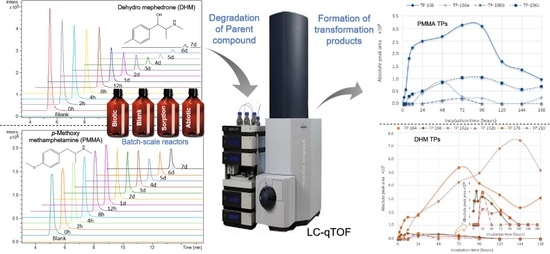
Investigation of Biotransformation Products of p-Methoxymethylamphetamine and Dihydromephedrone in Wastewater by High-Resolution Mass Spectrometry
Abstract
1. Introduction
2. Results
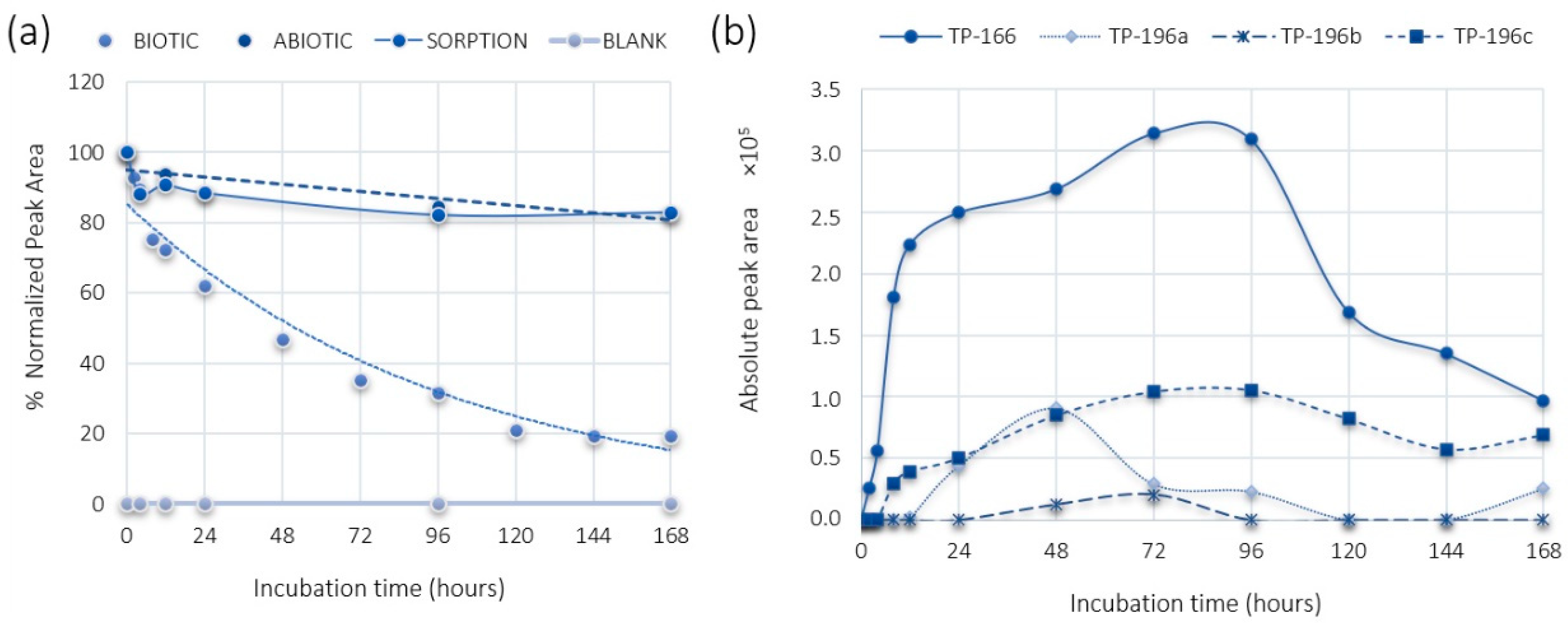
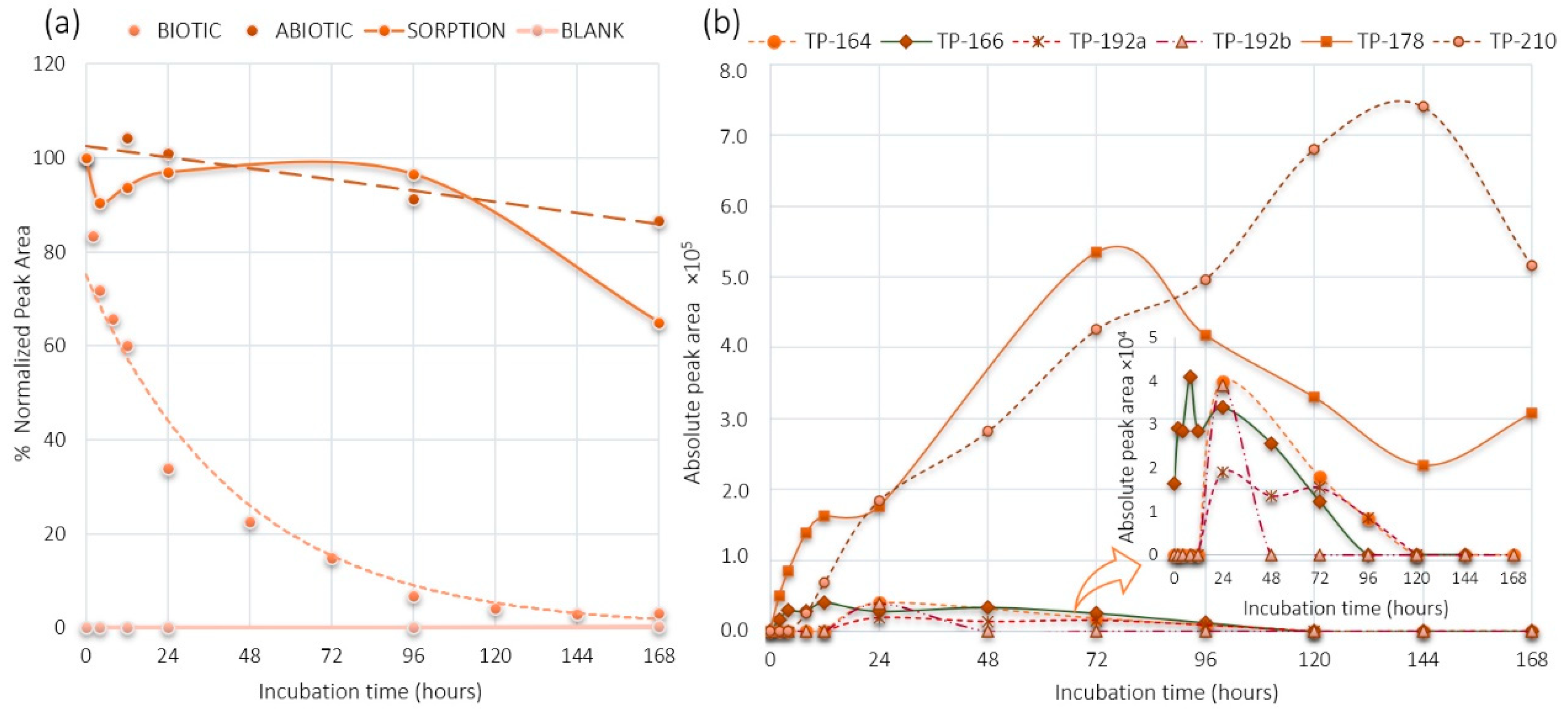
2.1. PMMA and DHM Degradation
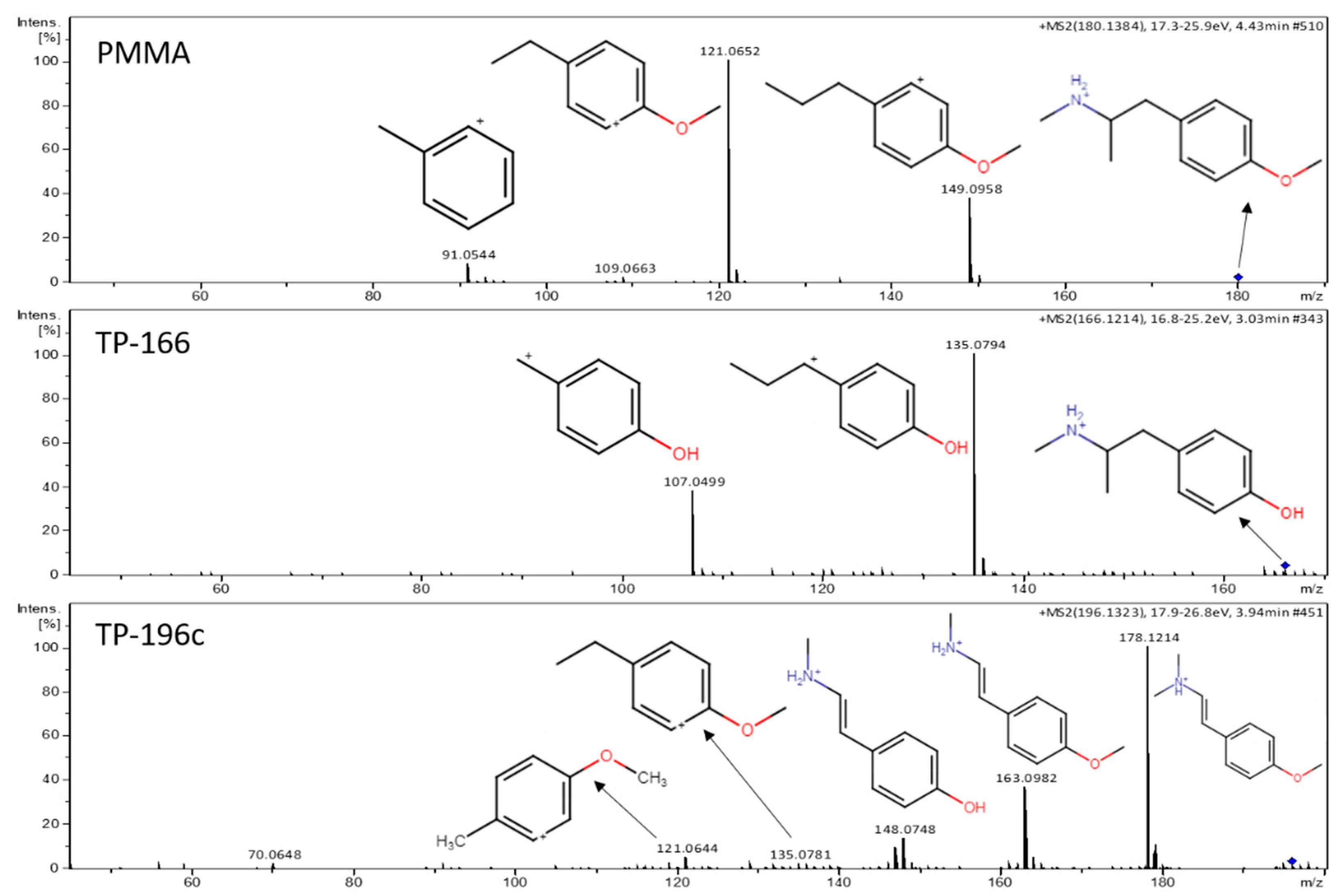
2.2. PMMA Biotransformation Products
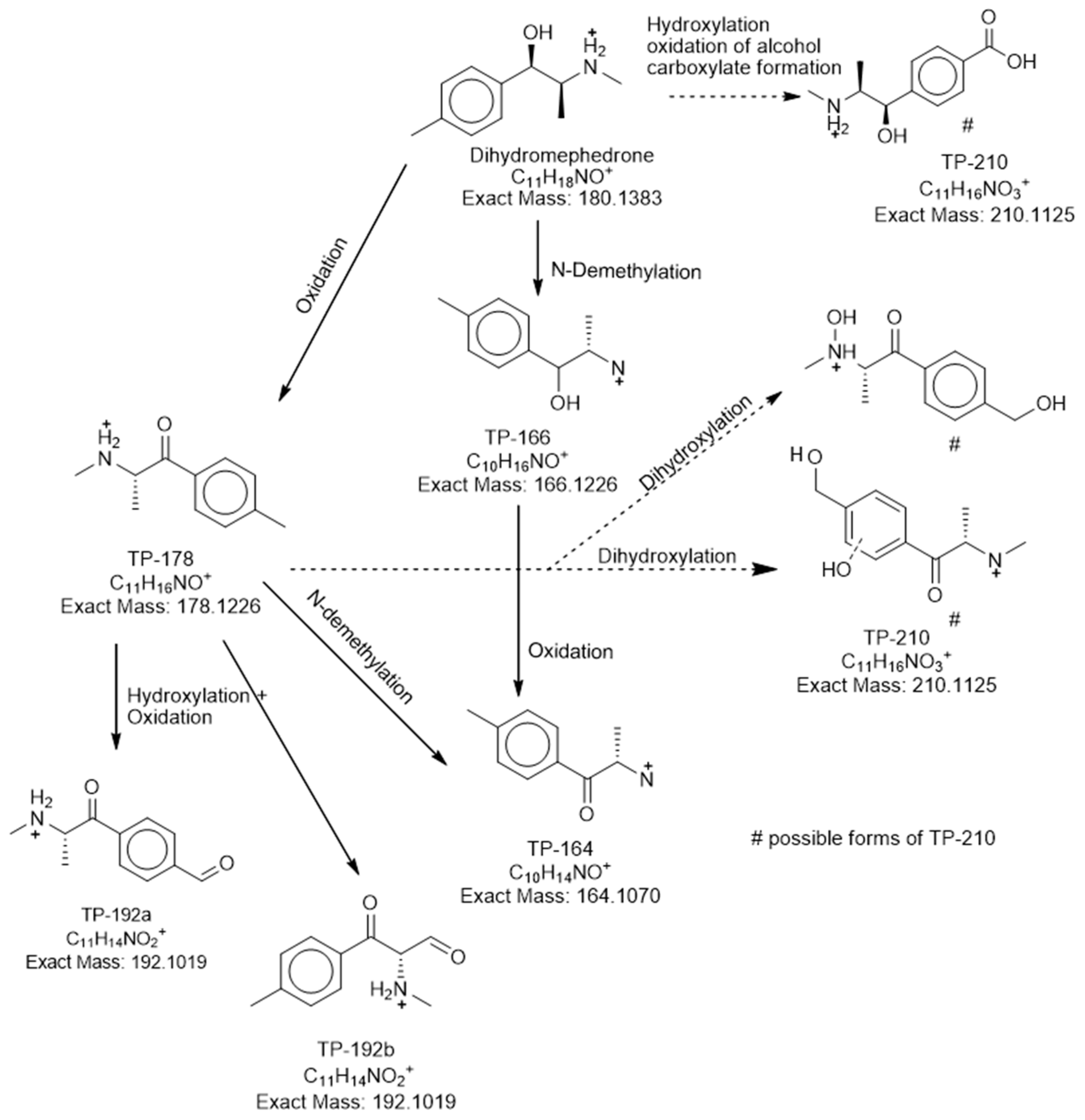
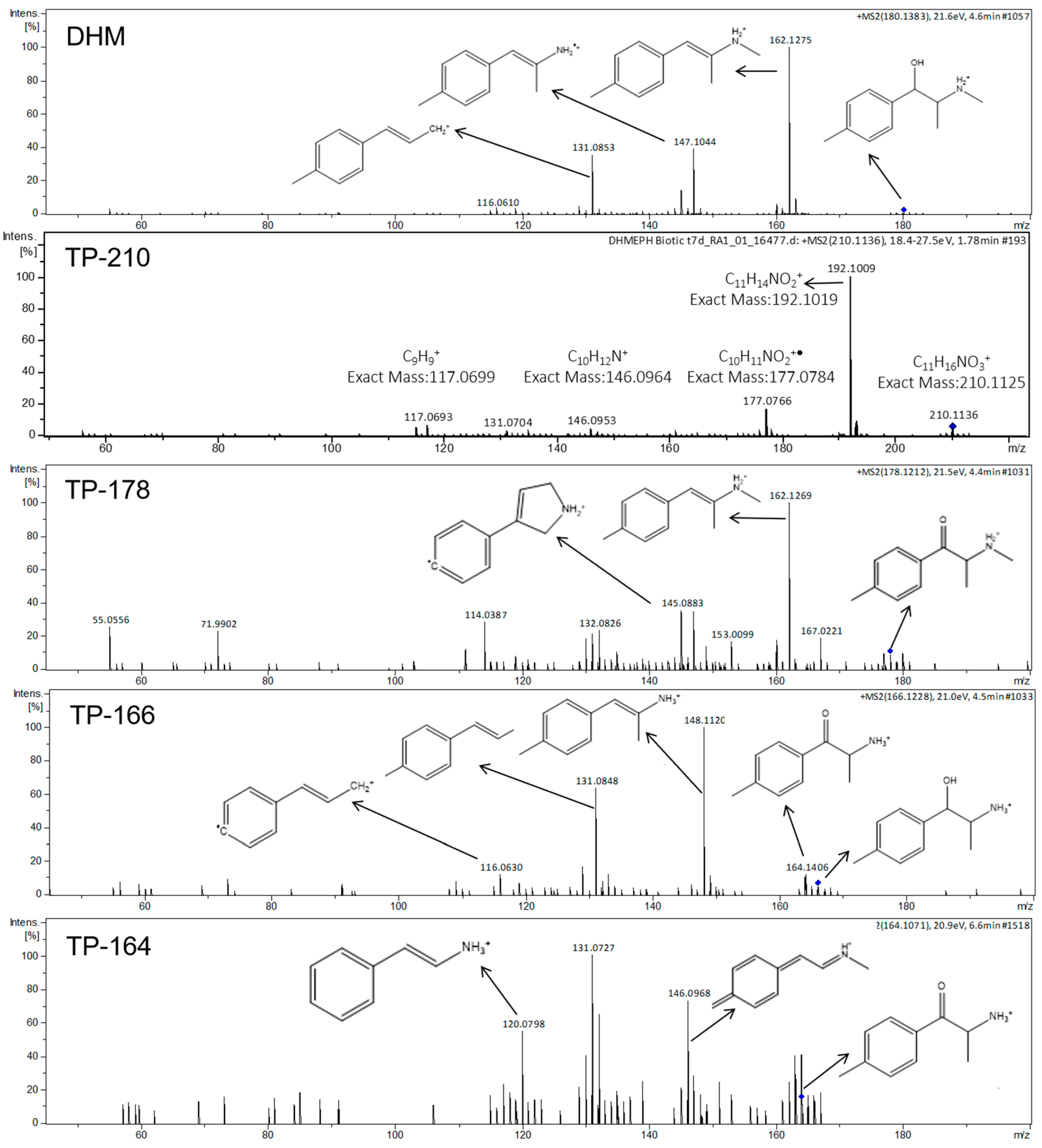
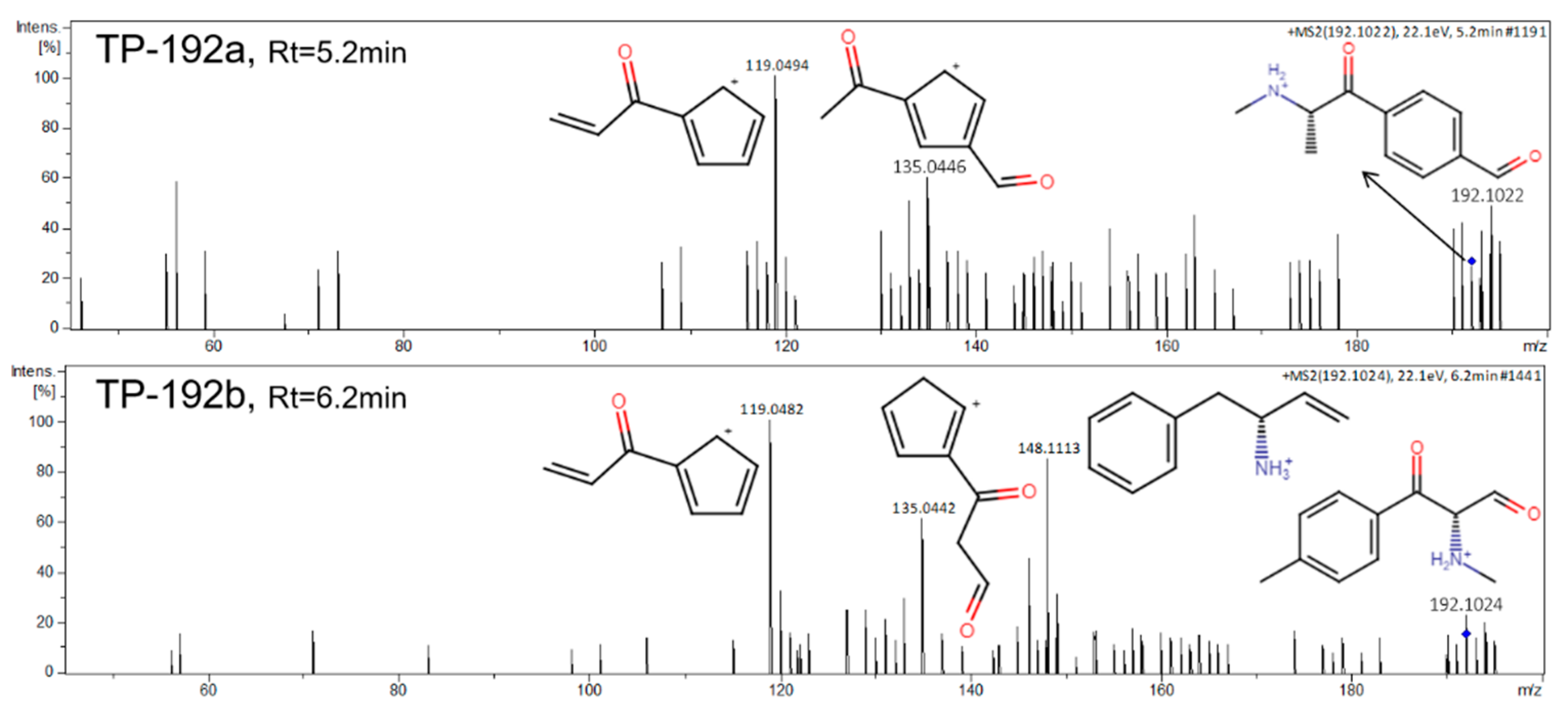
2.3. DHM Biotransformation Products
2.4. Occurrence of the Selected Compounds and Their TPs in Wastewater
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Samples and Sampling Procedure
4.3. Biotransformation Batch Experiments
4.4. Treatment of Wastewater Samples and Instrumental Analysis
4.5. Identification of Transformation Products by Suspect Screening
4.6. Retrospective Screening of the Compounds in Wastewater
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction. European Drug Report—Trends and Developments; EMCDDA: Lisbon, Portugal, 2020. [Google Scholar]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction. Report on the Risk Assessment of PMMA in the Framework of the Joint Action on New Synthetic Drugs; EMCDDA: Lisbon, Portugal, 2003; ISBN 9291681377. [Google Scholar]
- Vevelstad, M.; Øiestad, E.L.; Nerem, E.; Arnestad, M.; Bogen, I.L. Studies on Para-Methoxymethamphetamine (PMMA) Metabolite Pattern and Influence of CYP2D6 Genetics in Human Liver Microsomes and Authentic Samples from Fatal PMMA Intoxications. Drug Metab. Dispos. 2017, 45, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Kinyua, J.; Negreira, N.; McCall, A.-K.; Boogaerts, T.; Ort, C.; Covaci, A.; van Nuijs, A.L.N. Investigating in-sewer transformation products formed from synthetic cathinones and phenethylamines using liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Sci. Total Environ. 2018, 634, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.; Parrott, A. Mephedrone and MDMA: A comparative review. Brain Res. 2020, 1735, 146740. [Google Scholar] [CrossRef] [PubMed]
