Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
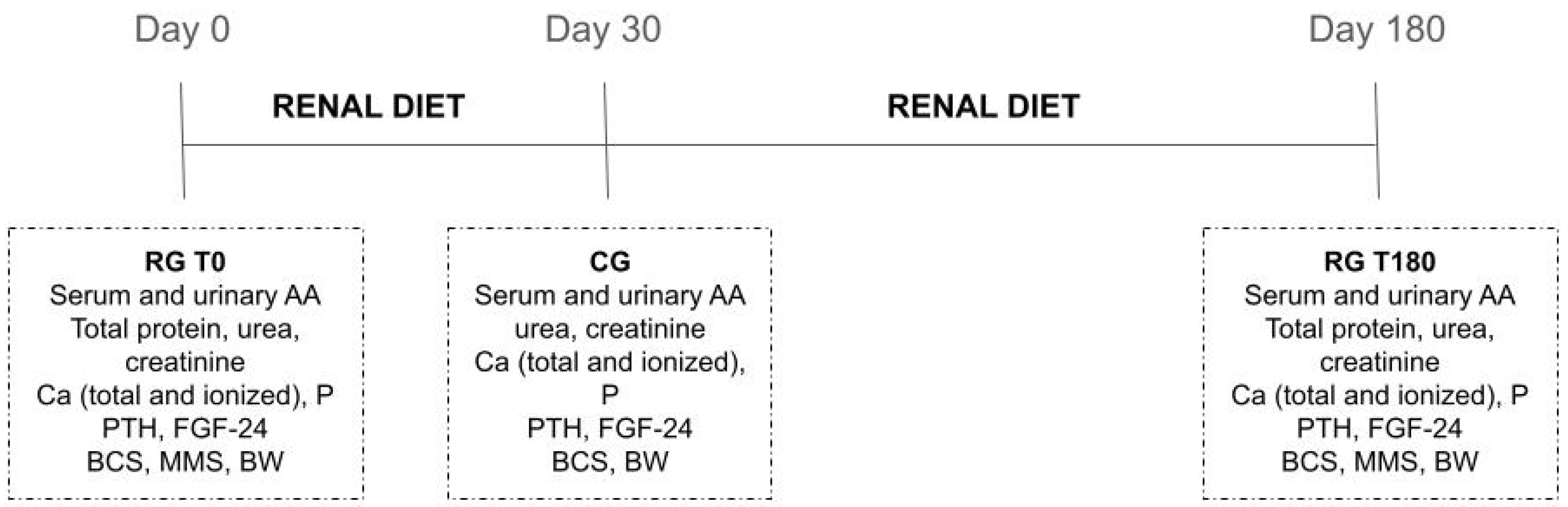
4.1. Animals and Study Site
4.2. Diet
4.3. Sample Collection and AA Determination
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polzin, D.J. Chronic Kidney Disease. In Textbook of Veterinary Internal Medicine; Ettinger, S.J., Feldman, E., Eds.; Saunders: St. Louis, MO, USA, 2010; pp. 2036–2067. [Google Scholar]
- Polzin, D.J. Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am.-Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Fritsch, M.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS-Stage 1 chronic kidney disease. J. Anim. Physiol. Anim. Nutr. 2018, 102, 297–307. [Google Scholar] [CrossRef]
- Hansen, B.; Dibartola, S.P.; Chew, D.J.; Brownie, C.; Berrie, H.K. Amino acid profiles in dogs with chronic renal failure fed two diets. Am. J. Vet. Res. 1992, 53, 335–341. [Google Scholar] [PubMed]
- Parker, V.J.; Fascetti, A.J.; Klamer, B.G. Amino acid status in dogs with protein-losing nephropathy. J. Vet. Intern. Med. 2019, 33, 680–685. [Google Scholar] [CrossRef]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- IRIS IRIS Staging of CKD. Available online: http://www.iris-kidney.com/guidelines/ (accessed on 6 March 2020).
