Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders
Abstract
1. Introduction
2. Results
2.1. Pre-Analytical Stage: Direct Assessment of CSF Sample Quality Prior to H2S Investigation
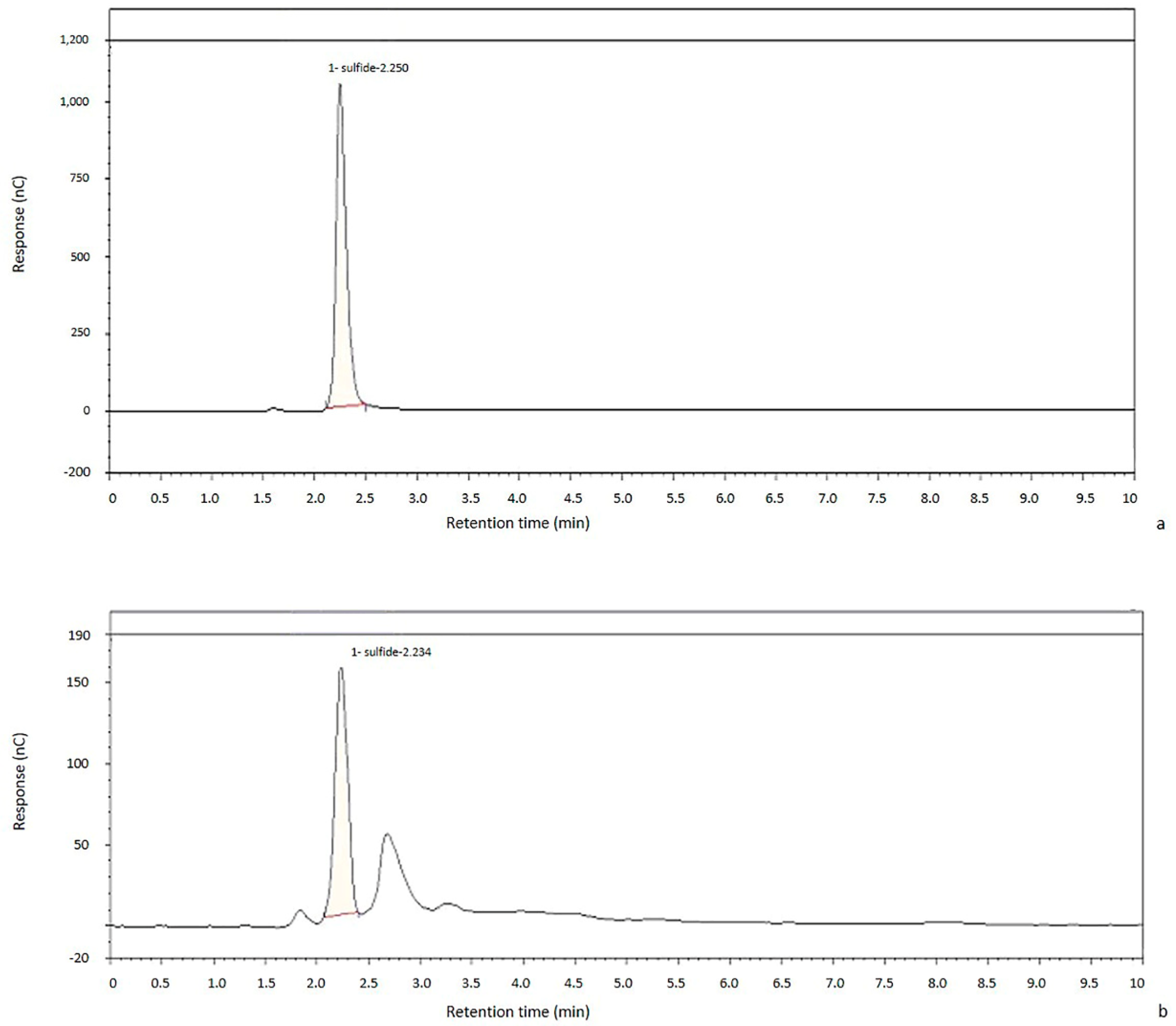
2.2. Chromatographic Method Validation
2.2.1. Linearity
2.2.2. Sensitivity
2.2.3. Robustness
2.2.4. Precision and Accuracy
2.2.5. Recovery
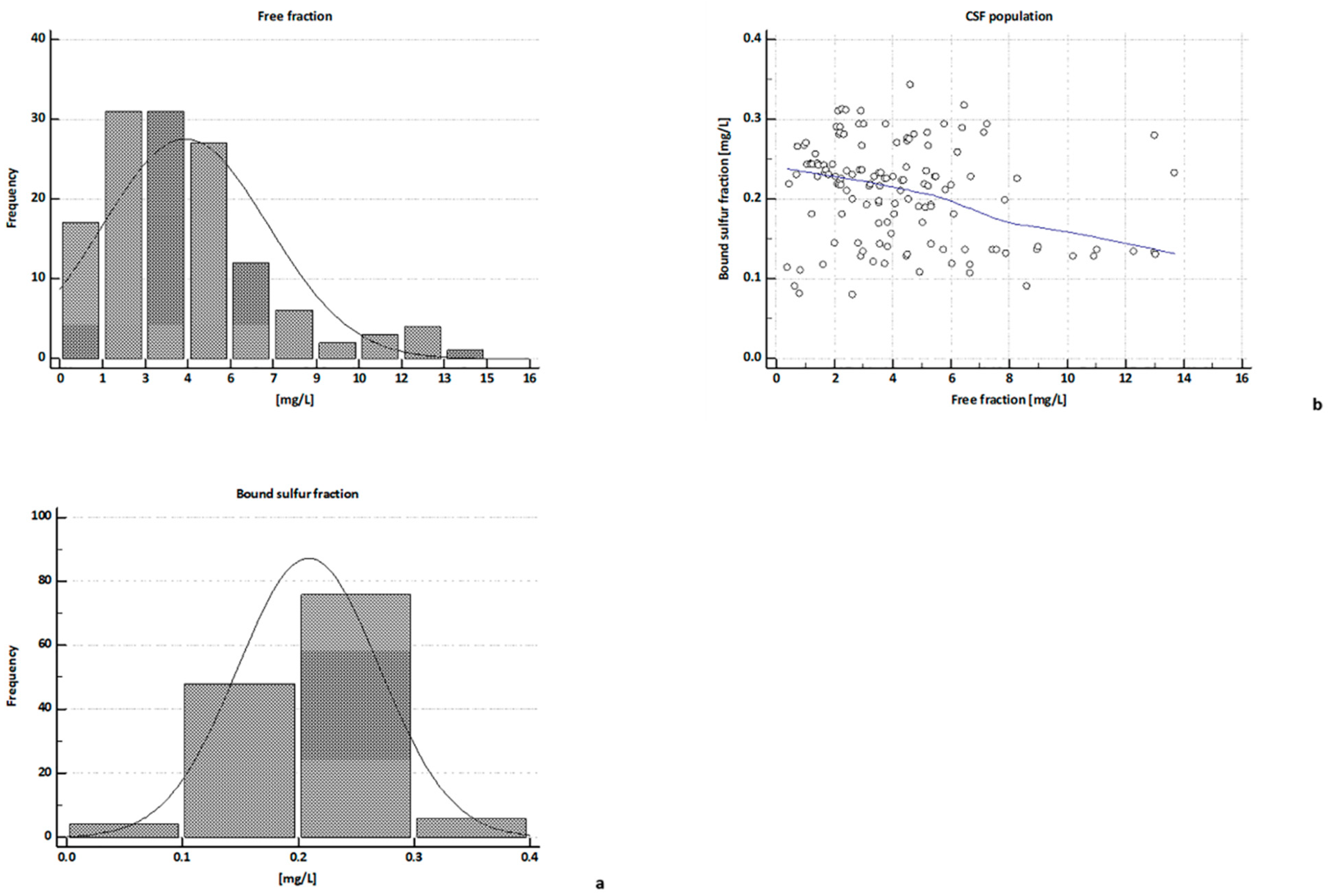
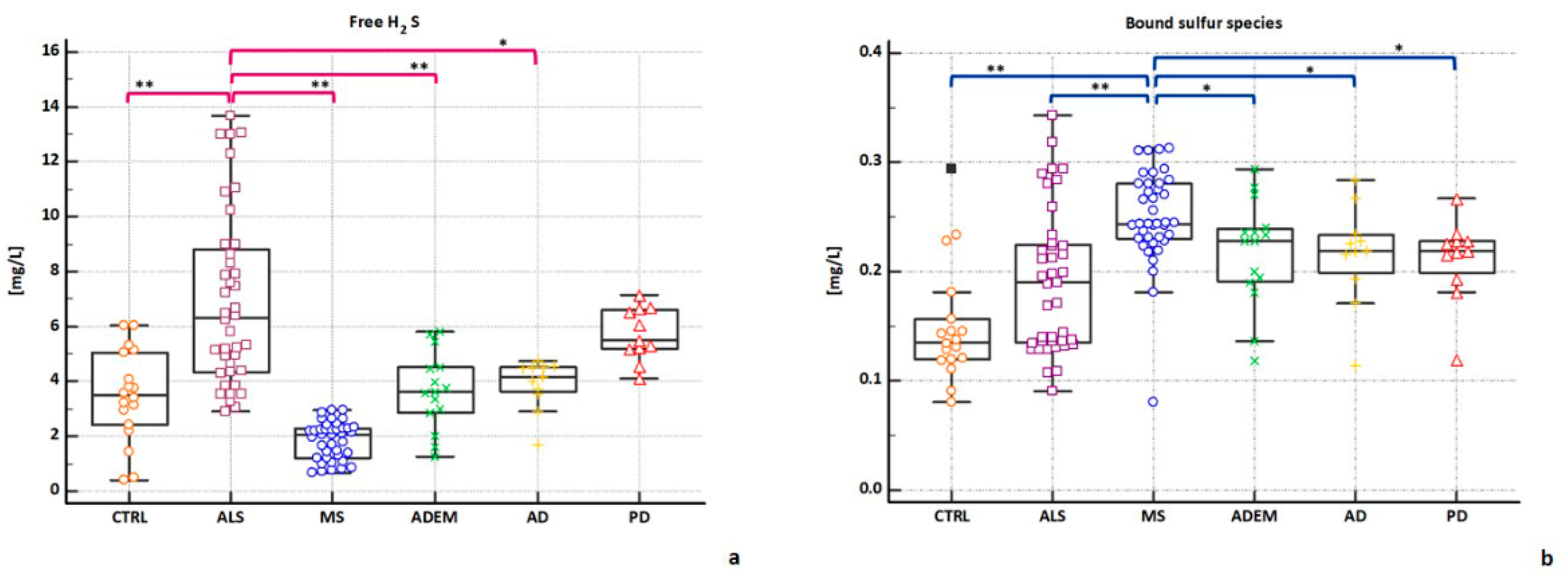
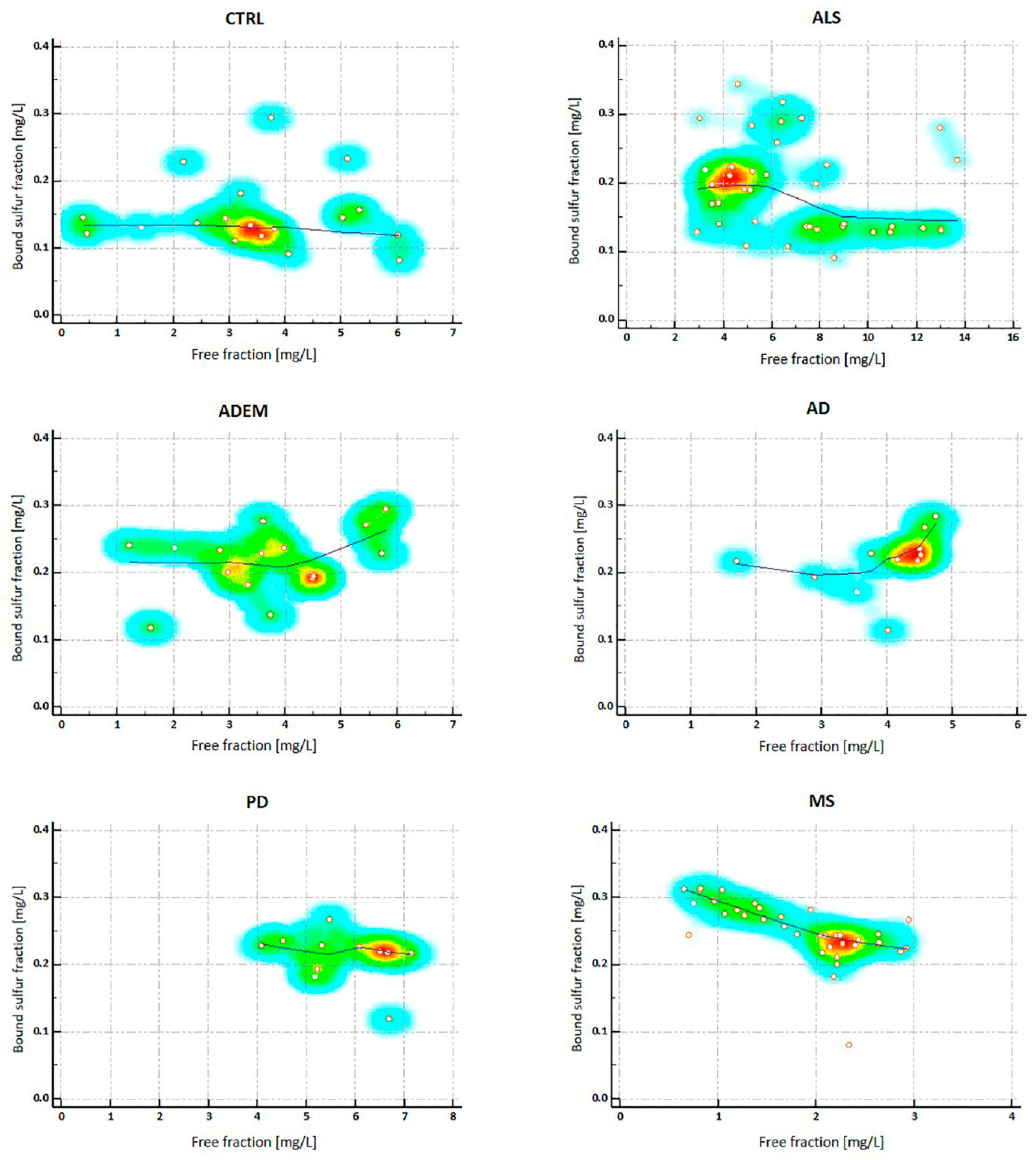
2.3. H2S Concentrations in CSFs
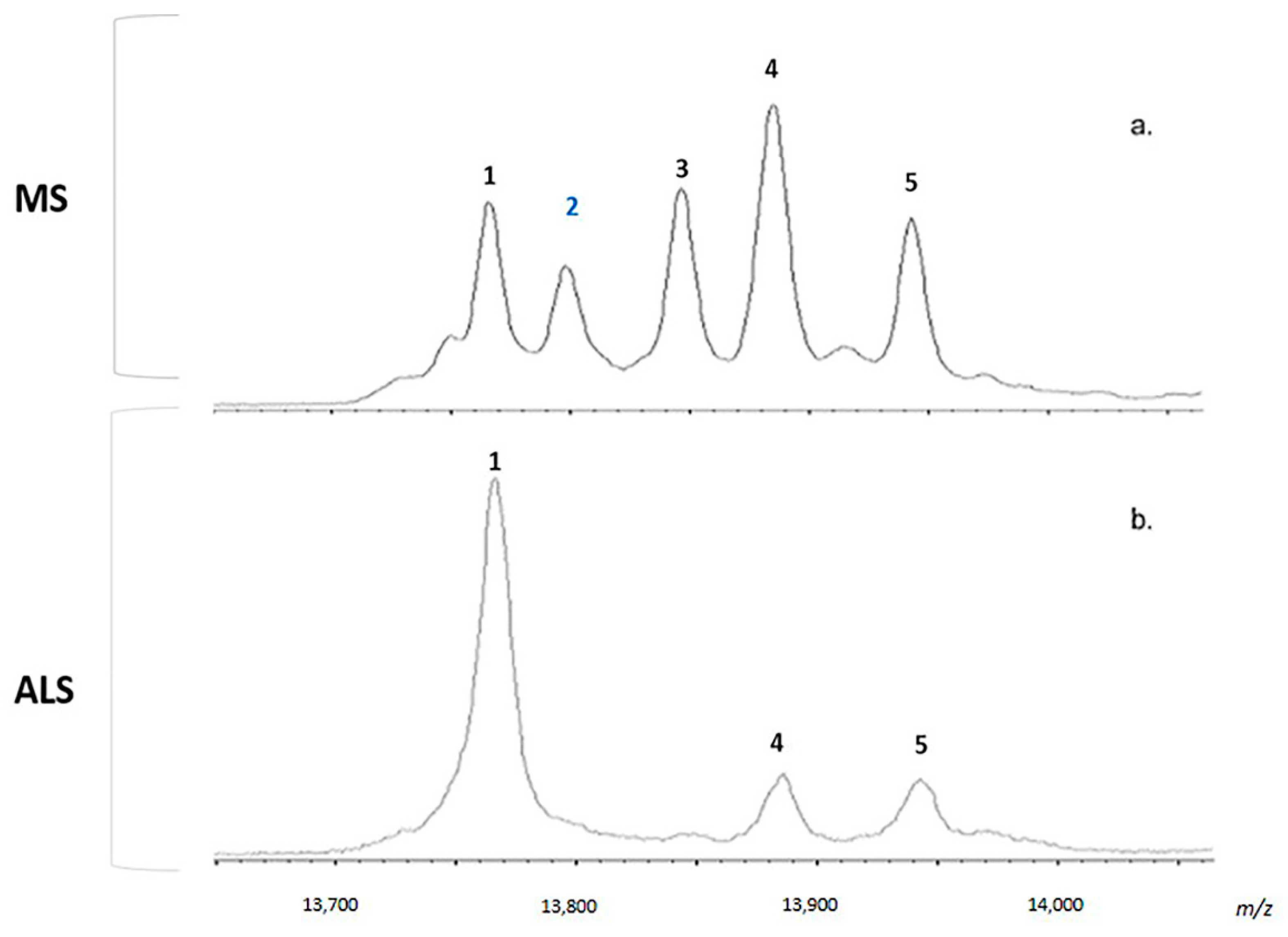
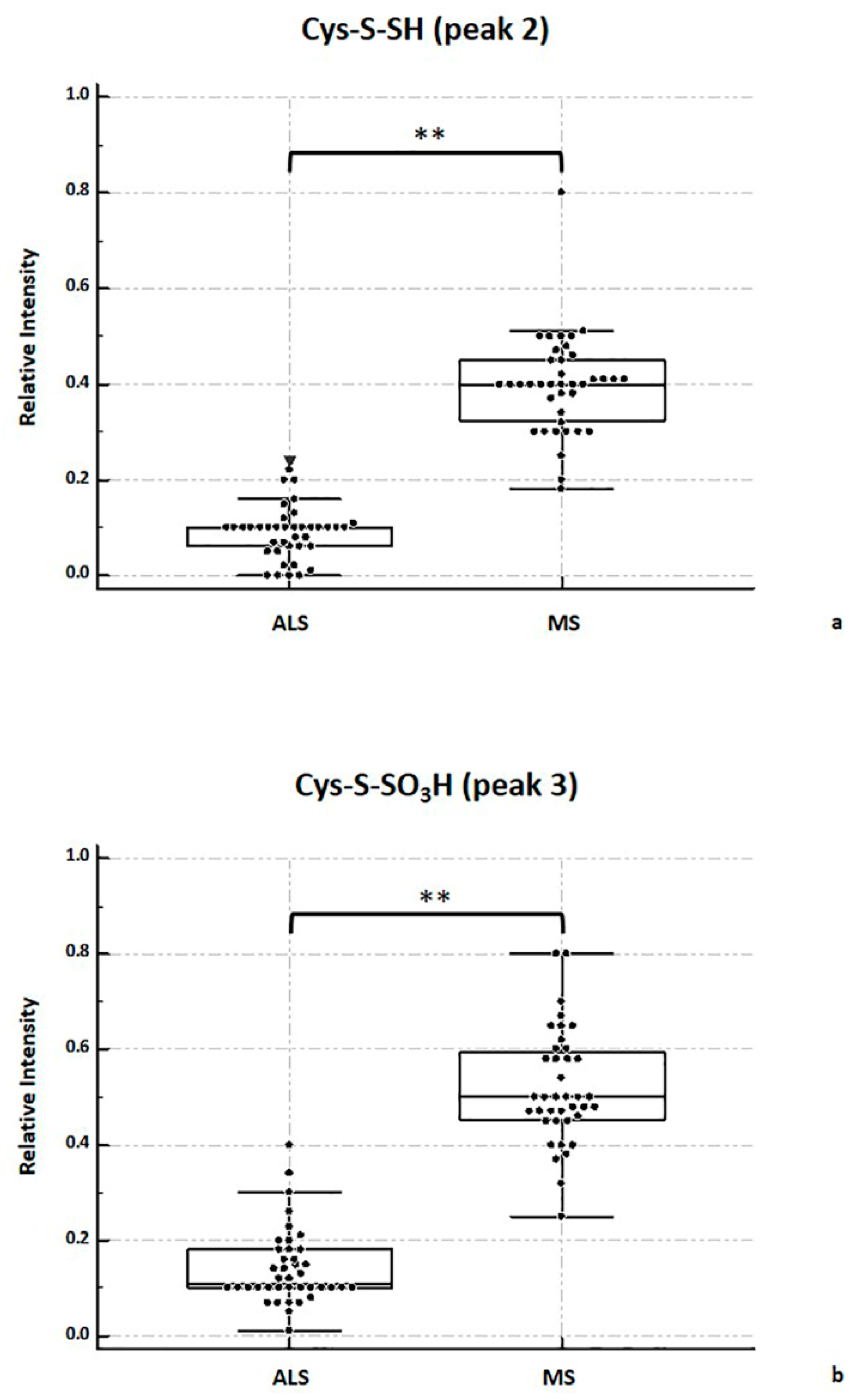
2.4. MALDI-TOF MS Analysis
2.5. Considerations
3. Materials and Methods
3.1. Materials
3.2. Sample Collection
3.3. Sample Preparation for MALDI-TOF MS Analysis
3.4. Sample Preparation for H2S Measurements (Free and Bound Sulfur Fractions)
3.5. Ion Chromatography (IC) for H2S Measurements
3.5.1. Chromatographic Condition: Instrumentation and Procedures
3.5.2. Standard Solutions
3.5.3. Linearity and Range
3.5.4. Intra and Inter-Day Precision
3.5.5. Recovery
3.6. Data and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Szabó, G.; Veres, G.; Radovits, T.; Gerő, D.; Módis, K.; Miesel-Gröschel, C.; Horkay, F.; Karck, M.; Szabó, C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide 2011, 25, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Wong, P.T.; Bian, J.S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [PubMed]
- Enokido, Y.; Suzuki, E.; Iwasawa, K.; Namekata, K.; Okazawa, H.; Kimura, H. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005, 19, 1854–1856. [Google Scholar] [CrossRef]
- Ichinohe, A.; Kanaumi, T.; Takashima, S.; Enokido, Y.; Nagai, Y.; Kimura, H. Cystathionine β-synthase is enriched in the brains of Down’s patients. Biochem. Biophys. Res. Commun. 2005, 338, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wong, P.T.; Moore, P.K.; Bian, J. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007, 100, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Hu, Y.; Hu, L.; Lu, Q.; Dawe, G.S.; Moore, P.K.; Wong, P.T.; Bian, J. Hydrogen sulphide regulates calcium homeostasis in microglial cells. GLIA 2006, 54, 116–124. [Google Scholar] [CrossRef]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Antonelli, E.; Roviezzo, F.; Morelli, A.; Cirino, G.; Wallace, J.L. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Ther. 2006, 316, 325–335. [Google Scholar] [CrossRef]