- Vevelstad, M.; Øiestad, E.L.; Middelkoop, G.; Hasvold, I.; Lilleng, P.; Delaveris, G.J.M.; Eggen, T.; Mørland, J.; Arnestad, M. The PMMA epidemic in Norway: Comparison of fatal and non-fatal intoxications. Forensic Sci. Int. 2012, 219, 151–157. [Google Scholar] [CrossRef]
- Lin, D.-L.; Liu, H.-C.; Yin, H.-L. Recent Paramethoxymethamphetamine (PMMA) Deaths in Taiwan. J. Anal. Toxicol. 2007, 31, 109–113. [Google Scholar] [CrossRef][Green Version]
- Becker, J.; Neis, P.; Röhrich, J.; Zörntlein, S. A fatal paramethoxymethamphetamine intoxication. Leg. Med. 2003, 5, S138–S141. [Google Scholar] [CrossRef]
- Johansen, S.S.; Carsten Hansen, A.; Müller, I.B.; Lundemose, J.B.; Franzmann, M.-B. Three Fatal Cases of PMA and PMMA Poisoning in Denmark. J. Anal. Toxicol. 2003, 27, 253–256. [Google Scholar] [CrossRef][Green Version]
- Lurie, Y.; Gopher, A.; Lavon, O.; Almog, S.; Sulimani, L.; Bentur, Y. Severe paramethoxymethamphetamine (PMMA) and paramethoxyamphetamine (PMA) outbreak in Israel. Clin. Toxicol. 2012, 50, 39–43. [Google Scholar] [CrossRef]
- Nicol, J.J.E.; Yarema, M.C.; Jones, G.R.; Martz, W.; Purssell, R.A.; MacDonald, J.C.; Wishart, I.; Durigon, M.; Tzemis, D.; Buxton, J.A. Deaths from exposure to paramethoxymethamphetamine in Alberta and British Columbia, Canada: A case series. CMAJ Open 2015, 3, E83–E90. [Google Scholar] [CrossRef]
- Tang, M.H.; Ching, C.; Tse, M.; Ng, C.; Lee, C.; Chong, Y.; Wong, W.; Mak, T.W.L. Surveillance of emerging drugs of abuse in Hong Kong: Validation of an analytical tools. Hong Kong Med. J. 2015, 21, 114–123. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Dalsgaard, P.W.; Rode, A.J.; Rasmussen, B.S.; Müller, I.B.; Johansen, S.S.; Linnet, K. Screening for illicit and medicinal drugs in whole blood using fully automated SPE and ultra-high-performance liquid chromatography with TOF-MS with data-independent acquisition. J. Sep. Sci. 2013, 36, 2081–2089. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Reitzel, L.A.; Johansen, S.S.; Linnet, K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test. Anal. 2013, 5, 430–438. [Google Scholar] [CrossRef]