- Pedrinelli, V.; Lima, D.M.; Duarte, C.N.; Teixeira, F.A.; Porsani, M.Y.H.; Zarif, C.; Amaral, A.R.; Vendramini, T.H.A.; Kogika, M.M.; Brunetto, M.A. Nutritional and laboratory parameters affect the survival of dogs with chronic kidney disease. PLoS ONE 2020, 15, e0234712. [Google Scholar] [CrossRef]
- Jacob, F.; Polzin, D.J.; Osborne, C.A.; Allen, T.A.; Kirk, C.A.; Neaton, J.D.; Lekcharoensuk, C.; Swanson, L.L. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J. Am. Vet. Med. Assoc. 2002, 220, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. N. Am. 2012, 42, 669–692. [Google Scholar] [CrossRef]
- Schefold, J.C.; Zeden, J.P.; Fotopoulou, C.; Von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transpl. 2009, 24, 1901–1908. [Google Scholar] [CrossRef]
- Peters, J.C. Tryptophan nutrition and metabolism: An overview. Adv. Exp. Med. Biol. 1991, 294, 345–358. [Google Scholar]
- Young, G.A. Amino acids and the kidney. Amino Acids 1991, 1, 183–192. [Google Scholar] [CrossRef]
- Trachtman, H.; Sturman, J.A. Taurine: A therapeutic agent in experimental kidney disease. Amino Acids 1996, 11, 1–13. [Google Scholar] [CrossRef]
- Van Guldener, C.; Stehouwer, C.D.A. Homocysteine and methionine metabolism in renal failure. Semin. Vasc. Med. 2005, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.C.; Sjoedsma, A. Clinical significance of a hydroxyproline-containing protein in human plasma. J. Clin. Investig. 1965, 44, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. Abnormal amino acid and protein metabolism in uremia. Kidney Int. 1978, 14, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Dubovský, J.; Pacovský, V.; Dubovska, E. Plasma hydroxyproline and proline in primary hyperparathyroidism and in chronic uremia. Clin. Chim. Acta 1965, 12, 230–232. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Berg, R.L.; Kopple, J.D.; Naito, H.; Walker, W.G.; Walser, M. Patterns of Fasting Plasma Amino Acid Levels in Chronic Renal Insufficiency: Results From the Feasibility Phase of the Modification of Diet in Renal Disease Study. Am. J. Kidney Dis. 1994, 23, 504–513. [Google Scholar] [CrossRef]
- Busque, S.M.; Wagner, C.A. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am. J. Physiol.-Ren. Physiol. 2009, 297, 440–450. [Google Scholar] [CrossRef]
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early alterations of plasma free amino acids in chronic renal failure. Clin. Chim. Acta 1990, 188, 101–108. [Google Scholar] [CrossRef]
- Mak, R.H.K. Effect of metabolic acidosis on branched-chain amino acids in uremia. Pediatr. Nephrol. 1999, 13, 319–322. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L.; Luciani, G. Mechanisms and treatment of anorexia in end-stage renal disease patients on hemodialysis. J. Ren. Nutr. 2009, 19, 2–9. [Google Scholar] [CrossRef]
