Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial
Abstract
1. Introduction
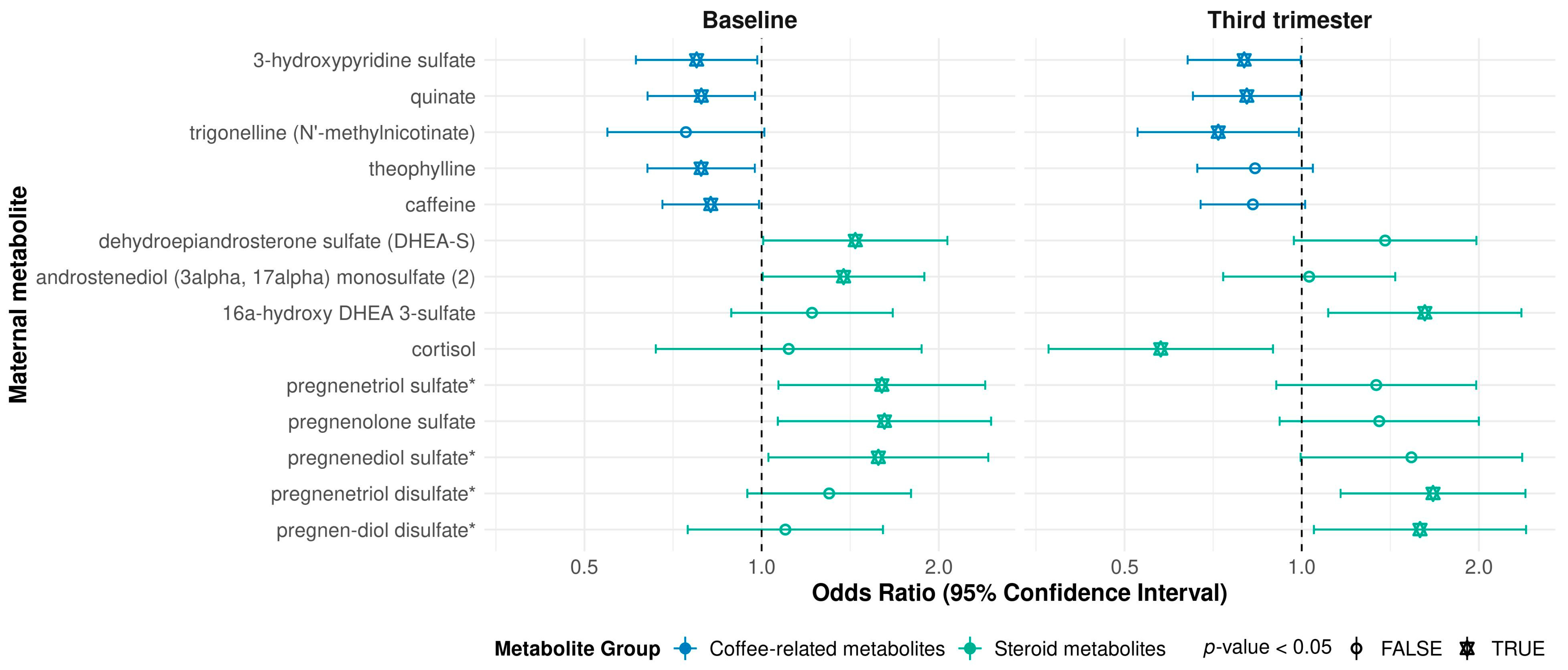
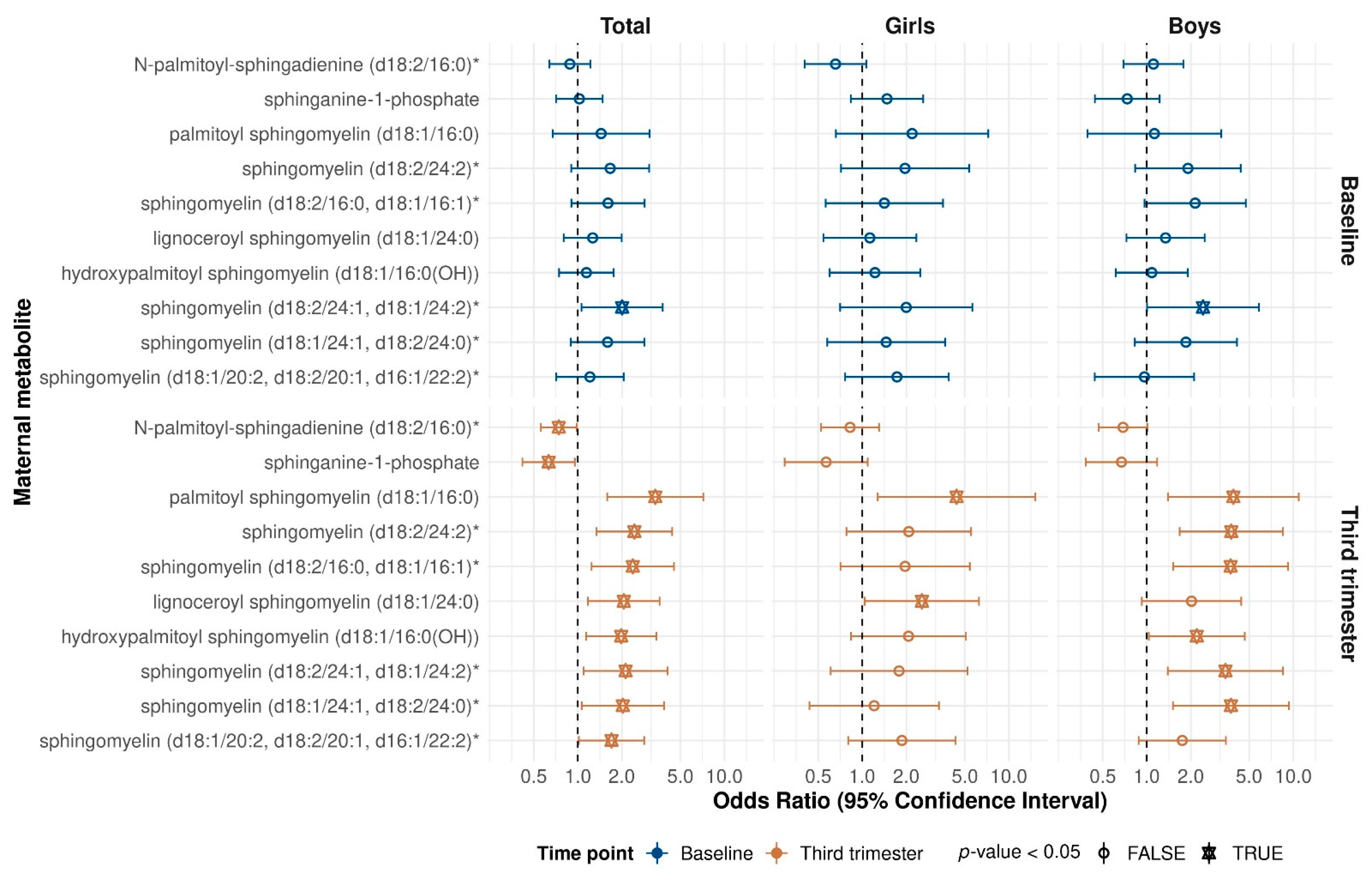
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Asthma or Recurrent Wheeze by the Age of Three Years in Children
4.3. Metabolomics Data
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiehn, O. Metabolomics the link between genotypes and phenotypes. Plant. Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Scheltema, N.; Bont, L.; Baraldi, E. Early-life origins of chronic respiratory diseases: Understanding and promoting healthy ageing. Eur. Respir. J. 2014, 44, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Wooldridge, A.L.; Kind, K.L.; Bischof, R.; Clifton, V.L. Pre-birth origins of allergy and asthma. J. Reprod. Immunol. 2017, 123, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Blighe, K.; Chawes, B.L.; Kelly, R.S.; Mirzakhani, H.; McGeachie, M.; Litonjua, A.A.; Weiss, S.T.; Lasky-Su, J.A. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y. Am. J. Clin. Nutr. 2017, 106, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; La Grutta, S. The Burden of Pediatric Asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Huang, W.Y.; Xiong, X.; Freedman, N.D.; Cross, A.J.; et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef]
- Playdon, M.C.; Sampson, J.N.; Cross, A.J.; Sinha, R.; Guertin, K.A.; Moy, K.A.; Rothman, N.; Irwin, M.L.; Mayne, S.T.; Stolzenberg-Solomon, R.; et al. Comparing metabolite profiles of habitual diet in serum and urine. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef]
- Wang, Y.; Gapstur, S.M.; Carter, B.D.; Hartman, T.J.; Stevens, V.L.; Gaudet, M.M.; McCullough, M.L. Untargeted Metabolomics Identifies Novel Potential Biomarkers of Habitual Food Intake in a Cross-Sectional Study of Postmenopausal Women. J. Nutr. 2018, 148, 932–943. [Google Scholar] [CrossRef]
