Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women
Abstract
1. Introduction
2. Results
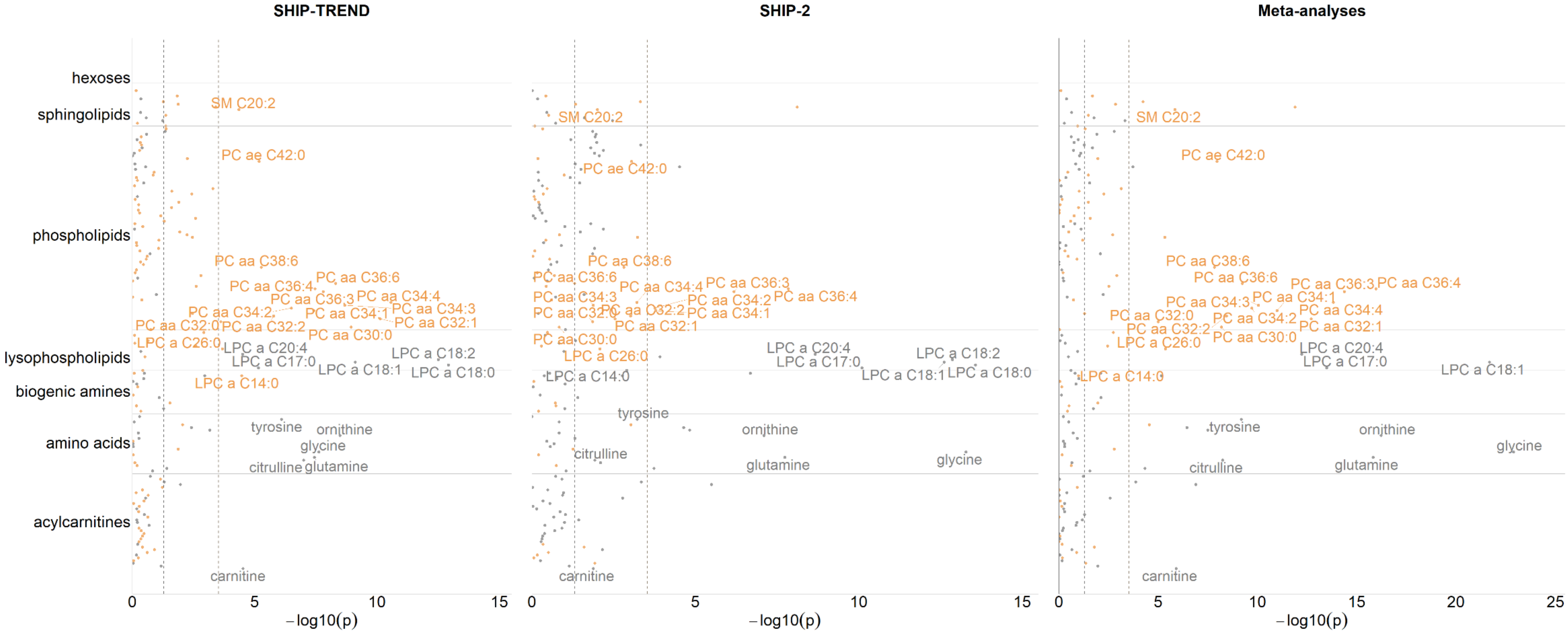
2.1. Associations between OC Intake and Blood Metabolite Concentrations
2.2. Mediation Effects of Cortisol
2.3. Extended Analyses To Assess the Impact of Menstrual Bleeding
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Interview and Psychometric Data
4.3. Blood Measurements
4.4. Metabolomic Profiling
4.5. Statistical Analyses
4.5.1. Associations between OC Intake and Blood Metabolite Concentrations
4.5.2. Mediation Effects of Cortisol
4.5.3. Extended Analyses to Assess the Impact of Menstrual Bleeding
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- P.D. World Contraceptive Use 2017; United Nations, Department of Economic, Social Affairs: New York, NY, USA, 2017.
- 20th WHO Model List of Essential Medicines; World Health Organization: Geneva, Switzerland, 2017.
- Sitruk–Ware, R.; Nath, A. Metabolic Effects of Contraceptive Steroids. Rev. Endocr. Metab. Disord. 2011, 12, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Wiegratz, I.; Lee, J.H.; Kutschera, E.; Bauer, H.H.; von Hayn, C.; Moore, C.; Mellinger, U.; Winkler, U.H.; Gross, W.; Kuhl, H. Effect of Dienogest–containing Oral Contraceptives on Lipid Metabolism. Contraception 2002, 65, 223–229. [Google Scholar] [CrossRef]
- Lidegaard, Ø.; Løkkegaard, E.; Svendsen, A.L.; Agger, C. Hormonal Contraception and Risk of Venous Thromboembolism: National Follow–up Study. BMJ 2009, 339, b2890. [Google Scholar] [CrossRef]
- Wiegratz, I.; Stahlberg, S.; Manthey, T.; Sänger, N.; Mittmann, K.; Lange, E.; Mellinger, U.; Kuhl, H. Effects of Conventional or Extended–cycle Regimen of an Oral Contraceptive Containing 30 mcg Ethinylestradiol and 2 mg Dienogest on Various Hemostasis Parameters. Contraception 2008, 78, 384–391. [Google Scholar] [CrossRef]
- Wiegratz, I.; Lee, J.H.; Kutschera, E.; Winkler, U.H.; Kuhl, H. Effect of Four Oral Contraceptives on Hemostatic Parameters. Contraception 2004, 70, 97–106. [Google Scholar] [CrossRef]
- Godsland, I.F.; Walton, C.; Felton, C.; Proudler, A.; Patel, A.; Wynn, V. Insulin Resistance, Secretion, and Metabolism in Users of Oral Contraceptives. J. Clin. Endocrinol. Metab. 1992, 74, 64–70. [Google Scholar] [CrossRef]
- Hertel, J.; König, J.; Homuth, G.; van der Auwera, S.; Wittfeld, K.; Pietzner, M.; Kacprowski, T.; Pfeiffer, L.; Kretschmer, A.; Waldenberger, M.; et al. Evidence for Stress–like Alterations in the HPA–Axis in Women Taking Oral Contraceptives. Sci. Rep. 2017, 7, 14111. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.R.; Parker, C.R.; Madden, J.D.; MacDonald, P.C.; Porter, J.C. Plasma Levels of Adrenocorticotropin and Cortisol in Women Receiving Oral Contraceptive Steroid Treatment. J. Clin. Endocrinol. Metab. 1979, 49, 346–349. [Google Scholar] [CrossRef]
- Meulenberg, P.M.; Ross, H.A.; Swinkels, L.M.; Benraad, T.J. The Effect of Oral Contraceptives on Plasma–free and Salivary Cortisol and Cortisone. Clin. Chim. Acta Int. J. Clin. Chem. 1987, 165, 379–385. [Google Scholar] [CrossRef]
- Skovlund, C.W.; Morch, L.S.; Kessing, L.V.; Lidegaard, O. Association of Hormonal Contraception with Depression. JAMA Psychiatry 2016, 73, 1154–1162. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen Controls Lipolysis by Up–regulating Alpha2a–adrenergic Receptors Directly in Human Adipose Tissue through the Estrogen Receptor Alpha. Implications for the Female Fat Distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef]
- Foryst–Ludwig, A.; Kintscher, U. Metabolic Impact of Estrogen Signalling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef]
- Ropero, A.B.; Alonso–Magdalena, P.; Quesada, I.; Nadal, A. The Role of Estrogen Receptors in the Control of Energy and Glucose Homeostasis. Steroids 2008, 73, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Mias, G.I.; Li–Pook–Than, J.; Jiang, L.; Lam, H.Y.K.; Chen, R.; Miriami, E.; Karczewski, K.J.; Hariharan, M.; Dewey, F.E.; et al. Personal Omics Profiling Reveals Dynamic Molecular and Medical Phenotypes. Cell 2012, 148, 1293–1307. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.H.; Kim, J.H.; Hwang, S.; Yoo, H.J. Understanding Metabolomics in Biomedical Research. Endocrinol. Metab. 2016, 31, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Klupczyńska, A.; Dereziński, P.; Kokot, Z.J. Metabolomics in Medical Sciences-Trends, Challenges and Perspectives. Acta Pol. Pharm. 2015, 72, 629–641. [Google Scholar]