- Garcia-Bereguiain, M.A.; Samhan-Arias, A.K.; Martin-Romero, F.J.; Gutierrez-Merino, C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxid. Redox Signal. 2008, 10, 31–42. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Ishii, K.; Togawa, T.; Tanabe, S. Determination of bound sulfur in serum by gas dialysis/high-performance liquid chromatography. Anal. Biochem. 1993. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Isoda, S.; Tanabe, S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kawamura, K.; Kimura, Y.; Shibuya, N.; Kimura, H.; Ogasawara, Y. Analysis of endogenous H2S and H2Sn in mouse brain by high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radic. Biol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.T.; Gazi, S.K.; Barrow, R.K.; Yang, G.D.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448. [Google Scholar] [CrossRef]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015. [Google Scholar] [CrossRef]
- Koike, S.; Ogasawara, Y. Sulfur atom in its bound state is a unique element involved in physiological functions in mammals. Molecules 2016, 21, 1753. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of hydrogen sulfide with disulfide and Sulfenic acid to form the strongly nucleophilic persulfide. J. Biol. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.I.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013. [Google Scholar] [CrossRef]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1626. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.S.; Peng, Z.F.; Chen, M.J.; Moore, P.K.; Whiteman, M. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology 2007, 53, 505–514. [Google Scholar] [CrossRef]
- Lu, M.; Hu, L.-F.; Hu, G.; Bian, J.-S. Hydrogen sulfide protects astrocytes against H(2)O(2)-induced neural injury via enhancing glutamate uptake. Free Radic. Biol. Med. 2008, 45, 1705–1713. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Sekiguchi, F.; Kubo, S.; Yamasaki, Y.; Matsuda, S.; Okamoto, Y.; Sekimoto, T.; Fukatsu, A.; Nishikawa, H.; Kume, T.; et al. Involvement of ERK in NMDA receptor-independent cortical neurotoxicity of hydrogen sulfide. Biochem. Biophys. Res. Commun. 2011, 414, 727–732. [Google Scholar] [CrossRef]
- Chen, M.J.; Peng, Z.F.; Manikandan, J.; Melendez, A.J.; Tan, G.S.; Chung, C.M.; Li, Q.-T.; Tan, T.M.; Deng, L.W.; Whiteman, M.; et al. Gene profiling reveals hydrogen sulphide recruits death signaling via the N-methyl-D-aspartate receptor identifying commonalities with excitotoxicity. J. Cell. Physiol. 2011, 226, 1308–1322. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Yuan, Z.; Lu, C. Colorimetric detection of biological hydrogen sulfide using fluorosurfactant functionalized gold nanorods. Analyst 2015, 140, 7443–7450. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mutus, B. Electrochemical detection of sulfide. Rev. Anal. Chem. 2013, 32, 247–256. [Google Scholar] [CrossRef]
