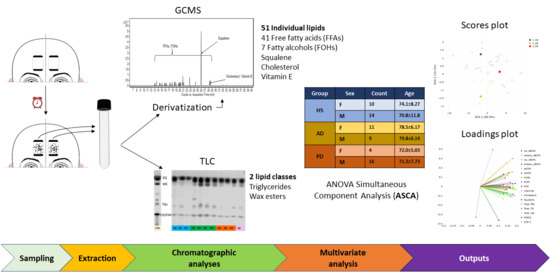
Application of Sebum Lipidomics to Biomarkers Discovery in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Results
2.1. Amounts of Lipid Species in the Sebum of HS, AD and PD Subjects
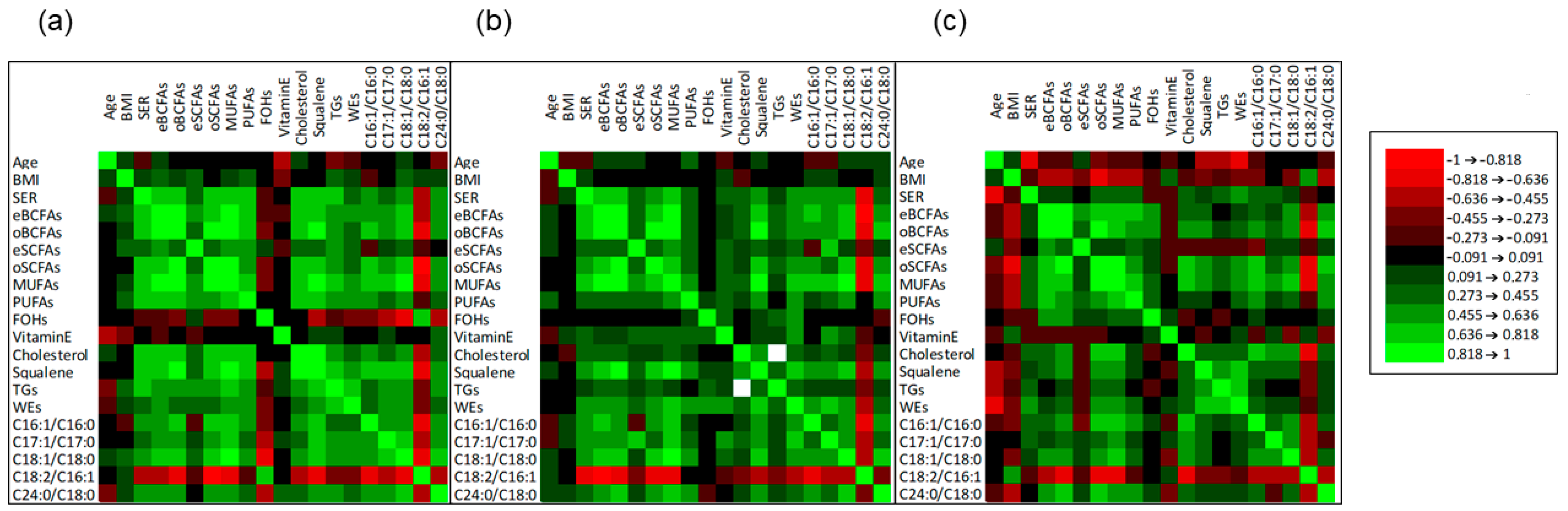
2.2. Correlation among Age, BMI, SER, and Sebum Lipid Components
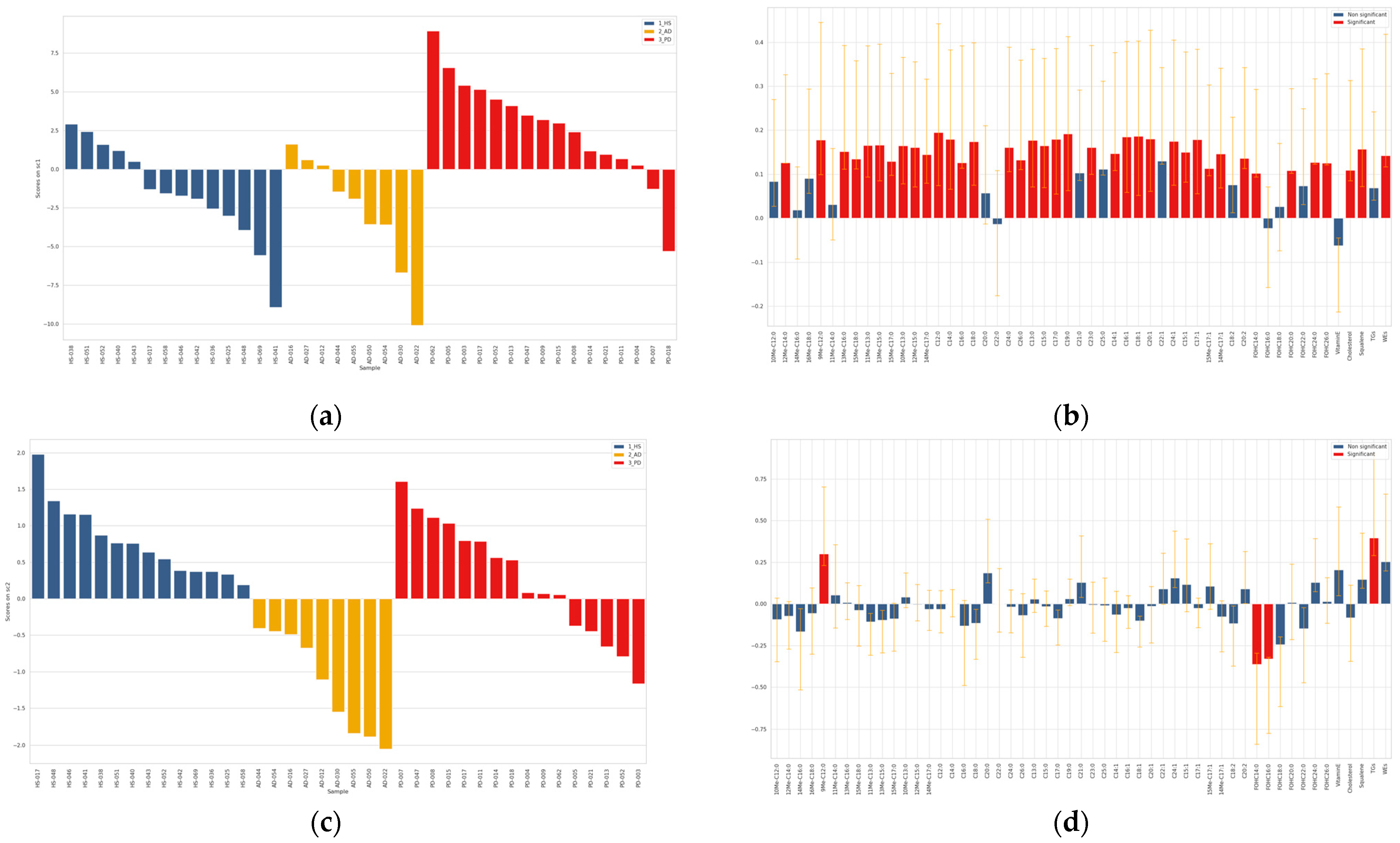
2.3. Chemometric Discrimination of Sebum in AD and PD from That of HS
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Sebum Collection
4.3. Materials, Chemicals, and Reagents
4.4. Sample Preparation and Sebum Analysis
4.5. Sebum Lipid Profiling
4.6. Data Analysis and Chemometric Modelling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avila, J.; Perry, G. A Multilevel View of the Development of Alzheimer’s Disease. Neuroscience 2020, 457, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A. The future burden of Parkinson’s disease. Mov. Disord. 2018, 33, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Ulane, C.M.; Burke, R. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef]
- Blennow, K. A Review of Fluid Biomarkers for Alzheimer’s Disease: Moving from CSF to Blood. Neurol. Ther. 2017, 6, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Paolillo, C.; Ferretti, G.; De Carlo, A.; Angiolillo, A.; Nasso, R.; Caputo, M.; Matrone, C.; Di Costanzo, A.; Corso, G. Challenges in LC–MS-based metabolomics for Alzheimer’s disease early detection: Targeted approaches versus untargeted approaches. Metabolomics 2021, 17, 78. [Google Scholar] [CrossRef]
- Calvano, C.D.; Palmisano, F.; Cataldi, T.R. Understanding neurodegenerative disorders by MS-based lipidomics. Bioanalysis 2018, 10, 787–790. [Google Scholar] [CrossRef]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G.; et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer’s Dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.V.; Savica, R.; Deuschle, C.; Gasser, T.; Hauser, A.K.; Graber-Sultan, S.; Schleicher, E.; et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: A pilot study. PLoS ONE 2013, 8, e73094. [Google Scholar]