- Altmaier, E.; Kastenmüller, G.; Römisch–Margl, W.; Thorand, B.; Weinberger, K.M.; Illig, T.; Adamski, J.; Döring, A.; Suhre, K. Questionnaire–based Self–reported Nutrition Habits Associate with Serum Metabolism as Revealed by Quantitative Targeted Metabolomics. Eur. J. Epidemiol. 2011, 26, 145–156. [Google Scholar] [CrossRef]
- Pohjanen, E.; Thysell, E.; Jonsson, P.; Eklund, C.; Silfver, A.; Carlsson, I.-B.; Lundgren, K.; Moritz, T.; Svensson, M.B.; Antti, H. A Multivariate Screening Strategy for Investigating Metabolic Effects of Strenuous Physical Exercise in Human Serum. J. Proteome Res. 2007, 6, 2113–2120. [Google Scholar] [CrossRef]
- Altmaier, E.; Kastenmüller, G.; Römisch–Margl, W.; Thorand, B.; Weinberger, K.M.; Adamski, J.; Illig, T.; Döring, A.; Suhre, K. Variation in the Human Lipidome Associated with Coffee Consumption as Revealed by Quantitative Targeted Metabolomics. Mol. Nutr. Food Res. 2009, 53, 1357–1365. [Google Scholar] [CrossRef]
- Wang–Sattler, R.; Yu, Y.; Mittelstrass, K.; Lattka, E.; Altmaier, E.; Gieger, C.; Ladwig, K.H.; Dahmen, N.; Weinberger, K.M.; Hao, P.; et al. Metabolic Profiling Reveals Distinct Variations Linked to Nicotine Consumption in Humans-First Results from the KORA Study. PLoS ONE 2008, 3, e3863. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah–Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A Global Biochemical Approach to Drug Response and Disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef]
- Ouyang, Y.; Tong, H.; Luo, P.; Kong, H.; Xu, Z.; Yin, P.; Xu, G. A High Throughput Metabolomics Method and Its Application in Female Serum Samples in a Normal Menstrual Cycle Based on Liquid Chromatography–mass Spectrometry. Talanta 2018, 185, 483–490. [Google Scholar] [CrossRef]
- Wallace, M.; Hashim, Y.Z.H.-Y.; Wingfield, M.; Culliton, M.; McAuliffe, F.; Gibney, M.J.; Brennan, L. Effects of Menstrual Cycle Phase on Metabolomic Profiles in Premenopausal Women. Hum. Reprod. 2010, 25, 949–956. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The Human Stress Response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Hucklebridge, F.H.; Clow, A.; Abeyguneratne, T.; Huezo-Diaz, P.; Evans, P. The Awakening Cortisol Response and Blood Glucose Levels. Life Sci. 1999, 64, 931–937. [Google Scholar] [CrossRef]
- Mendes, A.M.; Madon, R.J.; Flint, D.J. Effects of Cortisol and Progesterone on Insulin Binding and Lipogenesis in Adipocytes from Normal and Diabetic Rats. J. Endocrinol. 1985, 106, 225–231. [Google Scholar] [CrossRef]
- Korenblum, W.; Barthel, A.; Licinio, J.; Wong, M.-L.; Wolf, O.T.; Kirschbaum, C.; Bornstein, S.R. Elevated Cortisol Levels and Increased Rates of Diabetes and Mood Symptoms in Soviet Union–born Jewish Immigrants to Germany. Mol. Psychiatry 2005, 10, 974–975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muhtz, C.; Zyriax, B.-C.; Klähn, T.; Windler, E.; Otte, C. Depressive Symptoms and Metabolic Risk: Effects of Cortisol and Gender. Psychoneuroendocrinology 2009, 34, 1004–1011. [Google Scholar] [CrossRef]
- Mattsson, C.; Olsson, T. Estrogens and Glucocorticoid Hormones in Adipose Tissue Metabolism. Curr. Med. Chem. 2007, 14, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Jensen, M.D.; Dumesic, D.A.; Chapman, K.E.; Seckl, J.R.; Walker, B.R.; Morton, N.M. Omental 11beta–hydroxysteroid Dehydrogenase 1 Correlates with Fat Cell Size Independently of Obesity. Obesity 2007, 15, 1155–1163. [Google Scholar] [CrossRef]