- Tangerman, A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Xu, S.; Chen, W.; Xian, M. Hydrogen sulfide mediated tandem reaction of selenenyl sulfides and its application in fluorescent probe development. Org. Lett. 2019, 21, 7573–7576. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pacheco, A.; Takano, Y.; Day, J.J.; Hanaoka, K.; Xian, M. A single fluorescent probe to visualize hydrogen sulfide and hydrogen polysulfides with different fluorescence signals. Angewandte Chemie 2016, 55, 9993–9996. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef]
- Fan, H.; Guo, Y.; Liang, X.; Yuan, Y.; Qi, X.; Wang, M.; Ma, J.; Zhou, H. Hydrogen sulfide protects against amyloid beta-peptide induced neuronal injury via attenuating inflammatory responses in a rat model. J. Biomed. Res. 2013, 27, 296–304. [Google Scholar]
- Xue, X.; Bian, J.-S. Neuroprotective effects of hydrogen sulfide in Parkinson’s disease animal models: Methods and protocols. Methods Enzymol. 2015, 554, 169–186. [Google Scholar]
- Wang, M.; Zhu, J.; Pan, Y.; Dong, J.; Zhang, L.; Zhang, X.; Zhang, L. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2015, 93, 487–494. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Neurodegeneration in Huntington’s disease involves loss of cystathionine gamma-lyase. Cell Cycle 2014, 13, 2491–2493. [Google Scholar] [CrossRef]
- Spalloni, A.; Greco, V.; Ciriminna, G.; Corasolla Carregari, V.; Marini, F.; Pieroni, L.; Mercuri, N.B.; Urbani, A.; Longone, P. Impact of pharmacological inhibition of hydrogen sulphide production in the SOD1G93A-ALS mouse model. Int. J. Mol. Sci. 2019, 20, 2550. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-J.; Li, X.; Tang, X.-Q. Therapeutic benefits of H2S in Alzheimer’s disease. J. Clin. Neurosci. 2014, 21, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, Y.; Wang, Z.; Kong, Y.; Gao, R.; Chen, G. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med. Gas Res. 2017, 7, 113. [Google Scholar] [PubMed]
- Liu, X.Q.; Liu, X.Q.; Jiang, P.; Huang, H.; Yan, Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi 2008, 88, 2246. [Google Scholar] [PubMed]
- Kamoun, P.; Belardinelli, M.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. Part A 2003, 116, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Greco, V.; Pieragostino, D.; Piras, C.; Aebersold, R.; Wiltfang, J.; Caltagirone, C.; Bernardini, S.; Urbani, A. Direct analytical sample quality assessment for biomarker investigation: Qualifying cerebrospinal fluid samples. Proteomics 2014, 14, 1954–1962. [Google Scholar] [CrossRef]