- Chau, Y.P.; Au, P.C.M.; Li, G.H.Y.; Sing, C.W.; Cheng, V.K.F.; Tan, K.C.B.; Kung, A.W.C.; Cheung, C.L. Serum Metabolome of Coffee Consumption and its Association With Bone Mineral Density: The Hong Kong Osteoporosis Study. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.; Nodzenski, M.; Talbot, O.; Kuang, A.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; O’Neal, S.K.; Lowe, L.P.; et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia 2019, 62, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Maitre, L.; Villanueva, C.M.; Lewis, M.R.; Ibarluzea, J.; Santa-Marina, L.; Vrijheid, M.; Sunyer, J.; Coen, M.; Toledano, M.B. Maternal urinary metabolic signatures of fetal growth and associated clinical and environmental factors in the INMA study. BMC Med. 2016, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.F.B.; Morillon, A.C.; Melo Junior, E.F.; Souza, R.T.; McCarthy, F.P.; Khashan, A.; Baker, P.; Kenny, L.C.; Cecatti, J.G. Examining the predictive accuracy of metabolomics for small-for-gestational-age babies: A systematic review. BMJ Open 2019, 9, e031238. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E., Jr.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy and risk of pregnancy loss: A categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. 2016, 19, 1233–1244. [Google Scholar] [CrossRef]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: A systematic review and dose-response meta-analysis. BMC Med. 2014, 12, 174. [Google Scholar] [CrossRef]
- Jahanfar, S.; Jaafar, S.H. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Liu, X.; Liew, Z.; Olsen, J.; Pedersen, L.H.; Bech, B.H.; Agerbo, E.; Yuan, W.; Li, J. Association of prenatal exposure to acetaminophen and coffee with childhood asthma. Pharmacoepidemiol. Drug Saf. 2016, 25, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Buhrer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Levison, B.S.; Buffa, J.A.; Huang, Y.; Fu, X.; Wang, Z.; Gogonea, V.; DiDonato, J.A.; Hazen, S.L. Myeloperoxidase-mediated protein lysine oxidation generates 2-aminoadipic acid and lysine nitrile in vivo. Free Radic. Biol. Med. 2017, 104, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Bradshaw, H.B.; Hughes, H.V.; Chen, J.S.; Hu, S.S.; McHugh, D.; Vefring, E.; Jahnsen, J.A.; Thompson, E.L.; Masuda, K.; et al. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol. Pharmacol. 2008, 74, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Pijnenburg, M.W.; Smith, A.D.; De Jongste, J.C. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006, 61, 817–827. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Rifas-Shiman, S.L.; Carey, V.J.; et al. Fecal short-chain fatty acids in pregnancy and offspring asthma and allergic outcomes. J. Allergy Clin. Immunol. Pract. 2020, 8, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Strube, G.; Rudolf, M. For and against. Should steroids be the first line treatment for asthma? BMJ 2000, 320, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Ono, J.G.; Kim, B.I.; Zhao, Y.; Christos, P.J.; Tesfaigzi, Y.; Worgall, T.S.; Worgall, S. Decreased sphingolipid synthesis in children with 17q21 asthma-risk genotypes. J. Clin. Investig. 2020, 130, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Ono, J.G.; Worgall, T.S.; Worgall, S. Airway reactivity and sphingolipids-implications for childhood asthma. Mol. Cell. Pediatr. 2015, 2, 13. [Google Scholar] [CrossRef]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Kelly, R.S.; Boulin, A.; Laranjo, N.; Lee-Sarwar, K.; Chu, S.H.; Yadama, A.P.; Carey, V.; Litonjua, A.A.; Lasky-Su, J.; Weiss, S.T. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites 2019, 9, 42. [Google Scholar] [CrossRef]
- Huang, M.; Kelly, R.S.; Kachroo, P.; Chu, S.H.; Lee-Sarwar, K.; Chawes, B.L.; Bisgaard, H.; Litonjua, A.A.; Weiss, S.T.; Lasky-Su, J. Plasma 25-Hydroxyvitamin D Concentrations are Associated with Polyunsaturated Fatty Acid Metabolites in Young Children: Results from the Vitamin D Antenatal Asthma Reduction Trial. Metabolites 2020, 10, 151. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/index.html (accessed on 3 January 2021).