- Otto, L.; Budde, K.; Kastenmüller, G.; Kaul, A.; Völker, U.; Völzke, H.; Adamski, J.; Kühn, J.P.; Krumsiek, J.; Artati, A.; et al. Associations between Adipose Tissue Volume and Small Molecules in Plasma and Urine among Asymptomatic Subjects from the General Population. Sci. Rep. 2020, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Budde, K.; Homuth, G.; Kastenmüller, G.; Henning, A.-K.; Artati, A.; Krumsiek, J.; Völzke, H.; Adamski, J.; Lerch, M.M.; et al. Hepatic Steatosis Is Associated With Adverse Molecular Signatures in Subjects Without Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3856–3868. [Google Scholar] [CrossRef]
- Oinonen, K.A.; Mazmanian, D. To What Extent Do Oral Contraceptives Influence Mood and Affect? J. Affect. Disord. 2002, 70, 229–240. [Google Scholar] [CrossRef]
- Zgliczynska, M.; Szymusik, I.; Sierocinska, A.; Bajaka, A.; Rowniak, M.; Sochacki–Wojcicka, N.; Wielgos, M.; Kosinska–Kaczynska, K. Contraceptive Behaviors in Polish Women Aged 18–35–a Cross–Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 2723. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Weight Change with Oral Contraceptive Use and During the Menstrual Cycle. Contraception 1998, 58, 345–349. [Google Scholar] [CrossRef]
- Knopp, R.H.; Walden, C.E.; Wahl, P.W.; Hoover, J.J. Effects of Oral Contraceptives on Lipoprotein Triglyceride and Cholesterol: Relationships to Estrogen and Progestin Potency. Am. J. Obstet. Gynecol. 1982, 142, 725–731. [Google Scholar] [CrossRef]
- Khiat, A.; Bard, C.; Lacroix, A.; Boulanger, Y. Recovery of the Brain Choline Level in Treated Cushing’s Patients as Monitored by Proton Magnetic Resonance Spectroscopy. Brain Res. 2000, 862, 301–307. [Google Scholar] [CrossRef]
- Kol, S.; Ben–Shlomo, I.; Payne, D.W.; Ando, M.; Rohan, R.M.; Adashi, E.Y. Glucocorticoids Suppress Basal (but not Interleukin–1–supported) Ovarian Phospholipase A2 Activity: Evidence for Glucocorticoid Receptor–mediated Regulation. Mol. Cell. Endocrinol. 1998, 137, 117–125. [Google Scholar] [CrossRef]
- Akompong, T.; Spencer, R.L.; McEwen, B.S. Glucocorticoids Inhibit Soluble Phospholipase C Activity and Cytosolic Guanine Nucleotide Regulatory Protein–alpha i Immunoreactivity in Spleen. Endocrinology 1993, 133, 1963–1970. [Google Scholar] [CrossRef]
- Kougias, P.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Lysophosphatidylcholine and Secretory Phospholipase A2 in Vascular Disease: Mediators of Endothelial Dysfunction and Atherosclerosis. Med. Sci. Monit. 2006, 12, RA5-16. [Google Scholar]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.-M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine–Induced Endothelial Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence Supporting a Key Role of Lp–PLA2–generated Lysophosphatidylcholine in Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef]
- Akerele, O.A.; Cheema, S.K. Fatty Acyl Composition of Lysophosphatidylcholine is Important in Atherosclerosis. Med. Hypotheses 2015, 85, 754–760. [Google Scholar] [CrossRef]
- Husson, A.; Vaillant, R. Hormonal Regulation of Three Urea Cycle Enzymes in Rat Fetal Liver. Biol. Neonate 1979, 35, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Husson, A.; Vaillant, R. Effets des Glucocorticostéroïdes sur L’activité des Enzymes du Cycle de L’urée dans le Foie Foetal de Rat. Biochimie 1977, 59, 91–95. [Google Scholar] [CrossRef]
- Christowitz, D.; Mattheyse, F.J.; Balinsky, J.B. Dietary and Hormonal Regulation of Urea Cycle Enzymes in Rat Liver. Enzyme 1981, 26, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Landau, R.L.; Lugibihl, K. The Effect of Progesterone on Amino Acid Metabolism. J. Clin. Endocrinol. Metab. 1961, 21, 1355–1363. [Google Scholar] [CrossRef]