- Greco, V.; Piras, C.; Pieroni, L.; Ronci, M.; Putignani, L.; Roncada, P.; Urbani, A. Applications of MALDI-TOF mass spectrometry in clinical proteomics. Expert Rev. Proteom. 2018, 15, 683–696. [Google Scholar] [CrossRef]
- Du, S.; Jiang, X.; Feng, G.; Sun, L.; Wu, W.; Hong, T.; Du, J.; Tang, C.; Jin, H. Predictive role of cerebrospinal fluid hydrogen sulfide in central nervous system leukemia. Chin. Med. J. 2011, 124, 3450–3454. [Google Scholar]
- Han, M.; Liu, D.; Qiu, J.; Yuan, H.; Hu, Q.; Xue, H.; Li, T.; Ma, W.; Zhang, Q.; Li, G. Evaluation of H2S-producing enzymes in cerebrospinal fluid and its relationship with interleukin-6 and neurologic deficits in subarachnoid hemorrhage. Biomed. Pharmacother. 2020, 123, 109722. [Google Scholar] [CrossRef]
- Pieragostino, D.; Del Boccio, P.; Di Ioia, M.; Pieroni, L.; Greco, V.; De Luca, G.; D’Aguanno, S.; Rossi, C.; Franciotta, D.; Centonze, D.; et al. Oxidative modifications of cerebral transthyretin are associated with multiple sclerosis. Proteomics 2013, 13, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Del Boccio, P.; Panella, M.; Bernardini, S.; Di Ilio, C.; Gambi, D.; Stanzione, P.; Sacchetta, P.; Bernardi, G.; Martorana, A. Differential post-translational modifications of transthyretin in Alzheimer’s disease: A study of the cerebral spinal fluid. Proteomics 2006, 6, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Piras, C.; Pieroni, L.; Urbani, A. Direct assessment of plasma/serum sample quality for proteomics biomarker investigation. In Serum/Plasma Proteomics; Springer: New York, NY, USA, 2017; pp. 3–21. [Google Scholar]
- Greco, V.; Longone, P.; Spalloni, A.; Pieroni, L.; Urbani, A. Crosstalk between oxidative stress and mitochondrial damage: Focus on amyotrophic lateral sclerosis. In Mitochondria in Health and in Sickness; Springer: Singapore, 2019; pp. 71–82. [Google Scholar]
- You, J.; Gelfanova, V.; Knierman, M.D.; Witzmann, F.A.; Wang, M.; Hale, J.E. The impact of blood contamination on the proteome of cerebrospinal fluid. Proteomics 2005, 5, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.D.; DeLallo, L.J. Cerebrospinal fluid transthyretin in multiple sclerosis. Neurology 1987, 37, 1262. [Google Scholar] [CrossRef]
- Poulsen, K.; Bahl, J.M.C.; Tanassi, J.T.; Simonsen, A.H.; Heegaard, N.H.H. Characterization and stability of transthyretin isoforms in cerebrospinal fluid examined by immunoprecipitation and high-resolution mass spectrometry of intact protein. Methods 2012, 56, 284–292. [Google Scholar] [CrossRef]