- Van De Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Young, G.A.; Parsons, F.M. The effect of peritoneal dialysis upon the amino acids and other nitrogenous compounds in the blood and dialysates from patients with renal failure. Clin. Sci. 1969, 37, 1–10. [Google Scholar]
- Gulyassy, P.F.; Aviram, A.; Peters, J.H. Evaluation of Amino Acid and Protein Requirements in Chronic Uremia. Arch. Intern. Med. 1970, 126, 855–859. [Google Scholar] [CrossRef]
- Wang, M.; Vyhmeister, I.; Swendseid, M.E.; Kopple, J.D. Phenylalanine Hydroxylase and Tyrosine Aminotransferase Activities in Chronically Uremic Rats. J. Nutr. 1975, 105, 122–127. [Google Scholar] [CrossRef]
- Tizianello, A.; de Ferrari, G.; Garibotto, G.; Gurreri, G.; Robaudo, C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J. Clin. Investig. 1980, 65, 1162–1172. [Google Scholar] [CrossRef]
- Kopple, J.D. Amino acid metabolism in chronic renal failure. In Amino Acids-Metabolism and Medical Applications; Blackburn, G.L., Grant, J.P., Young, V.R., Wright, J., Eds.; John Wright-PSG Inc.: Boston, MA, USA, 1983; pp. 327–332. [Google Scholar]
- Fukuda, S.; Kopple, J.D. Chronic uremia syndrome in dogs induced with uranyl nitrate. Nephron 1980, 25, 139–143. [Google Scholar] [CrossRef]
- Chen, G.F.; Baylis, C. In vivo renal arginine release is impaired throughout development of chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2010, 298, 95–102. [Google Scholar] [CrossRef]
- Perfumo, F.; Verrina, E.; Romano, C.; Cerone, R.; Caruso, U.; Gusmano, R. Plasma amino acids in dialyzed children: Comparison of hemodialysis and continuous ambulatory peritoneal dialysis. Pediatr. Med. Chir. 1986, 8, 341–345. [Google Scholar] [PubMed]
- Canepa, A.; Divino Filho, J.C.; Gutierrez, A.; Carrea, A.; Forsberg, A.M.; Nilsson, E.; Verrina, E.; Perfumo, F.; Bergström, J. Free amino acids in plasma, red blood cells, polymorphonuclear leukocytes, and muscle in normal and uraemic children. Nephrol. Dial. Transplant. 2002, 17, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Qureshi, A.R.; Stenvinkel, P.; Pecoits-Filho, R.; Bárány, P.; Heimbürger, O.; Anderstam, B.; Ayala, E.R.; Divino Filho, J.C.; Alvestrand, A.; et al. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am. J. Clin. Nutr. 2005, 82, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Alvestrand, A.; Fürst, P. Plasma and muscle free amino acids in maintenance hemodialysis patients without protein malnutrition. Kidney Int. 1990, 38, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Divino Filho, J.C.; Bârâny, P.; Stehle, P.; Fürst, P.; Bergström, J. Free amino-acid levels simultaneously collected in plasma, muscle, and erythrocytes of uraemic patients. Nephrol. Dial. Transpl. 1997, 12, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006; ISBN 0309086280.
- FEDIAF—European Pet Food Industry Federation. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; FEDIAF: Brussels, Belgium, 2019. [Google Scholar]