- Craft, I.L.; Wise, I. Plasma Aminoacids and Oral Contraceptives. Lancet 1969, 2, 1138–1139. [Google Scholar] [CrossRef]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The Effects of Glucocorticoids on Adipose Tissue Lipid Metabolism. Metab. Clin. Exp. 2011, 60, 1500–1510. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Di Paolo, G.; et al. The Impact of Chronic Stress on the Rat Brain Lipidome. Mol. Psychiatry 2016, 21, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Santana, M.M.; Aveleira, C.A.; Simoes, C.; Maciel, E.; Melo, T.; Santinha, D.; Oliveira, M.M.; Peixoto, F.; Domingues, P.; et al. Alterations in Phospholipidomic Profile in the Brain of Mouse Model of Depression Induced by Chronic Unpredictable Stress. Neuroscience 2014, 273, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and Clinical Aspects of Cortisol as a Biochemical Marker of Chronic Stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Mondon, C.E.; Horner, J.A.; Weiser, J.N. Glucocorticoid and Estrogen Regulation of Corticosteroid–binding Globulin Production by Rat Liver. Am. J. Physiol. 1979, 237, E493–E499. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Ozel, B.; Jain, J.K.; Stanczyk, F.Z. Effects of Transdermal and Oral Contraceptives on Estrogen–sensitive Hepatic Proteins. Contraception 2006, 74, 293–296. [Google Scholar] [CrossRef]
- Qureshi, A.C.; Bahri, A.; Breen, L.A.; Barnes, S.C.; Powrie, J.K.; Thomas, S.M.; Carroll, P.V. The Influence of the Route of Oestrogen Administration on Serum Levels of Cortisol–binding Globulin and Total Cortisol. Clin. Endocrinol. 2007, 66, 632–635. [Google Scholar] [CrossRef]
- Goodyer, I.M.; Herbert, J.; Tamplin, A.; Altham, P.M. Recent Life Events, Cortisol, Dehydroepiandrosterone and the Onset of Major Depression in High–risk Adolescents. Br. J. Psychiatry J. Ment. Sci. 2000, 177, 499–504. [Google Scholar] [CrossRef]
- Herbert, J. Cortisol and Depression: Three Questions for Psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef]
- Anderl, C.; Li, G.; Chen, F.S. Oral Contraceptive Use in Adolescence Predicts Lasting Vulnerability to Depression in Adulthood. J. Child Psychol. Psychiatry Allied Discip. 2020, 61, 148–156. [Google Scholar] [CrossRef]
- de Wit, A.E.; Booij, S.H.; Giltay, E.J.; Joffe, H.; Schoevers, R.A.; Oldehinkel, A.J. Association of Use of Oral Contraceptives With Depressive Symptoms Among Adolescents and Young Women. JAMA Psychiatry 2019. [Google Scholar] [CrossRef]
- Herting, M.M.; Gautam, P.; Spielberg, J.M.; Kan, E.; Dahl, R.E.; Sowell, E.R. The Role of Testosterone and Estradiol in Brain Volume Changes Across Adolescence: A Longitudinal Structural MRI Study. Human Brain Mapp. 2014, 35, 5633–5645. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.K.; Curtis, J.R.; Saag, K.G. The Epidemiology of Glucocorticoid–associated Adverse Events. Curr. Opin. Rheumatol. 2008, 20, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cromer, B.A.; Stager, M.; Bonny, A.; Lazebnik, R.; Rome, E.; Ziegler, J.; Debanne, S.M. Depot Medroxyprogesterone Acetate, Oral Contraceptives and Bone Mineral Density in a Cohort of Adolescent Girls. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2004, 35, 434–441. [Google Scholar] [CrossRef]
- Park, H.; Kim, K. Associations between Oral Contraceptive Use and Risks of Hypertension and Prehypertension in a Cross–sectional Study of Korean Women. BMC Women’s Health 2013, 13, 39. [Google Scholar] [CrossRef]
- Stewart, P.M.; Petersenn, S. Rationale for Treatment and Therapeutic Options in Cushing’s Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23 (Suppl. S1), S15–S22. [Google Scholar] [CrossRef]
- Piltonen, T.; Puurunen, J.; Hedberg, P.; Ruokonen, A.; Mutt, S.J.; Herzig, K.H.; Nissinen, A.; Morin–Papunen, L.; Tapanainen, J.S. Oral, Transdermal and Vaginal Combined Contraceptives Induce an Increase in Markers of Chronic Inflammation and Impair Insulin Sensitivity in Young Healthy Normal–weight Women: A Randomized Study. Hum. Reprod. 2012, 27, 3046–3056. [Google Scholar] [CrossRef]
- Sheu, W.H.; Hsu, C.H.; Chen, Y.S.; Jeng, C.Y.; Fuh, M.M. Prospective Evaluation of Insulin Resistance and Lipid Metabolism in Women Receiving Oral Contraceptives. Clin. Endocrinol. 1994, 40, 249–255. [Google Scholar] [CrossRef]
- Polatti, F.; Perotti, F.; Filippa, N.; Gallina, D.; Nappi, R.E. Bone Mass and Long–term Monophasic Oral Contraceptive Treatment in Young Women. Contracept. 1995, 51, 221–224. [Google Scholar] [CrossRef]
- van Hylckama Vlieg, A.; Helmerhorst, F.M.; Vandenbroucke, J.P.; Doggen, C.J.M.; Rosendaal, F.R. The Venous Thrombotic Risk of Oral Contraceptives, Effects of Oestrogen Dose and Progestogen Type: Results of the MEGA Case–control Study. BMJ 2009, 339, b2921. [Google Scholar] [CrossRef]