- Simonsen, A.H.; Bahl, J.M.C.; Danborg, P.B.; Lindstrom, V.; Larsen, S.O.; Grubb, A.; Heegaard, N.H.H.; Waldemar, G. Pre-analytical factors influencing the stability of cerebrospinal fluid proteins. J. Neurosci. Methods 2013, 215, 234–240. [Google Scholar] [CrossRef]
- Whiteman, M.; Le Trionnaire, S.; Chopra, M.; Fox, B.; Whatmore, J. Emerging role of hydrogen sulfide in health and disease: Critical appraisal of biomarkers and pharmacological tools. Clin. Sci. 2011, 121, 459–488. [Google Scholar] [CrossRef] [PubMed]
- Hartle, M.D.; Pluth, M.D. A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Longen, S.; Richter, F.; Köhler, Y.; Wittig, I.; Beck, K.-F.; Pfeilschifter, J. Quantitative persulfide site identification (qPerS-SID) reveals protein targets of H2S releasing donors in mammalian cells. Sci. Rep. 2016, 6, 29808. [Google Scholar] [CrossRef]
- Hansen, R.E.; Roth, D.; Winther, J.R. Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar] [CrossRef] [PubMed]
- Giuriati, C.; Cavalli, S.; Gorni, A.; Badocco, D.; Pastore, P. Ion chromatographic determination of sulfide and cyanide in real matrices by using pulsed amperometric detection on a silver electrode. J. Chromatogr. A 2004, 1023, 105–112. [Google Scholar] [CrossRef]
- Xu, T.; Scafa, N.; Xu, L.-P.; Zhou, S.; Al-Ghanem, K.A.; Mahboob, S.; Fugetsu, B.; Zhang, X. Electrochemical hydrogen sulfide biosensors. Analyst 2016, 141, 1185–1195. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 2014, 41, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Longone, P.; Davoli, A.; Greco, V.; Spalloni, A.; Guatteo, E.; Neri, C.; Rizzo, G.R.; Cordella, A.; Romigi, A.; Cortese, C. Hydrogen sulphide “a double-faced Janus” in amyotrophic lateral sclerosis (ALS). Ther. Targets Neurol. Dis. 2015, 2, e749. [Google Scholar] [CrossRef]
- Hu, L.; Lu, M.; Tiong, C.X.; Dawe, G.S.; Hu, G.; Bian, J. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010, 9, 135–146. [Google Scholar] [CrossRef]
- Wu, R.; Yao, W.Z.; Chen, Y.H.; Geng, B.; Tang, C.S. Plasma level of endogenous hydrogen sulfide in patients with acute asthma. Beijing Da Xue Xue bao. Yi Xue Ban J. Peking Univ. Heal. Sci. 2008, 40, 505–508. [Google Scholar]
- Suzuki, K.; Sagara, M.; Aoki, C.; Tanaka, S.; Aso, Y. Clinical implication of plasma hydrogen sulfide levels in Japanese patients with type 2 diabetes. Intern. Med. 2017, 56, 17–21. [Google Scholar] [CrossRef]