- Chan, R.B.; Perotte, A.; Zhou, B.; Liong, C.; Shorr, E.J.; Marder, K.S.; Kang, U.J.; Waters, C.H.; Levy, O.A.; Xu, Y.; et al. Elevated GM3 plasma concentration in idiopathic Parkinson’s disease: A lipidomic analysis. PLoS ONE 2017, 12, e0172348. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanning, S.; Selkoe, D.; Dettmer, U. Parkinson’s disease: Proteinopathy or lipidopathy? NPJ Parkinsons Dis. 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proitsi, P.; Kim, M.; Whiley, L.; Pritchard, M.R.; Leung, R.; Soininen, H.; Kloszewska, I.; Mecocci, P.; Tsolaki, M.; Vellas, B.; et al. Plasma lipidomics analysis finds long chain cholesteryl esters to be associated with Alzheimer’s disease. Transl. Psychiatry 2015, 5, e494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.W.; Braidy, N.; Poljak, A.; Sachdev, P.S. The application of lipidomics to biomarker research and pathomecha-nisms in Alzheimer’s disease. Curr. Opin. Psychiatry. 2017, 30, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.; Miller, S. Parkinson’s disease and the skin. Pract. Neurol. 2015, 15, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Niemann, N.; Billnitzer, A.; Jankovic, J. Parkinson’s disease and skin. Parkinsonism Relat. Disord. 2021, 82, 61–76. [Google Scholar] [CrossRef]
- Arsic Arsenijevic, V.S.; Milobratovic, D.; Barac, A.M.; Vekic, B.; Marinkovic, J.; Kostic, V.S. A laboratory-based study on patients with Parkinson’s disease and seborrheic dermatitis: The presence and density of Malassezia yeasts, their different species and enzymes production. BMC Dermatol. 2014, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Antunes, I.; Purim, K.S.M.; Grande, L.L.; Alberton, N.C.; Navarro, T.F.R.; Winckler, T.C.D. Dermatoses in parkin-sonism: The importance of multidisciplinary follow-up. Rev. Assoc. Med. Bras. 2019, 65, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, D.K.; Sinclair, E.; Xu, Y.; Sarkar, D.; Walton-Doyle, C.; Liscio, C.; Banks, P.; Milne, J.; Silverdale, M.; Kunath, T.; et al. Discovery of Volatile Biomarkers of Parkinson’s Disease from Sebum. ACS Central Sci. 2019, 5, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, E.; Walton-Doyle, C.; Sarkar, D.; Hollywood, K.A.; Milne, J.; Lim, S.H.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Silverdale, M.; et al. Validating Differential Volatilome Profiles in Parkinson’s Disease. ACS Central Sci. 2021, 7, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef]