- Chasan–Taber, L.; Willett, W.C.; Manson, J.E.; Spiegelman, D.; Hunter, D.J.; Curhan, G.; Colditz, G.A.; Stampfer, M.J. Prospective Study of Oral Contraceptives and Hypertension among Women in the United States. Circulation 1996, 94, 483–489. [Google Scholar] [CrossRef]
- Hertel, J.; Rotter, M.; Frenzel, S.; Zacharias, H.U.; Krumsiek, J.; Rathkolb, B.; Hrabe de Angelis, M.; Rabstein, S.; Pallapies, D.; Brüning, T.; et al. Dilution Correction for Dynamically Influenced Urinary Analyte Data. Anal. Chim. Acta 2018, 1032, 18–31. [Google Scholar] [CrossRef]
- Völzke, H.; Alte, D.; Schmidt, C.O.; Radke, D.; Lorbeer, R.; Friedrich, N.; Aumann, N.; Lau, K.; Piontek, M.; Born, G.; et al. Cohort Profile: The Study of Health in Pomerania. Int. J. Epidemiol. 2011, 40, 294–307. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI–related Errors in the Measurement of Obesity. Int. J. Obes. 2008, 32 (Suppl. S3), S56–S59. [Google Scholar] [CrossRef]
- ATC–Index. Anatomisch–Therapeutisch–Chemische Klassifikation mit Tagesdosen. Amtliche Fassung des ATC–Index mit DD–Angaben für Deutschland (2016). In Proceedings of the 15. Sitzung der Arbeitsgruppe ATC/DDD des Kuratoriums für Fragen der Klassifikation im Gesundheitswesen, Berlin, Germany, 25 November 2016. [Google Scholar]
- Beck, A.T.; Steer, R.A. Beck Depression Inventory–Manual; The Psychological Coporation: San Antonio, CA, USA, 1987. [Google Scholar]
- Wahl, I.; Löwe, B.; Bjorner, J.B.; Fischer, F.; Langs, G.; Voderholzer, U.; Aita, S.A.; Bergemann, N.; Brähler, E.; Rose, M. Standardization of Depression Measurement: A Common Metric was Developed for 11 Self–report Depression Measures. J. Clin. Epidemiol. 2014, 67, 73–86. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and Validation of a Brief Screening Version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Masuch, A.; Budde, K.; Kastenmüller, G.; Artati, A.; Adamski, J.; Völzke, H.; Nauck, M.; Pietzner, M. Metabolic Signature Associated with Parameters of the Complete Blood Count in Apparently Healthy Individuals. J. Cell. Mol. Med. 2019, 23, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Do, K.T.; Pietzner, M.; Rasp, D.J.; Friedrich, N.; Nauck, M.; Kocher, T.; Suhre, K.; Mook–Kanamori, D.O.; Kastenmüller, G.; Krumsiek, J. Phenotype–driven Identification of Modules in a Hierarchical Map of Multifluid Metabolic Correlations. NPJ Syst. Biol. Appl. 2017, 3, 669. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta–analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Harrell, J.F.E.; Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed.; Springer: New York, NY, USA, 2015; ISBN 9783319194240. [Google Scholar]
- Mehmetoglu, M. Medsem: A Stata Package for Statistical Mediation Analysis. IJCEE 2018, 8, 63. [Google Scholar] [CrossRef]
- Kenny, D.A.; Judd, C.M. Power Anomalies in Testing Mediation. Psychol. Sci. 2014, 25, 334–339. [Google Scholar] [CrossRef]


| Variables | SHIP-TREND (N = 232) | SHIP-2 (N = 159) | |||||
|---|---|---|---|---|---|---|---|
| miss. % | M | SD | miss. % | M | SD | p-Value | |
| Age (years) | 0.00 | 38.940 | 7.959 | 0.00 | 42.340 | 5.202 | 5.14 × 10−7 a |
| Cortisol (nmol/l) | 0.43 | 378.477 | 191.299 | 0.43 | 319.569 | 156.745 | 9.72 × 10−4 a |
| Time of Blood Sampling (h:min) | 0.00 | 9:17 | 0:55 | 0.00 | 9:31 | 0:58 | 0.017 a |
| Fasting Time (h:min) | 0.00 | 13:28 | 1:32 | 0.00 | 4:49 | 4:40 | 1.56 × 10−54 a |
| Waist circumference (cm) | 0.00 | 79.835 | 11.588 | 0.00 | 79.209 | 10.344 | 0.576 a |
| BMI | 0.00 | 25.933 | 5.051 | 0.00 | 25.783 | 4.672 | 0.763 a |
| Triglycerides (mmol/l) | 0.00 | 1.173 | 0.601 | 0.00 | 1.237 | 0.741 | 0.368 a |
| HbA1c (%) | 0.00 | 4.917 | 0.480 | 0.00 | 4.987 | 0.517 | 0.176 a |
| Glucose (mmol/l) | 0.00 | 5.031 | 0.444 | 0.00 | 5.056 | 0.924 | 0.756 a |
| RBC (Tpt/l) | 0.00 | 4.435 | 0.312 | 0.00 | 4.427 | 0.301 | 0.803 a |
| WBC (Gpt/l) | 0.00 | 6.088 | 1.614 | 0.00 | 6.532 | 1.829 | 0.014 a |
| PLT (Gpt/l) | 0.00 | 246.427 | 52.371 | 0.00 | 253.270 | 56.298 | 0.225 a |