- Karunya, R.; Jayaprakash, K.S.; Gaikwad, R.; Sajeesh, P.; Ramshad, K.; Muraleedharan, K.M.; Dixit, M.; Thangaraj, P.R.; Sen, A.K. Rapid measurement of hydrogen sulphide in human blood plasma using a microfluidic method. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Perluigi, M. Redox proteomics: A key tool for new insights into protein modification with relevance to disease. Antioxid. Redox Signal. 2017, 26, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Davoli, A.; Greco, V.; Spalloni, A.; Guatteo, E.; Neri, C.; Rizzo, G.R.; Cordella, A.; Romigi, A.; Cortese, C.; Bernardini, S.; et al. Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann. Neurol. 2015, 77, 697–709. [Google Scholar] [CrossRef]
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar] [CrossRef]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef]
- Han, Y.; Shang, Q.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 293. [Google Scholar] [CrossRef]
- Belardinelli, M.-C.; Chabli, A.; Chadefaux-Vekemans, B.; Kamoun, P. Urinary sulfur compounds in Down syndrome. Clin. Chem. 2001, 47, 1500–1501. [Google Scholar] [CrossRef]
- Kamoun, P. Mental retardation in Down syndrome: A hydrogen sulfide hpothesis. Med. Hypotheses 2001, 57, 389–392. [Google Scholar] [CrossRef]
- Szabo, C. The re-emerging pathophysiological role of the cystathionine-β-synthase-hydrogen sulfide system in Down syndrome. FEBS J. 2020, 287, 3150–3160. [Google Scholar] [CrossRef]
- Panagaki, T.; Randi, E.B.; Augsburger, F.; Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 18769–18771. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Protein sulfhydration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 555, pp. 79–90. ISBN 0076-6879. [Google Scholar]
- Wright, C.M.; Christman, G.D.; Snellinger, A.M.; Johnston, M.V.; Mueller, E.G. Direct evidence for enzyme persulfide and disulfide intermediates during 4-thiouridine biosynthesis. Chem. Commun. 2006, 29, 3104–3106. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid. Redox Signal. 2015, 22, 411–423. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Persichilli, S.; Gervasoni, J.; Di Napoli, A.; Fuso, A.; Nicolia, V.; Giardina, B.; Scarpa, S.; Desiderio, C.; Cavallaro, R.A. Plasma thiols levels in Alzheimer’s disease mice under diet-induced hyperhomocysteinemia: Effect of s-adenosylmethionine and superoxide-dismutase supplementation. J. Alzheimers Dis. 2015, 44, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sánchez, E.D. Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Vural, G.; Gümüşyayla, Ş.; Deniz, O.; Neşelioğlu, S.; Erel, Ö. Relationship between thiol-disulphide homeostasis and visual evoked potentials in patients with multiple sclerosis. Neurol. Sci. 2019, 40, 385–391. [Google Scholar] [CrossRef]