- Michelerio, A.; Tomasini, C.F. The Alzheimer patient from the dermatologist’s point of view. G. Ital. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, W.; Jiang, L.; Zhou, W.; Liu, Z.; Li, S.; Lu, H. Overexpression of Amyloid Precursor Protein Promotes the Onset of Seborrhoeic Keratosis and is Related to Skin Ageing. Acta Derm. Venereol. 2018, 98, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Martignoni, E.; Godi, L.; Pacchetti, C.; Berardesca, E.; Vignoli, G.P.; Albani, G.; Mancini, F.; Nappi, G. Is seborrhea a sign of autonomic impairment in Parkinson’s disease? J. Neural Transm. 1997, 104, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.-H.R.; Thyssen, J.P.; Egeberg, A. Skin disorders in Parkinson’s disease: Potential biomarkers and risk factors. Clin. Cosmet. Investig. Dermatol. 2017, 10, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.; Billings, J.K.; Frantz, R.A.; Kinney, C.K.; Stewart, M.E.; Downing, D.T. Age-Related Changes in Sebaceous Wax Ester Secretion Rates in Men and Women. J. Investig. Dermatol. 1985, 85, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Makrantonaki, E.; Adjaye, J.; Herwig, R.; Brink, T.C.; Groth, D.; Hultschig, C.; Lehrach, H.; Zouboulis, C.P.D. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell 2006, 5, 331–344. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. The skin as a mirror of the aging process in the human organism--state of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2). Exp. Gerontol. 2007, 42, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Nitkowska, M.; Tomasiuk, R.; Czyzyk, M.; Friedman, A. Prolactin and sex hormones levels in males with Parkinson’s disease. Acta Neurol. Scand. 2015, 131, 411–416. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; He, Y.; Huang, D. Lipidomics demonstrates the association of sex hormones with sebum. J. Cosmet. Dermatol. 2021, 20, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Sim, D.; O’Donnell-Megaro, A.M.; Bauman, D.E.; Barbano, D.M.; Brenna, J.T. Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 2011, 46, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.; Brenna, J.T. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Paris, D.; Melck, D.; Angiolillo, A.; Corso, G.; Maniscalco, M.; Motta, A. Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Sci. Rep. 2020, 10, 15612. [Google Scholar] [CrossRef]