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Michel, K.E.; Anderson, W.M.; Cupp, C.J.; Laflamme, D.P. Correlation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometry. Brit. J. Nutr. 2011, 106, S57–S59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, J.A.; Hart, R.J.; Fry, J.C. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Autom. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef]
| Variables | Treatments | RG T0 vs. RG T180 | CG T30 vs. RG T180 | ||||
|---|---|---|---|---|---|---|---|
| CG T30 | RG T0 | RG T180 | p | SEM 2 | p | SEM 2 | |
| Urea (mg/dL) | 39.29 | 206.07 | 235.34 | 0.1871 | 13.75 | 0.0002 1 | - |
| Creatinine (mg/dL) | 1.12 | 3.11 | 4.30 | 0.0022 | 0.34 | <0.0001 | 0.45 |
| Total calcium (mg/dL) | 10.73 | 11.33 | 11.94 | 0.3129 | 0.34 | 0.0847 | 0.34 |
| Ionized calcium (mmol/L) | 1.41 | 1.41 | 1.38 | 0.2327 | 0.2 | 0.3442 | 0.02 |
| Phosphorus (mg/dL) | 3.34 | 5.15 | 5.36 | 0.6309 | 0.28 | <0.0001 | 0.30 |
| Parathormone (pg/mL) | - | 145.81 | 336.48 | 0.2077 | 70.62 | - | - |
| FGF-23 (pg/mL) | - | 5645.67 | 5788.56 | 0.8580 | 1157.67 | - | - |
| Variables | Treatments | RG T0 vs. RG T180 | ||
|---|---|---|---|---|
| RG T0 | RG T180 | p | SEM 2 | |
| Total protein (g/dL) | 6.12 | 6.17 | 0.7772 | 0.12 |
| Albumin (g/dL) | 3.23 | 3.18 | 1.0000 1 | 0.06 |
| BCS | 5.70 | 5.30 | 0.3750 | 0.20 |
| MMS | 2.20 | 2.40 | 0.6250 1 | 0.10 |
| Body weight (kg) | 16.34 | 14.78 | 0.0453 | 3.05 |
| Variables | Serum | Urine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CG T30 1 | RG T0 2 | RG T180 3 | p RGT0 vs. RG T180 | p RG T180 vs. CG T30 | CG T30 1 | RG T0 2 | RG T180 3 | p RG T0 vs. RG T180 | p RG T180 vs. CG T30 | |
| Essential Amino Acids (µmol/L) | ||||||||||
| Arginine | 367.78 ± 40.67 | 165.98 ± 24.06 | 153.36 ± 24.06 | 0.435 | <0.0001 | 36.73 ± 6.51 | 31.75 ± 24.52 | 27.12 ± 12.70 | 0.568 | 0.284 |
| Histidine | 53.68 ± 6.25 | 65.02 ± 10.38 | 74.77 ± 15.64 | 0.079 | 0.001 | 8.20 ± 3.09 | 49.20 ± 52.41 | 56.05 ± 90.16 | 0.842 | 0.165 |
| Isoleucine | 15.76 ± 1.14 | 44.11 ± 12.97 | 45.83 ± 14.56 | 0.750 | <0.0001 | 1.34 ± 0.59 | 23.17 ± 10.16 | 33.84 ± 13.84 | 0.049 | <0.0001 |
| Leucine | 16.62 ± 2.79 | 87.65 ± 28.54 | 86.22 ± 25.39 | 0.893 | <0.0001 | 1.27 ± 0.24 | 16.82 ± 11.62 | 21.08 ± 33.26 | 0.659 | 0.069 |
| Lysine | 33.34 ± 3.44 | 107.27 ± 27.14 | 110.51 ± 32.39 | 0.782 | <0.0001 | 9.68 ± 1.28 | 32.84 ± 44.86 | 28.38 ± 19.56 | 0.751 | 0.233 |
| Methionine | 51.31 ± 11.45 | 44.79 ± 10.38 | 41.57 ± 9.92 | 0.500 | 0.071 | 6.73 ± 3.97 | 22.04 ± 19.58 | 23.20 ± 20.71 | 0.899 | 0.879 |
| Phenylalanine | 1.82 ± 0.90 | 76.64 ± 10.06 | 66.23 ± 8.37 | 0.007 | <0.0001 | 1.74 ± 1.37 | 18.49 ± 13.25 | 27.89 ± 21.08 | 0.202 | 0.0028 |
| Threonine | 117.68 ± 46.39 | 150.57 ± 38.02 | 164.85 ± 82.04 | 0.599 | 0.123 | 13.21 ± 9.45 | 92.13 ± 146.79 | 35.99 ± 17.98 | 0.223 | 0.646 |
