± 0.38 | −38.6 ± 27.7 | 0.0003 | 0.0010 | 0.703 | ↓ | HMDB0004814 | Fatty acid metabolism (β-oxidation) [12] |
| Terpenoids | ||||||||
| Carvone (2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one) d, L1 | −0.66 ± 0.39 | −62.3 ± 25.5 | 0.0001 | 0.0004 | 0.715 | ↓ | HMDB0035824 | Lipid and carbohydrate metabolisms [16] |
| Piperitone (3-methyl-6-propan-2-ylcyclohex-2-en-1-one) d, L2 | −0.44 ± 0.38 | −57.5 ± 34.1 | 0.0019 | 0.0037 | 0.673 | ↓ | HMDB0034975 | Lipid metabolism [17] |
| Unknowns | ||||||||
| Unknown 1 d, L4 | 0.45 ± 0.38 | 75.0 ± 23.5 | 0.0157 | 0.0225 | 0.634 | ↑ | − | − |
| Unknown 2 d, L4 | 0.72 ± 0.39 | 153.2 ± 24.0 | <0.0001 | 0.0003 | 0.726 | ↑ | − | − |
| Unknown 3 d, L4 | 0.63 ± 0.38 | 68.1 ± 16.0 | 0.0061 | 0.0101 | 0.653 | ↑ | − | − |
| Unknown 4 d, L4 | 0.41 ± 0.38 | 269.6 ± 55.8 | 0.0001 | 0.0003 | 0.725 | ↑ | − | − |
| Unknown 5 d, L4 | −0.50 ± 0.38 | −58.2 ± 30.8 | 0.0003 | 0.0010 | 0.702 | ↓ | − | − |
| Unknown 6 e, L4 | −0.46 ± 0.38 | −23.9 ± 11.2 | 0.0303 | 0.0383 | 0.621 | ↓ | − | − |
| Groups Compared | AUC | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Stage Ta/Tis (n = 26) vs. controls (n = 56) | 0.761 | 65% | 84% | 78% |
| Stage T1 (n = 17) vs. controls (n = 56) | 0.910 | 94% | 80% | 84% |
| Stages ≥ T2 (n = 10) vs. controls (n = 56) | 0.820 | 60% | 91% | 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Carapito, Â.; Amaro, F.; Lima, A.R.; Carvalho-Maia, C.; Martins, M.C.; Jerónimo, C.; Henrique, R.; Bastos, M.d.L.; Guedes de Pinho, P. Discovery of Volatile Biomarkers for Bladder Cancer Detection and Staging through Urine Metabolomics. Metabolites 2021, 11, 199. https://doi.org/10.3390/metabo11040199
Pinto J, Carapito Â, Amaro F, Lima AR, Carvalho-Maia C, Martins MC, Jerónimo C, Henrique R, Bastos MdL, Guedes de Pinho P. Discovery of Volatile Biomarkers for Bladder Cancer Detection and Staging through Urine Metabolomics. Metabolites. 2021; 11(4):199. https://doi.org/10.3390/metabo11040199
Chicago/Turabian StylePinto, Joana, Ângela Carapito, Filipa Amaro, Ana Rita Lima, Carina Carvalho-Maia, Maria Conceição Martins, Carmen Jerónimo, Rui Henrique, Maria de Lourdes Bastos, and Paula Guedes de Pinho. 2021. "Discovery of Volatile Biomarkers for Bladder Cancer Detection and Staging through Urine Metabolomics" Metabolites 11, no. 4: 199. https://doi.org/10.3390/metabo11040199
APA StylePinto, J., Carapito, Â., Amaro, F., Lima, A. R., Carvalho-Maia, C., Martins, M. C., Jerónimo, C., Henrique, R., Bastos, M. d. L., & Guedes de Pinho, P. (2021). Discovery of Volatile Biomarkers for Bladder Cancer Detection and Staging through Urine Metabolomics. Metabolites, 11(4), 199. https://doi.org/10.3390/metabo11040199