- Gostner, J.M.; Becker, K.; Kofler, H.; Strasser, B.; Fuchs, D. Tryptophan Metabolism in Allergic Disorders. Int. Arch. Allergy Immunol. 2016, 169, 203–215. [Google Scholar] [CrossRef]
| No Asthma/Wheeze by Age 3 (n = 485) | Asthma/Wheeze by Age 3 (n = 200) | p-Value | |
|---|---|---|---|
| Maternal Characteristics | |||
| Age, mean (SD) | 27.9 (5.3) | 26.1 (5.6) | <0.001 |
| Pre-pregnancy BMI, mean (SD) | 28.2 (7.5) a | 29.0 (9.0) b | 0.323 |
| Baseline vitamin D level, mean (SD) | 23.4 (10.5) | 21.2 (9.6) | 0.008 |
| Third trimester vitamin D level, mean (SD) | 34.0 (14.4) | 30.4 (15.1) | 0.004 |
| Exact baseline gestational week, mean (SD) | 14.0 (2.7) | 14.5 (2.8) | 0.032 |
| Treatment group, n (%) | 0.140 | ||
| 4400 IU/day vitamin D | 250 (51.5%) | 90 (45.0%) | |
| 400 IU/day vitamin D | 235 (48.5%) | 110 (55.0%) | |
| Site, n (%) | 0.003 | ||
| Boston | 126 (26.0%) | 67 (33.5%) | |
| San Diego | 178 (36.7%) | 47 (23.5%) | |
| St Louis | 181 (37.3%) | 86 (43.0%) | |
| Race, n (%) | 0.002 | ||
| Black | 195 (40.2%) | 109 (54.5%) | |
| Other | 82 (16.9%) | 23 (11.5%) | |
| White | 208 (42.9%) | 68 (34.0%) | |
| Asthma, n (%) | <0.001 | ||
| Yes | 177 (36.5%) | 104 (52.0%) | |
| No | 308 (63.5%) | 96 (48.0%) | |
| Education level, n (%) | 0.013 | ||
| ≥College graduate | 187 (38.6%) | 52 (26.0%) | |
| Some college | 105 (21.6%) | 46 (23.0%) | |
| High/Tech school | 136 (28.0%) | 70 (35.0%) | |
| <High school | 57 (11.8%) | 32 (16.0%) | |
| Income level, n (%) | 0.001 | ||
| <$30,000 | 132 (27.2%) | 76 (38.0%) | |
| $30,000–$74,999 | 123 (25.4%) | 48 (24.0%) | |
| $75,000–$99,999 | 59 (12.2%) | 9 (4.5%) | |
| ≥$100,000 | 62 (12.8%) | 15 (7.5%) | |
| Prefer not to answer/do not know | 109 (22.5%) | 52 (26.0%) | |
| Gestational diabetes, n (%) | 0.105 | ||
| Yes | 28 (5.8%) | 5 (2.5%) | |
| No | 457 (94.2%) | 195 (97.5%) | |
| Preeclampsia, n (%) | 0.018 | ||
| Yes | 15 (3.1%) | 15 (7.5%) | |
| No | 470 (96.9%) | 185 (92.5%) | |
| Paternal Characteristics | |||
| Asthma, n (%) | 0.992 | ||
| Yes | 114 (23.5%) | 46 (23.1%) | |
| No | 371 (76.5%) | 153 (76.9%) c | |
| Characteristics at Birth | |||
| Mode of delivery, n (%) | 0.697 | ||
| Cesarean | 139 (28.7%) | 61 (30.5%) | |
| Vaginal | 346 (71.3%) | 139 (69.5%) | |
| Delivery <37 weeks, n (%) | <0.001 | ||
| Yes | 24 (4.9%) | 26 (13.0%) | |
| No | 461 (95.1%) | 174 (87.0%) | |
| Child sex, n (%) | 0.007 | ||
| Female | 248 (51.1%) | 79 (39.5%) | |
| Male | 237 (48.9%) | 121 (60.5%) | |
| Birth weight, kg, mean (SD) | 3.3 (0.5) | 3.2 (0.6) | 0.034 |
| Birth length, cm, mean (SD) | 50.9 (2.7) | 50.6 (3.1) | 0.176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Kelly, R.S.; Chu, S.H.; Kachroo, P.; Gürdeniz, G.; Chawes, B.L.; Bisgaard, H.; Weiss, S.T.; Lasky-Su, J. Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial. Metabolites 2021, 11, 65. https://doi.org/10.3390/metabo11020065
Huang M, Kelly RS, Chu SH, Kachroo P, Gürdeniz G, Chawes BL, Bisgaard H, Weiss ST, Lasky-Su J. Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial. Metabolites. 2021; 11(2):65. https://doi.org/10.3390/metabo11020065
Chicago/Turabian StyleHuang, Mengna, Rachel S. Kelly, Su H. Chu, Priyadarshini Kachroo, Gözde Gürdeniz, Bo L. Chawes, Hans Bisgaard, Scott T. Weiss, and Jessica Lasky-Su. 2021. "Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial" Metabolites 11, no. 2: 65. https://doi.org/10.3390/metabo11020065
APA StyleHuang, M., Kelly, R. S., Chu, S. H., Kachroo, P., Gürdeniz, G., Chawes, B. L., Bisgaard, H., Weiss, S. T., & Lasky-Su, J. (2021). Maternal Metabolome in Pregnancy and Childhood Asthma or Recurrent Wheeze in the Vitamin D Antenatal Asthma Reduction Trial. Metabolites, 11(2), 65. https://doi.org/10.3390/metabo11020065