| Cystatin C (mg/l) | 0.00 | 0.629 | 0.073 | 0.00 | 0.614 | 0.094 | 0.094 a |
| BDI–II | 0.00 | 8.831 | 6.329 | 0.00 | 4.918 | 5.963 | 1.44 × 10−9 a |
| CTQ | 13.79 | 32.658 | 10.457 | 0.00 | 32.246 | 10.501 | 0.712 a |
| Menstruation (% Yes) | 0.00 | 18.10 | 0.00 | 19.50 | 0.792 b | ||
| OC use (% Yes) | 0.00 | 31.47 | 0.00 | 27.04 | 0.369 b | ||
| Variables | Nonusers (N = 275) | Users (N = 116) | |||||
|---|---|---|---|---|---|---|---|
| miss. % | M | SD | miss. % | M | SD | p–Value | |
| Age (years) | 0.00 | 41.236 | 6.543 | 0.00 | 38.155 | 8.071 | 3.58 × 10−4 a |
| Cortisol (nmol/l) | 0.36 | 287.289 | 136.503 | 0.86 | 514.808 | 170.651 | 1.04 × 10−26 a |
| Time of Blood Sampling (h:min) | 0.00 | 9:28 | 0:58 | 0.00 | 9:11 | 0:52 | 0.003 a |
| Fasting Time (h:min) | 0.00 | 9:52 | 5:23 | 0.00 | 10:10 | 5:10 | 0.596 a |
| Waist circumference (cm) | 0.00 | 80.529 | 11.210 | 0.00 | 77.331 | 10.505 | 0.008 a |
| BMI | 0.00 | 26.145 | 4.978 | 0.00 | 25.226 | 4.649 | 0.082 a |
| Triglycerides (mmol/l) | 0.00 | 1.149 | 0.675 | 0.00 | 1.320 | 0.613 | 0.015 a |
| HbA1c (%) | 0.00 | 4.983 | 0.484 | 0.00 | 4.859 | 0.513 | 0.028 a |
| Glucose (mmol/l) | 0.00 | 5.038 | 0.536 | 0.00 | 5.049 | 0.941 | 0.907 a |
| RBC (Tpt/l) | 0.00 | 4.421 | 0.308 | 0.00 | 4.458 | 0.305 | 0.276 a |
| WBC (Gpt/l) | 0.00 | 6.279 | 1.754 | 0.00 | 6.243 | 1.631 | 0.844 a |
| PLT (Gpt/l) | 0.00 | 249.611 | 53.928 | 0.00 | 248.259 | 54.514 | 0.822 a |
| Cystatin C (mg/l) | 0.00 | 0.628 | 0.085 | 0.00 | 0.612 | 0.075 | 0.065 a |
| BDI–II | 0.00 | 7.954 | 7.021 | 0.00 | 5.547 | 4.508 | 6.58 × 10−5 a |
| CTQ | 9.09 | 33.163 | 11.186 | 6.03 | 30.900 | 8.418 | 0.036 a |
| Menstruation (% Yes) | 0.00 | 20.36 | 0.00 | 14.66 | 0.203 b | ||
| Metabolites | SHIP−TREND | SHIP−2 | Meta−Analysis | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | 95%−CI | p−Value | b | 95%−CI | p−Value | b | 95%−CI | p−Value | b | p−Value | |
| Carnitine | −0.161 | (−0.236; −0.087) | 2.995 × 10−5 | −0.107 | (−0.191; −0.023) | 0.013 | −0.137 | (−0.193; −0.082) | 1.225 × 10−6 | 0.000 | 0.338 |
| Citrulline | −0.304 | (−0.413; −0.195) | 9.932 × 10−8 | −0.160 | (−0.283; −0.036) | 0.012 | −0.241 | (−0.322; −0.160) | 5.719 × 10−9 | 66.637 | 0.083 |
| Glutamine | −0.221 | (−0.297; −0.145) | 3.647 × 10−8 | −0.221 | (−0.295; −0.148) | 1.833 × 10−8 | −0.221 | (−0.274; −0.169) | 1.506 × 10−16 | 0.000 | 0.997 |
| Glycine | −0.406 | (−0.544; −0.268) | 2.393 × 10−8 | −0.567 | (−0.702; −0.433) | 5.219 × 10−14 | −0.489 | (−0.584; −0.393) | 1.302 × 10−23 | 63.400 | 0.098 |
| Ornithine | −0.374 | (−0.493; −0.254) | 3.262 × 10−9 | −0.337 | (−0.455; −0.219) | 8.020 × 10−8 | −0.355 | (−0.438; −0.272) | 5.973 × 10−17 | 0.000 | 0.666 |
| Tyrosine | −0.252 | (−0.350; −0.154) | 8.019 × 10−7 | −0.256 | (−0.400; −0.112) | 6.050 × 10−4 | −0.253 | (−0.334; −0.173) | 6.590 × 10−10 | 0.000 | 0.968 |
| LPC a C14:0 | 0.172 | (0.092; 0.252) | 3.409 × 10−5 | −0.022 | (−0.074; 0.031) | 0.418 | 0.036 | (−0.007; 0.080) | 0.101 | 93.705 | 6.733 × 10−5 |
| LPC a C17:0 | −0.219 | (−0.313; −0.125) | 6.939 × 10−6 | −0.547 | (−0.701; −0.393) | 7.979 × 10−11 | −0.308 | (−0.388; −0.229) | 3.282 × 10−14 | 92.229 | 3.341 × 10−4 |
| LPC a C18:0 | −0.351 | (−0.438; −0.264) | 1.201 × 10−13 | −0.602 | (−0.743; −0.462) | 2.657 × 10−14 | −0.421 | (−0.495; −0.348) | 3.767 × 10−29 | 88.879 | 0.003 |
| LPC a C18:1 | −0.284 | (−0.371; −0.197) | 7.642 × 10−10 | −0.566 | (−0.704; −0.427) | 2.438 × 10−13 | −0.364 | (−0.437; −0.290) | 2.086 × 10−22 | 91.345 | 6.761 × 10−4 |
| LPC a C18:2 | −0.418 | (−0.524; −0.312) | 3.081 × 10−13 | −0.650 | (−0.807; −0.492) | 1.371 × 10−13 | −0.490 | (−0.578; −0.403) | 3.688 × 10−28 | 82.877 | 0.016 |
| LPC a C20:4 | −0.184 | (−0.269; −0.099) | 3.052 × 10−5 | −0.378 | (−0.494; −0.261) | 2.136 × 10−9 | −0.251 | (−0.319; −0.183) | 5.892 × 10−13 | 85.715 | 0.008 |
| LPC a C26:0 | 0.198 | (0.095; 0.302) | 2.052 × 10−4 | 0.157 | (0.041; 0.273) | 0.008 | 0.180 | (0.103; 0.257) | 4.243 × 10−6 | 0.000 | 0.597 |
| PC aa C30:0 | 0.417 | (0.288; 0.546) | 1.151 × 10−9 | 0.113 | (−0.040; 0.267) | 0.147 | 0.291 | (0.193; 0.389) | 6.495 ×10−9 | 88.780 | 0.003 |