- Fabelo, N.; Martín, M.V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe Alterations in Lipid Composition of Frontal Cortex Lipid Rafts from Parkinson’s Disease and Incidental Parkinson’s Disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.L.M.; Picardo, M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J. Lipid Res. 2010, 51, 3377–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, A. Epidermal surface lipids. Dermato-Endocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Camera, E.; Picardo, M. Lipids in Serum and Sebum. In Pathogenesis and Treatment of Acne and Rosacea; Zouboulis, C., Katsambas, A., Kligman, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 305–313. [Google Scholar]
- Ludovici, M.; Kozul, N.; Materazzi, S.; Risoluti, R.; Picardo, M.; Camera, E. Influence of the sebaceous gland density on the stratum corneum lipidome. Sci. Rep. 2018, 8, 11500. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.J.; Kydonieus, A. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 176–187. [Google Scholar] [CrossRef]
- Drake, D.R.; Brogden, K.A.; Dawson, D.V.; Wertz, P.W. Thematic Review Series: Skin Lipids. Antimicrobial lipids at the skin surface. J. Lipid Res. 2008, 49, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermato Endocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Park, H.G.; Wang, D.; Kitano, R.; Kothapalli, K.S.; Brenna, J.T. Fatty acid desaturase 2 (FADS2) but not FADS1 desaturates branched chain and odd chain saturated fatty acids. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1865, 158572. [Google Scholar] [CrossRef] [PubMed]
- Okoro, O.E.; Adenle, A.; Ludovici, M.; Truglio, M.; Marini, F.; Camera, E. Lipidomics of facial sebum in the comparison between acne and non-acne adolescents with dark skin. Sci. Rep. 2021. [Google Scholar] [CrossRef]
- Lovászi, M.; Mattii, M.; Eyerich, S.; Gácsi, A.; Csányi, E.; Kovács, D.; Rühl, R.; Szegedi, A.; Kemény, L.; Ståhle, M.; et al. Sebum lipids influence macrophage polarization and activation. Br. J. Dermatol. 2017, 177, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Downing, D.T.; Stewart, M.E.; Strauss, J.S. Estimation of Sebum Production Rates in Man by Measurement of the Squalene Content of Skin Biopsies. J. Investig. Dermatol. 1981, 77, 358–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouboulis, C.C.; Baron, J.; Böhm, M.; Kippenberger, S.; Kurzen, H.; Reichrath, J.; Thielitz, A. Frontiers in sebaceous gland biology and pathology. Exp. Dermatol. 2008, 17, 542–551. [Google Scholar] [CrossRef]