- Meyer, M.R.; Wilhelm, J.; Peters, F.T.; Maurer, H.H. Beta-keto amphetamines: Studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2010, 397, 1225–1233. [Google Scholar] [CrossRef]
- Kinyua, J.; Negreira, N.; Miserez, B.; Causanilles, A.; Emke, E.; Gremeaux, L.; de Voogt, P.; Ramsey, J.; Covaci, A.; van Nuijs, A.L.N. Qualitative screening of new psychoactive substances in pooled urine samples from Belgium and United Kingdom. Sci. Total Environ. 2016, 573, 1527–1535. [Google Scholar] [CrossRef]
- Lee, H.H.; Chen, S.C.; Lee, J.F.; Lin, H.Y.; Chen, B.H. Simultaneous drug identification in urine of sexual assault victims by using liquid chromatography tandem mass spectrometry. Forensic Sci. Int. 2018, 282, 35–40. [Google Scholar] [CrossRef]
- Bijlsma, L.; Celma, A.; López, F.J.; Hernández, F. Monitoring new psychoactive substances use through wastewater analysis: Current situation, challenges and limitations. Curr. Opin. Environ. Sci. Health 2019, 9, 1–12. [Google Scholar] [CrossRef]
- González-Mariño, I.; Baz-Lomba, J.A.; Alygizakis, N.A.; Andrés-Costa, M.J.; Bade, R.; Bannwarth, A.; Barron, L.P.; Been, F.; Benaglia, L.; Berset, J.; et al. Spatio-temporal assessment of illicit drug use at large scale: Evidence from 7 years of international wastewater monitoring. Addiction 2020, 115, 109–120. [Google Scholar] [CrossRef]
- O’Rourke, C.E.; Subedi, B. Occurrence and Mass Loading of Synthetic Opioids, Synthetic Cathinones, and Synthetic Cannabinoids in Wastewater Treatment Plants in Four U.S. Communities. Environ. Sci. Technol. 2020, 54, 6661–6670. [Google Scholar] [CrossRef]
- Bade, R.; White, J.M.; Nguyen, L.; Tscharke, B.J.; Mueller, J.F.; O’Brien, J.W.; Thomas, K.V.; Gerber, C. Determining changes in new psychoactive substance use in Australia by wastewater analysis. Sci. Total Environ. 2020, 731, 139209. [Google Scholar] [CrossRef]
- Diamanti, K.; Aalizadeh, R.; Alygizakis, N.; Galani, A.; Mardal, M.; Thomaidis, N.S. Wide-scope target and suspect screening methodologies to investigate the occurrence of new psychoactive substances in influent wastewater from Athens. Sci. Total Environ. 2019, 685, 1058–1065. [Google Scholar] [CrossRef]