| PC aa C32:0 | 0.162 | (0.076; 0.247) | 2.387 × 10−4 | 0.113 | (0.023; 0.202) | 0.014 | 0.138 | (0.077; 0.200) | 9.959 × 10−6 | 0.000 | 0.432 |
| PC aa C32:1 | 0.597 | (0.425; 0.769) | 7.411 × 10−11 | 0.376 | (0.131; 0.621) | 0.003 | 0.524 | (0.384; 0.664) | 1.977 × 10−13 | 53.103 | 0.144 |
| PC aa C32:2 | 0.349 | (0.210; 0.489) | 1.649 × 10−6 | 0.255 | (0.105; 0.404) | 9.568 × 10−4 | 0.305 | (0.204; 0.406) | 3.649 × 10−9 | 0.000 | 0.361 |
| PC aa C34:1 | 0.268 | (0.175; 0.360) | 3.861 × 10−8 | 0.193 | (0.095; 0.292) | 1.629 × 10−4 | 0.233 | (0.166; 0.300) | 1.020 × 10−11 | 15.374 | 0.277 |
| PC aa C34:2 | 0.219 | (0.137; 0.301) | 3.159 × 10−7 | 0.138 | (0.067; 0.208) | 1.719 × 10−4 | 0.172 | (0.119; 0.226) | 1.947 × 10−10 | 54.672 | 0.137 |
| PC aa C34:3 | 0.356 | (0.244; 0.468) | 2.084 × 10−9 | 0.185 | (0.039; 0.330) | 0.013 | 0.292 | (0.204; 0.380) | 9.071 × 10−11 | 70.558 | 0.065 |
| PC aa C34:4 | 0.523 | (0.377; 0.670) | 2.720 × 10−11 | 0.333 | (0.145; 0.521) | 6.211 × 10−4 | 0.451 | (0.336; 0.566) | 1.510 × 10−14 | 59.707 | 0.115 |
| PC aa C36:3 | 0.280 | (0.186; 0.374) | 1.562 × 10−8 | 0.269 | (0.167; 0.371) | 6.559 × 10−7 | 0.275 | (0.206; 0.344) | 4.254 × 10−15 | 0.000 | 0.877 |
| PC aa C36:4 | 0.291 | (0.191; 0.391) | 3.364 × 10−8 | 0.305 | (0.205; 0.405) | 1.438 × 10−8 | 0.298 | (0.227; 0.368) | 1.061 × 10−16 | 0.000 | 0.841 |
| PC aa C36:6 | 0.400 | (0.270; 0.529) | 4.941 × 10−9 | 0.202 | (4.075 × 10−4; 0.403) | 0.050 | 0.342 | (0.233; 0.450) | 5.853×10−10 | 62.569 | 0.102 |
| PC aa C38:6 | 0.254 | (0.147; 0.361) | 5.319 × 10−6 | 0.229 | (0.089; 0.369) | 0.002 | 0.245 | (0.160; 0.329) | 1.464 × 10−8 | 0.000 | 0.779 |
| PC ae C42:0 | 0.149 | (0.085; 0.213) | 6.620 × 10−6 | 0.129 | (0.054; 0.205) | 9.087 × 10−4 | 0.141 | (0.093; 0.189) | 1.107 × 10−8 | 0.000 | 0.694 |
| SM C20:2 | 0.265 | (0.140; 0.390) | 4.368 × 10−5 | 0.174 | (0.042; 0.306) | 0.010 | 0.222 | (0.132; 0.312) | 1.412 × 10−6 | 0.000 | 0.326 |
| Metabolites | SHIP–TREND | SHIP–2 | Meta–Analysis | Heterogeneity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ind. Effect | 95%–CI | p–Value | ind. Effect | 95%–CI | p–Value | ind. Effect | 95%–CI | p–Value | % Mediated | 95%–CI | p–Value | ind. Effect | p–Value | % Mediated | p–Value | |
| Carnitine | −0.071 | (−0.129; −0.013) | 0.016 | −0.012 | (−0.072; 0.049) | 0.708 | −0.043 | (−0.085; −8.483 × 10−4) | 0.046 | 38.8 | (−132.074; 209.589) | 0.657 | 48.391 | 0.164 | 0.0 | 0.894 |
| Citrulline | −0.043 | (−0.113; 0.027) | 0.232 | 0.006 | (−0.072; 0.084) | 0.890 | −0.021 | (−0.073; 0.031) | 0.427 | 14.1 | (−10.725; 38.872) | 0.266 | 0.000 | 0.366 | 0.0 | 0.973 |
| Glutamine | −0.059 | (−0.110; −0.009) | 0.022 | 0.007 | (−0.038; 0.053) | 0.759 | −0.022 | (−0.056; 0.011) | 0.195 | 9.2 | (−7.389; 25.752) | 0.277 | 72.704 | 0.056 | 67.3 | 0.080 |
| Glycine | −0.170 | (−0.263; −0.077) | 3.329 × 10−4 | −0.077 | (−0.187; 0.034) | 0.173 | −0.131 | (−0.202; −0.060) | 2.919 × 10−4 | 23.3 | (6.980; 39.549) | 0.005 | 37.887 | 0.204 | 64.0 | 0.096 |
| Ornithine | −0.118 | (−0.190; −0.047) | 0.001 | −0.003 | (−0.106; 0.099) | 0.953 | −0.081 | (−0.139; −0.022) | 0.007 | 20.3 | (1.739; 38.868) | 0.032 | 69.378 | 0.071 | 59.8 | 0.115 |
| Tyrosine | −0.073 | (−0.151; 0.006) | 0.070 | 0.078 | (−0.017; 0.173) | 0.106 | −0.011 | (−0.072; 0.049) | 0.717 | 9.3 | (−20.772; 39.385) | 0.544 | 82.659 | 0.016 | 68.6 | 0.075 |
| LPC a C17:0 | −0.040 | (−0.114; 0.034) | 0.293 | −0.111 | (−0.222; −0.001) | 0.048 | −0.062 | (−0.124; −5.429 × 10−4) | 0.048 | 19.6 | (1.022; 38.258) | 0.039 | 10.691 | 0.290 | 0.0 | 0.949 |
| LPC a C18:0 | −0.045 | (−0.111; 0.021) | 0.179 | −0.072 | (−0.156; 0.011) | 0.090 | −0.055 | (−0.107; −0.004) | 0.035 | 12.1 | (0.474; 23.752) | 0.041 | 0.000 | 0.614 | 0.0 | 0.918 |
| LPC a C18:1 | −0.036 | (−0.102; 0.031) | 0.293 | −0.064 | (−0.162; 0.035) | 0.204 | −0.044 | (−0.099; 0.011) | 0.114 | 11.5 | (−2.964; 25.864) | 0.119 | 0.000 | 0.643 | 0.0 | 0.913 |
| LPC a C18:2 | −0.096 | (−0.178; −0.014) | 0.021 | −0.078 | (−0.185; 0.029) | 0.154 | −0.089 | (−0.154; −0.024) | 0.007 | 15.8 | (2.619; 28.971) | 0.019 | 0.000 | 0.794 | 0.0 | 0.408 |