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221. [Google Scholar] [CrossRef]
- Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L.; Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S. Hydrogen sulfide is an endogenous modulator of leukocyte mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Bos, E.M.; Calzia, E.; Van Goor, H.; Coletta, C.; Papapetropoulos, A.; Hellmich, M.R.; Radermacher, P.; Bouillaud, F.; Szabo, C. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br. J. Pharmacol. 2014, 171, 2123–2146. [Google Scholar] [CrossRef]
- Greco, V.; Spalloni, A.; Corasolla Carregari, V.; Pieroni, L.; Persichilli, S.; Mercuri, N.B.; Urbani, A.; Longone, P. Proteomics and toxicity analysis of spinal-cord primary cultures upon hydrogen sulfide treatment. Antioxidants 2018, 7, 87. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Ding, L.; Bian, J. A new hope for a devastating disease: Hydrogen sulfide in Parkinson’s disease. Mol. Neurobiol. 2018, 55, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Yamada, M.; Tokuda, K.; Marutani, E.; Kakinohana, M.; Kaneki, M.; Ichinose, F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid. Redox Signal. 2011, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.; Waraska, J.; Acworth, I. Direct determination of tissue aminothiol, disulfide, and thioether levels using HPLC-ECD with a novel stable boron-doped diamond working electrode. Methods Mol. Biol. 2010, 594, 327–339. [Google Scholar] [CrossRef]
| (a) | ||||||||||||
| Sulfide | NaOH 70 mM | NaOH 75 mM | NaOH 80 mM | |||||||||
| [mg/L] | [mg/L] | RSD% | R T | RSD% | [mg/L] | RSD% | R T | RSD% | [mg/L] | RSD% | R T | RSD% |
| 2 | 1.98 | 3.9 | 2.28 | 3.9 | 2.1 | 10 | 2.17 | 0.31 | 1.95 | 6.4 | 2.08 | 6.4 |
| 5 | 5.6 | 6.3 | 2.28 | 6.3 | 5.2 | 6.8 | 2.15 | 1.02 | 5.2 | 3.5 | 2.08 | 3.49 |
| 10 | 9.4 | 4.8 | 2.28 | 4.8 | 10.2 | 2.4 | 2.17 | 0.23 | 9.7 | 7.0 | 2.11 | 7.10 |
| (b) | ||||||||||||
| Sulfide | 0.9 mL/min | 1.0 mL/min | 1.1 mL/min | |||||||||
| [mg/L] | [mg/L] | RSD% | R T | RSD% | [mg/L] | RSD% | R T | RSD% | [mg/L] | RSD% | R T | RSD% |
| 2 | 2.19 | 8.0 | 2.33 | 0.52 | 2.1 | 10 | 2.17 | 0.31 | 2.04 | 5.1 | 2.02 | 0.19 |
| 5 | 5.1 | 6.0 | 2.34 | 0.41 | 5.2 | 6.8 | 2.15 | 1.02 | 4.79 | 7.9 | 2.02 | 0.5 |
| 10 | 10.7 | 8.7 | 2.36 | 2.71 | 10.2 | 2.4 | 2.17 | 0.23 | 10.7 | 6.4 | 2.01 | 0.39 |
| Sulfide | Intra-Day | Inter-Day | ||