- Trivedi, N.R.; Cong, Z.; Nelson, A.; Albert, A.J.; Rosamilia, L.L.; Sivarajah, S.; Gilliland, K.L.; Liu, W.; Mauger, D.T.; Gabbay, R.A.; et al. Peroxisome Proliferator-Activated Receptors Increase Human Sebum Production. J. Investig. Dermatol. 2006, 126, 2002–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.S.; Lee, J.S.; Kim, M.; Ahn, B.Y.; Jung, H.S.; Lee, H.M.; Kim, J.W.; Park, K.S. Regulation of Wnt/beta-catenin signaling by CCAAT/enhancer binding protein beta during adipogenesis. Obesity 2012, 20, 482–487. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Camera, E.; Picardo, M.; Zouboulis, C.P.D.; Chan, L.; Chang, B.H.-J.; Schneider, M.R. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 4642–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camera, E.; Dahlhoff, M.; Ludovici, M.; Zouboulis, C.P.D.; Schneider, M.R. Perilipin 3 modulates specific lipogenic pathways in SZ95 sebocytes. Exp. Dermatol. 2014, 23, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Clayton, R.W.; Langan, E.A.; Ansell, D.; de Vos, I.; Göbel, K.; Schneider, M.R.; Picardo, M.; Lim, X.; Van Steensel, M.A.M.; Paus, R. Neuroendocrinology and neurobiology of sebaceous glands. Biol. Rev. 2020, 95, 592–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Jung, H.D.; Lee, H.J.; Kim, B.S.; Lee, S.-J.; Kim, D.W. Influence of substance-P on cultured sebocytes. Arch. Dermatol. Res. 2008, 300, 311–316. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Kagoura, M.; Morohashi, M. Sebaceous glands in acne patients express high levels of neutral endopeptidase. Exp. Dermatol. 2002, 11, 241–247. [Google Scholar] [CrossRef]
- Kim, J.Y.; Illigens, B.M.; McCormick, M.P.; Wang, N.; Gibbons, C.H. Alpha-Synuclein in Skin Nerve Fibers as a Biomarker for Alpha-Synucleinopathies. J. Clin. Neurol. 2019, 15, 135–142. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, I.; Calderon-Garciduenas, A.L.; Jimenez-Capdeville, M.E.; Renteria-Palomo, A.A.; Hernandez-Rodriguez, H.G.; Valdes-Rodriguez, R.; Fuentes-Ahumada, C.; Torres-Alvarez, B.; Sepulveda-Saavedra, J.; Soto-Dominguez, A.; et al. α-Synuclein inclusions in the skin of Parkinson’s disease and parkinsonism. Ann. Clin. Transl. Neurol. 