- Sulej-Suchomska, A.M.; Klupczynska, A.; Dereziński, P.; Matysiak, J.; Przybyłowski, P.; Kokot, Z.J. Urban wastewater analysis as an effective tool for monitoring illegal drugs, including new psychoactive substances, in the Eastern European region. Sci. Rep. 2020, 10, 4885. [Google Scholar] [CrossRef]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Causanilles, A.; Gracia-Lor, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Liquid chromatography-tandem mass spectrometry determination of synthetic cathinones and phenethylamines in influent wastewater of eight European cities. Chemosphere 2017, 168, 1032–1041. [Google Scholar] [CrossRef]
- Kinyua, J.; Covaci, A.; Maho, W.; McCall, A.-K.; Neels, H.; van Nuijs, A.L.N. Sewage-based epidemiology in monitoring the use of new psychoactive substances: Validation and application of an analytical method using LC-MS/MS. Drug Test. Anal. 2015, 7, 812–818. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Castiglioni, S.; Bade, R.; Been, F.; Castrignanò, E.; Covaci, A.; González-Mariño, I.; Hapeshi, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Measuring biomarkers in wastewater as a new source of epidemiological information: Current state and future perspectives. Environ. Int. 2017, 99, 131–150. [Google Scholar] [CrossRef]
- McCall, A.-K.; Bade, R.; Kinyua, J.; Lai, F.Y.; Thai, P.K.; Covaci, A.; Bijlsma, L.; van Nuijs, A.L.N.; Ort, C. Critical review on the stability of illicit drugs in sewers and wastewater samples. Water Res. 2016, 88, 933–947. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Rousis, N.I.; Bade, R.; Bijlsma, L.; Zuccato, E.; Sancho, J.V.; Hernandez, F.; Castiglioni, S. Monitoring a large number of pesticides and transformation products in water samples from Spain and Italy. Environ. Res. 2017, 156, 31–38. [Google Scholar] [CrossRef]
- Gallé, T.; Koehler, C.; Plattes, M.; Pittois, D.; Bayerle, M.; Carafa, R.; Christen, A.; Hansen, J. Large-scale determination of micropollutant elimination from municipal wastewater by passive sampling gives new insights in governing parameters and degradation patterns. Water Res. 2019, 160, 380–393. [Google Scholar] [CrossRef]