|---|---|---|---|---|
| (mg/L) | RSD% | RE% | RSD% | RE% |
| 2 | 5.07 | 2.14 | 7.65 | 2.48 |
| 5 | 7.89 | 5.24 | 7.70 | 3.89 |
| 10 | 6.37 | 2.40 | 7.72 | 1.47 |
| Spiked Concentration | Batch 1 | Batch 2 | Batch 3 | |||
|---|---|---|---|---|---|---|
| [mg/L] | Mean% | RSD % | Mean% | RSD % | Mean% | RSD % |
| 2 | 98.9 | 0.6 | 104.7 | 0.7 | 100.3 | 0.7 |
| 5 | 100.6 | 2.1 | 99.4 | 3.2 | 98.4 | 1.5 |
| 10 | 97.9 | 5.4 | 94.8 | 2.9 | 96.7 | 3.1 |
| CSF Group (n = 134) | CSF Groups | ||||||
|---|---|---|---|---|---|---|---|
| CTRL (n = 18) | ALS (n = 40) | MS (n = 39) | ADEM (n = 15) | AD (n = 11) | PD (n = 11) | ||
| free H2S | 3.74 | 3.48 ** | 6.32 | 2.07 ** | 3.62 * | 4.17 * | 5.48 |
| (13.69–0.39) | (6.04–0.39) | (13.69–2.92) | (2.95–0.66) | (5.8–1.23) | (4.75–1.71) | (7.16–4.09) | |
| Bound Sufur | 0.21 | 0.13 §§ | 0.19 §§ | 0.24 | 0.22 § | 0.22 § | 0.22 § |
| (0.34–0.08) | (0.29–0.08) | (0.34–0.09) | (0.31–0.08) | (0.29–0.12) | (0.28–0.11) | (0.27–0.12) | |
| Column | IonPac™ AS15 Hydroxide Selective Anion-Exchange Column 5 µm (3 × 150 mm, Thermo Fisher Scientific) + IonPac™ AG15 Guard Columns 5 µm (3 × 30 mm, Thermo Fisher Scientific) | ||
| Eluent | NaOH 75 mM solution prepared by NaOH Eluent Generator (Thermo Fisher Scientific) | ||
| Injection volume | 25 μL | ||
| Detection | disposable silver electrode | ||
| PAD sequence | Time | Potential | Integration |
| 0 | −0.1 | Begin End | |
| 0.2 | −0.1 | ||
| 0.3 | −0.1 | ||
| 0.31 | 0.1 | ||
| 0.4 | 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, V.; Neri, C.; Pieragostino, D.; Spalloni, A.; Persichilli, S.; Gastaldi, M.; Mercuri, N.B.; Longone, P.; Urbani, A. Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders. Metabolites 2021, 11, 152. https://doi.org/10.3390/metabo11030152
Greco V, Neri C, Pieragostino D, Spalloni A, Persichilli S, Gastaldi M, Mercuri NB, Longone P, Urbani A. Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders. Metabolites. 2021; 11(3):152. https://doi.org/10.3390/metabo11030152
Chicago/Turabian StyleGreco, Viviana, Cristina Neri, Damiana Pieragostino, Alida Spalloni, Silvia Persichilli, Matteo Gastaldi, Nicola B. Mercuri, Patrizia Longone, and Andrea Urbani. 2021. "Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders" Metabolites 11, no. 3: 152. https://doi.org/10.3390/metabo11030152
APA StyleGreco, V., Neri, C., Pieragostino, D., Spalloni, A., Persichilli, S., Gastaldi, M., Mercuri, N. B., Longone, P., & Urbani, A. (2021). Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders. Metabolites, 11(3), 152. https://doi.org/10.3390/metabo11030152