| Tryptophan | 10.27 ± 41.64 | 199.59 ± 30.00 | 150.66 ± 2.11 | 0.001 | <0.0001 | 3.85 ± 2.51 | 78.25 ± 24.06 | 82.96 ± 23.48 | 0.614 | <0.0001 |
| Valine | 133.33 ± 12.44 | 113.67 ± 32.56 | 122.68 ± 29.41 | 0.472 | 0.440 | 3.00 ± 1.17 | 14.77 ± 23.26 | 18.55 ± 35.97 | 0.759 | 0.245 |
| Non-Essential Amino Acids (µmol/L) | ||||||||||
| Alanine | 322.79 ± 92.36 | 351.64 ± 123.89 | 361.34 ± 178.00 | 0.878 | 0.583 | 23.36 ± 5.19 | 157.17 ± 201.62 | 115.97 ± 75.83 | 0.527 | 0.189 |
| Asparagine | 100.97 ± 22.45 | 70.45 ± 22.45 | 72.30 ± 18.27 | 0.832 | 0.006 | 23.94 ± 4.97 | 53.19 ± 43.73 | 56.92 ± 45.18 | 0.837 | 0.107 |
| Aspartate | 15.05 ± 14.89 | 7.31 ± 3.76 | 6.25 ± 1.15 | 0.763 | 0.031 | 4.68 ± 0.64 | 19.45 ± 14.43 | 18.13 ± 12.08 | 0.800 | 0.026 |
| Cysteine | 1.28 ± 0.13 | 7.65 ± 3.46 | 7.90 ± 5.11 | 0.884 | 0.001 | 1.36 ± 0.75 | 15.44 ± 9.30 | 11.62 ± 6.28 | 0.278 | 0.011 |
| Citrulline | 10.64 ± 1.84 | 95.47 ± 21.55 | 117.23 ± 44.62 | 0.130 | <0.0001 | 40.03 ± 9.92 | Not detected | Not detected | - | - |
| Glycine | 225.57 ± 60.48 | 253.75 ± 98.37 | 247.49 ± 114.45 | 0.886 | 0.651 | 22.04 ± 9.35 | 157.05 ± 181.38 | 96.37 ± 93.60 | 0.288 | 0.239 |
| Glutamate | 32.61 ± 9.27 | 27.59 ± 8.10 | 25.22 ± 6.24 | 0.502 | 0.066 | 6.76 ± 1.75 | 17.67 ± 12.26 | 14.82 ± 19.84 | 0.721 | 0.348 |
| Glutamine | 727.71 ± 111.12 | 506.40 ± 87.97 | 547.11 ± 161.42 | 0.475 | 0.007 | 33.83 ± 23.18 | 167.49 ± 261.62 | 116.65 ± 123.53 | 0.528 | 0.353 |
| Hydroxyproline | 11.27 ± 3.54 | 32.75 ± 22.51 | 16.35 ± 6.85 | 0.018 | 0.484 | 15.73 ± 5.49 | 53.09 ± 71.81 | 37.30 ± 14.50 | 0.460 | 0.359 |
| Ornithine | 6.39 ± 1.13 | 17.72 ± 5.95 | 15.15 ± 4.20 | 0.213 | 0.001 | 3.15 ± 0.55 | 9.24 ± 8.00 | 8.46 ± 3.49 | 0.780 | 0.081 |
| Proline | 127.03 ± 15.41 | 164.83 ± 73.24 | 133.27 ± 37.40 | 0.178 | 0.806 | 5.10 ± 1.92 | 91.89 ± 81.76 | 39.04 ± 30.97 | 0.095 | 0.235 |
| Serine | 124.98 ± 66.79 | 80.11 ± 30.26 | 74.92 ± 16.55 | 0.771 | 0.016 | 43.01 ± 12.44 | 94.36 ± 69.32 | 88.85 ± 59.98 | 0.829 | 0.112 |
| Taurine | 49.38 ± 186.50 | 333.30 ± 116.91 | 236.06 ± 17.64 | 0.120 | 0.009 | 1764.26 ± 381.01 | 2404.53 ± 1605.12 | 620.51 ± 919.43 | 0.003 | 0.057 |
| Tyrosine | 39.02 ± 9.85 | 33.11 ± 10.62 | 28.41 ± 6.08 | 0.252 | 0.024 | 2.40 ± 1.69 | 12.89 ± 14.40 | 8.28 ± 7.37 | 0.349 | 0.263 |
| BCAA 4 | 165.71 ± 11.09 | 254.44 ±72.86 | 254.74 ± 67.68 | 0.736 | 0.006 | 5.43 ± 1.95 | 46.66 ± 3.57 | 65.30 ± 72.82 | 0.465 | 0.023 |
| Total EAA 5 | 801.59 ± 77.76 | 1055.29 ± 170.79 | 1016.69 ± 163.89 | 0.570 | 0.007 | 85.60 ± 21.00 | 347.09 ± 308.55 | 280.09 ± 171.54 | 0.495 | 0.080 |
| Total NEAA 6 | 1755.67 ± 227.14 | 1948.98 ± 520.03 | 1860.58 ± 457.51 | 0.656 | 0.632 | 1987.32 ± 419.52 | 2935.81 ± 1756.05 | 1097.30 ± 934.51 | 0.002 | 0.157 |
| Amino Acids Ratio | ||||||||||
| EAA 5/NEAA 6 | 0.46 ± 0.04 | 0.56 ± 0.09 | 0.57 ± 0.13 | 0.853 | 0.032 | 0.04 ± 0.01 | 0.17 ± 0.14 | 0.42 ± 0.32 | 0.012 | 0.001 |