- Kramer, R.D.; Filippe, T.C.; Prado, M.R.; de Azevedo, J.C.R. The influence of solid-liquid coefficient in the fate of pharmaceuticals and personal care products in aerobic wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 25515–25525. [Google Scholar] [CrossRef]
- Mardal, M.; Meyer, M.R. Studies on the microbial biotransformation of the novel psychoactive substance methylenedioxypyrovalerone (MDPV) in wastewater by means of liquid chromatography-high resolution mass spectrometry/mass spectrometry. Sci. Total Environ. 2014, 493, 588–595. [Google Scholar] [CrossRef]
- Wick, A.; Wagner, M.; Ternes, T.A. Elucidation of the Transformation Pathway of the Opium Alkaloid Codeine in Biological Wastewater Treatment. Environ. Sci. Technol. 2011, 45, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, A.; Mardal, M.; Linnet, K.; Dalsgaard, P.W. How to perform spectrum-based LC-HR-MS screening for more than 1,000 NPS with HighResNPS consensus fragment ions. PLoS ONE 2020, 15, e0242224. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Schymanski, E.L.; Bletsou, A.A.; Aalizadeh, R.; Hollender, J.; Thomaidis, N.S. Extended Suspect and Non-Target Strategies to Characterize Emerging Polar Organic Contaminants in Raw Wastewater with LC-HRMS/MS. Environ. Sci. Technol. 2015, 49, 12333–12341. [Google Scholar] [CrossRef]
- Aalizadeh, R.; Nika, M.-C.; Thomaidis, N.S. Development and application of retention time prediction models in the suspect and non-target screening of emerging contaminants. J. Hazard. Mater. 2019, 363, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Abbate, V.; Abdelaziz, A.; Nguyen, L.; Trobbiani, S.; Stockham, P.; Elliott, S.; White, J.M.; Gerber, C. The complexities associated with new psychoactive substances in influent wastewater: The case of 4-ethylmethcathinone. Drug Test. Anal. 2020, 12, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.Y.; Erratico, C.; Kinyua, J.; Mueller, J.F.; Covaci, A.; van Nuijs, A.L.N. Liquid chromatography-quadrupole time-of-flight mass spectrometry for screening in vitro drug metabolites in humans: Investigation on seven phenethylamine-based designer drugs. J. Pharm. Biomed. Anal. 2015, 114, 355–375. [Google Scholar] [CrossRef]