| LPC a C20:4 | 0.037 | (−0.031; 0.105) | 0.288 | −0.053 | (−0.134; 0.027) | 0.196 | −5.618 × 10−4 | (−0.052; 0.051) | 0.983 | 7.0 | (−12.660; 26.673) | 0.485 | 64.309 | 0.094 | 45.4 | 0.176 |
| LPC a C26:0 | 0.081 | (0.005; 0.157) | 0.037 | 0.075 | (−0.004; 0.154) | 0.062 | 0.078 | (0.023; 0.133) | 0.005 | 40.2 | (−13.432; 93.807) | 0.142 | 0.000 | 0.919 | 0.0 | 0.988 |
| PC aa C32:0 | 0.101 | (0.034; 0.168) | 0.003 | 0.031 | (−0.039; 0.101) | 0.388 | 0.068 | (0.019; 0.116) | 0.006 | 60.4 | (−13.577; 134.281) | 0.110 | 50.930 | 0.153 | 0.0 | 0.867 |
| PC aa C32:1 | 0.184 | (0.067; 0.300) | 0.002 | 0.262 | (0.090; 0.435) | 0.003 | 0.208 | (0.112; 0.305) | 2.387 × 10−5 | 31.2 | (7.617; 54.729) | 0.009 | 0.000 | 0.460 | 0.0 | 0.733 |
| PC aa C32:2 | 0.073 | (−0.017; 0.163) | 0.110 | 0.092 | (−0.021; 0.205) | 0.110 | 0.080 | (0.010; 0.151) | 0.025 | 23.9 | (−2.330; 50.108) | 0.074 | 0.000 | 0.797 | 0.0 | 0.689 |
| PC aa C34:1 | 0.137 | (0.068; 0.205) | 9.963 × 10−5 | 0.071 | (0.006; 0.135) | 0.031 | 0.101 | (0.054; 0.148) | 2.335 × 10−5 | 49.6 | (17.485; 81.697) | 0.002 | 47.150 | 0.169 | 0.0 | 0.831 |
| PC aa C34:2 | 0.109 | (0.051; 0.168) | 2.305 × 10−4 | 0.033 | (−0.013; 0.079) | 0.158 | 0.062 | (0.026; 0.098) | 7.017 × 10−4 | 40.0 | (11.929; 68.131) | 0.005 | 75.473 | 0.043 | 0.0 | 0.399 |
| PC aa C34:3 | 0.127 | (0.056; 0.198) | 4.660 × 10−4 | 0.098 | (−0.010; 0.206) | 0.075 | 0.118 | (0.059; 0.178) | 9.501 × 10−5 | 35.5 | (11.077; 59.937) | 0.004 | 0.000 | 0.663 | 0.0 | 0.979 |
| PC aa C34:4 | 0.243 | (0.139; 0.347) | 4.848 × 10−6 | 0.130 | (−0.003; 0.263) | 0.055 | 0.200 | (0.118; 0.282) | 1.721 × 10−6 | 44.3 | (22.211; 66.454) | 8.571 × 10−5 | 41.377 | 0.192 | 0.0 | 0.720 |
| PC aa C36:3 | 0.160 | (0.092; 0.228) | 4.283 × 10−6 | 0.093 | (0.026; 0.160) | 0.007 | 0.126 | (0.078; 0.174) | 2.511 × 10−7 | 43.3 | (21.085; 65.441) | 1.317 × 10−4 | 46.161 | 0.173 | 3.2 | 0.309 |
| PC aa C36:4 | 0.193 | (0.116; 0.269) | 7.582 × 10−7 | 0.082 | (0.022; 0.143) | 0.007 | 0.125 | (0.077; 0.172) | 2.334 × 10−7 | 36.9 | (19.170; 54.588) | 4.472 × 10−5 | 79.710 | 0.026 | 73.7 | 0.051 |
| PC aa C36:6 | 0.143 | (0.046; 0.239) | 0.004 | 0.103 | (−0.042; 0.248) | 0.164 | 0.130 | (0.050; 0.211) | 0.002 | 35.7 | (7.449; 63.917) | 0.013 | 0.000 | 0.657 | 0.0 | 0.984 |
| PC aa C38:6 | 0.104 | (0.023; 0.186) | 0.012 | 0.086 | (−0.013; 0.185) | 0.088 | 0.097 | (0.034; 0.160) | 0.002 | 38.5 | (−0.517; 77.553) | 0.053 | 0.000 | 0.779 | 0.0 | 0.900 |
| PC ae C42:0 | 0.097 | (0.049; 0.144) | 7.618 × 10−5 | 0.055 | (0.011; 0.098) | 0.015 | 0.074 | (0.041; 0.106) | 7.957 × 10−6 | 55.4 | (21.783; 89.112) | 0.001 | 38.023 | 0.204 | 0.0 | 0.488 |
| SM C20:2 | 0.104 | (0.026; 0.182) | 0.009 | 0.088 | (−0.012; 0.188) | 0.084 | 0.098 | (0.037; 0.160) | 0.002 | 39.3 | (−2.271; 80.930) | 0.064 | 0.000 | 0.800 | 0.0 | 0.944 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eick, C.; Klinger-König, J.; Zylla, S.; Hannemann, A.; Budde, K.; Henning, A.K.; Pietzner, M.; Nauck, M.; Völzke, H.; Grabe, H.J.; et al. Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women. Metabolites 2021, 11, 193. https://doi.org/10.3390/metabo11040193
Eick C, Klinger-König J, Zylla S, Hannemann A, Budde K, Henning AK, Pietzner M, Nauck M, Völzke H, Grabe HJ, et al. Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women. Metabolites. 2021; 11(4):193. https://doi.org/10.3390/metabo11040193
Chicago/Turabian StyleEick, Clara, Johanna Klinger-König, Stephanie Zylla, Anke Hannemann, Kathrin Budde, Ann Kristin Henning, Maik Pietzner, Matthias Nauck, Henry Völzke, Hans J. Grabe, and et al. 2021. "Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women" Metabolites 11, no. 4: 193. https://doi.org/10.3390/metabo11040193
APA StyleEick, C., Klinger-König, J., Zylla, S., Hannemann, A., Budde, K., Henning, A. K., Pietzner, M., Nauck, M., Völzke, H., Grabe, H. J., & Hertel, J. (2021). Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women. Metabolites, 11(4), 193. https://doi.org/10.3390/metabo11040193