| Valine/Glycine | 0.63 ± 0.22 | 0.50 ± 0.18 | 0.59 ± 0.31 | 0.391 | 0.735 | 0.15 ± 0.05 | 0.09 ± 0.08 | 0.14 ± 0.12 | 0.220 | 0.980 |
| Arginine/Citrulline | 29.05 ± 3.66 | 1.80 ± 0.57 | 1.48 ± 0.59 | 0.694 | <0.0001 | 0.95 ± 0.25 | - | - | - | - |
| Tyrosine/Phenylalanine | 36.94 ± 47.07 | 0.43 ± 0.11 | 0.43 ± 0.08 | 1.000 | 0.004 | 2.93 ± 1.92 | 0.68 ± 0.33 | 1.03 ± 1.66 | 0.011 | 0.062 |
| Serine/Glycine | 0.65 ± 0.59 | 0.32 ± 0.07 | 0.33 ± 0.07 | 0.966 | 0.041 | 2.05 ± 0.37 | 1.12 ± 0.97 | 1.47 ± 1.55 | 0.109 | 0.310 |
| Nutrient | Diet | NRC | FEDIAF |
|---|---|---|---|
| Dry matter (%) | 90.00 | - | - |
| Crude protein (%) | 14.50 | 10.00 | 21.00 |
| Fat (%) | 18.00 | 5.50 | 5.50 |
| Ash (%) | 5.50 | - | - |
| Crude fiber (%) | 3.50 | - | - |
| Calcium (mg/kg) | 0.50 | 0.40 | 0.58 |
| Phosphorus (mg/kg) | 0.30 | 0.30 | 0.46 |
| Essential Amino Acids (g/100 g) | |||
| Arginine | 0.93 | 0.35 | 0.60 |
| Phenylalanine | 0.63 | 0.45 | 0.63 |
| Histidine | 0.34 | 0.19 | 0.27 |
| Isoleucine | 0.60 | 0.38 | 0.53 |
| Leucine | 1.08 | 0.68 | 0.95 |
| Lysine | 0.90 | 0.35 | 0.46 |
| Methionine | 0.65 | 0.33 | 0.46 |
| Threonine | 0.49 | 0.43 | 0.60 |
| Tryptophan | 0.17 | 0.14 | 0.20 |
| Valine | 0.75 | 0.49 | 0.68 |
| Non-Essential Amino Acids (g/100 g) | |||
| Alanine | 1.07 | - | - |
| Aspartate | 1.25 | - | - |
| Cysteine | 0.07 | - | - |
| Glycine | 1.02 | - | - |
| Glutamate | 2.13 | - | - |
| Proline | 0.52 | - | - |
| Serine | 0.76 | - | - |
| Taurine | - | - | - |
| Metabolizable energy (Kcal/g) | 4.072 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetto, M.A.; Halfen, D.P.; Risolia, L.W.; Pedrinelli, V.; Caragelasco, D.S.; Vendramini, T.H.A.; de Carvalho Balieiro, J.C.; Pontieri, C.F.F.; Jeremias, J.T.; Ruberti, B.; et al. Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet. Metabolites 2021, 11, 844. https://doi.org/10.3390/metabo11120844
Brunetto MA, Halfen DP, Risolia LW, Pedrinelli V, Caragelasco DS, Vendramini THA, de Carvalho Balieiro JC, Pontieri CFF, Jeremias JT, Ruberti B, et al. Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet. Metabolites. 2021; 11(12):844. https://doi.org/10.3390/metabo11120844
Chicago/Turabian StyleBrunetto, Marcio Antonio, Doris Pereira Halfen, Larissa Wunsche Risolia, Vivian Pedrinelli, Douglas Segalla Caragelasco, Thiago Henrique Annibale Vendramini, Julio César de Carvalho Balieiro, Cristiana Fonseca Ferreira Pontieri, Juliana Toloi Jeremias, Bruna Ruberti, and et al. 2021. "Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet" Metabolites 11, no. 12: 844. https://doi.org/10.3390/metabo11120844
APA StyleBrunetto, M. A., Halfen, D. P., Risolia, L. W., Pedrinelli, V., Caragelasco, D. S., Vendramini, T. H. A., de Carvalho Balieiro, J. C., Pontieri, C. F. F., Jeremias, J. T., Ruberti, B., & Kogika, M. M. (2021). Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet. Metabolites, 11(12), 844. https://doi.org/10.3390/metabo11120844