- Czerwinska, J.; Jang, M.; Costa, C.; Parkin, M.C.; George, C.; Kicman, A.T.; Bailey, M.J.; Dargan, P.I.; Abbate, V. Detection of mephedrone and its metabolites in fingerprints from a controlled human administration study by liquid chromatography-tandem mass spectrometry and paper spray-mass spectrometry. Analyst 2020, 145, 3038–3048. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Bletsou, A.A.; Damalas, D.E.; Aalizadeh, R.; Alygizakis, N.A.; Singer, H.P.; Hollender, J.; Thomaidis, N.S. Wide-scope target screening of >2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J. Hazard. Mater. 2020, 387, 121712. [Google Scholar] [CrossRef]
- Huntscha, S.; Hofstetter, T.B.; Schymanski, E.L.; Spahr, S.; Hollender, J. Biotransformation of Benzotriazoles: Insights from Transformation Product Identification and Compound-Specific Isotope Analysis. Environ. Sci. Technol. 2014, 48, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man; Biomedical Publications: Seal Beach, CA, USA, 2014. [Google Scholar]
- Ort, C.; van Nuijs, A.L.N.; Berset, J.-D.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; de Voogt, P.; Emke, E.; Fatta-Kassinos, D.; Griffiths, P.; et al. Spatial differences and temporal changes in illicit drug use in Europe quantified by wastewater analysis. Addiction 2014, 109, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Castrignanò, E.; Yang, Z.; Bade, R.; Baz-Lomba, J.A.; Castiglioni, S.; Causanilles, A.; Covaci, A.; Gracia-Lor, E.; Hernandez, F.; Kinyua, J.; et al. Enantiomeric profiling of chiral illicit drugs in a pan-European study. Water Res. 2018, 130. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Gracia-Lor, E.; Rousis, N.I.; Castrignanò, E.; Thomas, K.V.; Quintana, J.B.; Kasprzyk-Hordern, B.; Zuccato, E.; Castiglioni, S. Wastewater-Based Epidemiology To Monitor Synthetic Cathinones Use in Different European Countries. Environ. Sci. Technol. 2016, 50, 10089–10096. [Google Scholar] [CrossRef] [PubMed]