2014, 1, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Juceviciute, N.; Banaityte, I.; Vaitkus, A.; Balnyte, R. Preclinical signs of Parkinson’s disease: A possible association of Parkinson’s disease with skin and hair features. Med. Hypotheses 2019, 127, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.; Ingram, K.D. Microbicidal Activity of Tripotassium Phosphate and Fatty Acids toward Spoilage and Pathogenic Bacteria Associated with Poultry. J. Food Prot. 2005, 68, 1462–1466. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.C.; Gallo, R.L.; Zhang, L.; Hsieh, M.-F.; Huang, C.-M. An Innate Bactericidal Oleic Acid Effective Against Skin Infection of Methicillin-Resistant Staphylococcus aureus: A Therapy Concordant with Evolutionary Medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Janůšová, B.; Zbytovská, J.; Lorenc, P.; Vavrysová, H.; Palát, K.; Hrabálek, A.; Vávrová, K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim. et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Ludovici, M.; Tortorella, S.; Sinagra, J.L.M.; Capitanio, B.; Goracci, L.; Picardo, M. Use of lipidomics to investigate sebum dysfunction in juvenile acne. J. Lipid Res. 2016, 57, 1051–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimache, A.; Șalaru, D.; Sascău, R.; Stătescu, C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Bertinetto, C.; Engel, J.; Jansen, J. ANOVA simultaneous component analysis: A tutorial review. Anal. Chim. Acta: X 2020, 6, 100061. [Google Scholar] [CrossRef]
- Marini, F.; de Beer, D.; Walters, N.A.; de Villiers, A.; Joubert, E.; Walczak, B. Multivariate analysis of variance of designed chromatographic data. A case study involving fermentation of rooibos tea. J. Chromatogr. A 2017, 1489, 115–125. [Google Scholar] [CrossRef]
- Vis, D.J.; Westerhuis, J.A.; Smilde, A.K.; Van Der Greef, J. Statistical validation of megavariate effects in ASCA. BMC Bioinform. 2007, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.-J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]

| CATEGORIES | COUNTS | AGE (YEARS) | BMI (KG/M2) | SMOKING | ALCOHOL INTAKE | EDUCATION (YEARS) | MMSE | SER |
|---|---|---|---|---|---|---|---|---|
| HS | 24 | 72.2 ± 10.4 | 26.6 ± 1.64 | 14 (58%) | 12 (50%) | 12.5 ± 3.79 | 29.8 ± 1.09 | 4.91 ± 2.76 |
| HS|F | 10 | 74.1 ± 8.27 | 26.2 ± 1.23 | 6 (60%) | 3 (30%) | 10.7 ± 3.8 | 30.3 ± 1.35 | 3.33 ± 1.44 |
| HS|M | 14 | 70.8 ± 11.8 | 26.9 ± 1.87 | 8 (57%) | 9 (64%) | 13.8 ± 3.33 | 29.5 ± 0.74 | 6.04 ± 2.96 # |
| AD | 20 | 79.1 ± 6.03 | 26.1 ± 3.56 | 6 (30%) | 10 (50%) | 8.35 ± 3.33 ** | 21.4 ± 3.20 *** | 5.37 ± 2.68 |