- Fallati, L.; Castiglioni, S.; Galli, P.; Riva, F.; Gracia-Lor, E.; González-Mariño, I.; Rousis, N.I.; Shifah, M.; Messa, M.C.; Strepparava, M.G.; et al. Use of legal and illegal substances in Malé (Republic of Maldives) assessed by wastewater analysis. Sci. Total Environ. 2020, 698, 134207. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Banks, A.; Li, J.; Jiang, G.; Lai, F.Y.; Mueller, J.F.; Thai, P.K. Evaluation of in-sewer transformation of selected illicit drugs and pharmaceutical biomarkers. Sci. Total Environ. 2017, 609, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Linhart, I.; Himl, M.; Židková, M.; Balíková, M.; Lhotková, E.; Páleníček, T. Metabolic profile of mephedrone: Identification of nor-mephedrone conjugates with dicarboxylic acids as a new type of xenobiotic phase II metabolites. Toxicol. Lett. 2016, 240, 114–121. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of Mephedrone and Its Metabolites in Whole Blood and Plasma after Controlled Intranasal Administration to Healthy Human Volunteers. J. Anal. Toxicol. 2020, 14, 5. [Google Scholar] [CrossRef]
- Beretsou, V.G.; Psoma, A.K.; Gago-Ferrero, P.; Aalizadeh, R.; Fenner, K.; Thomaidis, N.S. Identification of biotransformation products of citalopram formed in activated sludge. Water Res. 2016, 103, 205–214. [Google Scholar] [CrossRef]
- Staack, R.; Fehn, J.; Maurer, H. New designer drug p-methoxymethamphetamine: Studies on its metabolism and toxicological detection in urine using gas chromatography–mass spectrometry1. J. Chromatogr. B 2003, 789, 27–41. [Google Scholar] [CrossRef]
| Compound | Retention Time (min.) | Measured m/z [M + H]+ | Chemical Formula | Mass Error (Δm, ppm) | Diagnostic Product Ions (m/z) | Id. Level * |
|---|---|---|---|---|---|---|
| PMMA | 4.38 | 180.1376 | [C11H18NO]+ | −3.89 | 149.0953, 121.0650, 109.0661, 91.0544 | 1 |
| TP-166 | 3.01 | 166.1215 | [C10H16NO]+ | −6.62 | 135.0797, 107.0491 | 2a |
| TP-196a | 3.03 | 196.1317 | [C11H17NO2]+ | −7.65 | MS/MS spectra not obtained | 4 |
| TP-196b | 3.53 | 196.1324 | [C11H17NO2]+ | −4.08 | MS/MS spectra not obtained | 4 |
| TP-196c | 3.93 | 196.1323 | [C11H17NO2]+ | −4.59 | 178.1216, 163.0981, 148.0736, 121.0654 | 3 |
| Compound | Retention Time (min.) | Measured m/z [M + H]+ | Chemical Formula | Mass Error (Δm, ppm) | Diagnostic Product Ions (m/z) | Id. Level * |
|---|---|---|---|---|---|---|
| DHM | 4.64 | 180.1382 | [C11H18NO]+ | −0.56 | 162.1286, 147.1047, 131.0861 | 1 |
| TP-210 | 1.82 | 210.1130 | [C11H16NO3]+ | 2.38 | 192.1011, 177.0722, 161.0577 | 3 |
| TP-178 (mephedrone) | 4.64 | 178.1212 | [C11H16NO]+ | −7.86 | 162.1269, 145.0883, 132.0826 | 1 |
| TP-166 | 4.49 | 166.1228 | [C10H16NO]+ | 1.20 | 148.112, 131.0848, 116.063 | 2b |
| TP-164 | 6.50 | 164.1071 | [C10H14NO]+ | 0.61 | 146.0968, 131.0727, 120.0798 | 2b |
| TP-192a | 5.10 | 192.1024 | [C11H14NO2]+ | 2.60 | 148.1112, 135.0432, 119.049 | 3 |
| TP-192b | 6.15 | 192.1022 | [C11H14NO2]+ | 1.56 | 148.1113, 135.0435, 119.0482 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinyua, J.; Psoma, A.K.; Rousis, N.I.; Nika, M.-C.; Covaci, A.; van Nuijs, A.L.N.; Τhomaidis, Ν.S. Investigation of Biotransformation Products of p-Methoxymethylamphetamine and Dihydromephedrone in Wastewater by High-Resolution Mass Spectrometry. Metabolites 2021, 11, 66. https://doi.org/10.3390/metabo11020066
Kinyua J, Psoma AK, Rousis NI, Nika M-C, Covaci A, van Nuijs ALN, Τhomaidis ΝS. Investigation of Biotransformation Products of p-Methoxymethylamphetamine and Dihydromephedrone in Wastewater by High-Resolution Mass Spectrometry. Metabolites. 2021; 11(2):66. https://doi.org/10.3390/metabo11020066
Chicago/Turabian StyleKinyua, Juliet, Aikaterini K. Psoma, Nikolaos I. Rousis, Maria-Christina Nika, Adrian Covaci, Alexander L. N. van Nuijs, and Νikolaos S. Τhomaidis. 2021. "Investigation of Biotransformation Products of p-Methoxymethylamphetamine and Dihydromephedrone in Wastewater by High-Resolution Mass Spectrometry" Metabolites 11, no. 2: 66. https://doi.org/10.3390/metabo11020066
APA StyleKinyua, J., Psoma, A. K., Rousis, N. I., Nika, M.-C., Covaci, A., van Nuijs, A. L. N., & Τhomaidis, Ν. S. (2021). Investigation of Biotransformation Products of p-Methoxymethylamphetamine and Dihydromephedrone in Wastewater by High-Resolution Mass Spectrometry. Metabolites, 11(2), 66. https://doi.org/10.3390/metabo11020066