| AD|F | 11 | 78.5 ± 6.17 | 25.1 ± 4.29 | 2 (18%) | 5 (45%) | 7.09 ± 2.95 | 21.5 ± 2.95 °°° | 4.79 ± 2.67 |
| AD|M | 9 | 79.8 ± 6.14 | 27.3 ± 2.03 | 4 (44%) | 5 (56%) | 9.89 ± 3.26 # | 21.4 ± 3.67 °°° | 6.09 ± 2.65 |
| PD | 20 | 71.5 ± 7.16 | 26.1 ± 2.50 | 8 (40%) | 10 (50%) | 11.3 ± 4.52 | 26.3 ± 1.99 ** | 8.05 ± 4.09 * |
| PD|F | 4 | 72.0 ± 5.03 | 28.5 ± 2.02 | 1 (25%) | 2 (50%) | 10.5 ± 3.69 | 27.3 ± 1.21 | 5.51 ± 2.86 |
| PD|M | 16 | 71.3 ± 7.73 | 25.5 ± 2.28 # | 7 (44%) | 8 (50%) | 11.5 ± 4.79 | 26.1 ± 2.10 ° | 8.69 ± 4.17 |
| Average ± SD (HS) | Average ± SD (AD) | Average ± SD (PD) | FC AD vs. HS | p-Value | FC PD vs. HS | p-Value | |
|---|---|---|---|---|---|---|---|
| 10Me-C12:0 | 0.143 ± 0.159 | 0.164 ± 0.216 | 0.296 ± 0.292 | 1.152 | >0.05 | 2.07 | 0.036 |
| 12Me-C14:0 | 1.38 ± 1.91 | 1.63 ± 2.06 | 4.98 ± 7.41 | 1.179 | >0.05 | 3.61 | 0.014 |
| 14Me-C16:0 | 1.49 ± 1.93 | 2.31 ± 2.98 | 1.96 ± 1.36 | 1.538 | >0.05 | 1.32 | 0.057 |
| 16Me-C18:0 | 0.851 ± 1.33 | 1.17 ± 1.81 | 1.96 ± 2.69 | 1.376 | >0.05 | 2.30 | >0.05 |
| 9Me-C12:0 | 0.111 ± 0.136 | 0.0488 ± 0.0442 | 0.393 ± 0.774 | 0.443 | >0.05 | 3.54 | >0.05 |
| 11Me-C14:0 | 0.357 ± 0.923 | 0.389 ± 0.773 | 0.387 ± 0.647 | 1.091 | >0.05 | 1.08 | >0.05 |
| 13Me-C16:0 | 1.59 ± 2.08 | 1.47 ± 1.16 | 5.01 ± 6.59 | 0.920 | >0.05 | 3.15 | 0.006 |
| 15Me-C18:0 | 2.40 ± 2.63 | 2.54 ± 1.97 | 5.78 ± 4.77 | 1.057 | >0.05 | 2.41 | 0.008 |
| 11Me-C13:0 | 0.365 ± 0.336 | 0.412 ± 0.297 | 0.969 ± 1.37 | 1.129 | >0.05 | 2.65 | 0.006 |
| 13Me-C15:0 | 3.452 ± 3.37 | 4.21 ± 2.97 | 9.393 ± 15.2 | 1.220 | >0.05 | 2.72 | 0.004 |
| 15Me-C17:0 | 0.996 ± 0.847 | 0.944 ± 0.524 | 1.92 ± 3.02 | 0.948 | >0.05 | 1.93 | >0.05 |
| 10Me-C13:0 | 1.17 ± 1.21 | 1.01 ± 0.864 | 2.45 ± 1.61 | 0.859 | >0.05 | 2.09 | 0.010 |
| 12Me-C15:0 | 9.34 ± 10.1 | 8.84 ± 7.24 | 19.8 ± 12.4 | 0.947 | >0.05 | 2.12 | 0.004 |
| 14Me-C17:0 | 3.83 ± 3.08 | 3.56 ± 2.31 | 6.52 ± 3.42 | 0.929 | >0.05 | 1.70 | 0.010 |
| C12:0 | 3.32 ± 2.34 | 4.17 ± 2.51 | 6.09 ± 3.36 | 1.256 | >0.05 | 1.83 | 0.003 |
| C14:0 | 36.1 ± 39.6 | 38.1 ± 31.4 | 91.3 ± 73.2 | 1.057 | >0.05 | 2.53 | 0.001 |
| C16:0 | 136 ± 120 | 159 ± 123 | 291 ± 212 | 1.171 | >0.05 | 2.14 | 0.002 |
| C18:0 | 41.5 ± 27.7 | 41.3 ± 17.2 | 49.5 ± 20.1 | 0.996 | >0.05 | 1.19 | >0.05 |
| C20:0 | 2.63 ± 2.21 | 2.21 ± 1.45 | 3.63 ± 3.91 | 0.842 | >0.05 | 1.38 | >0.05 |
| C22:0 | 2.12 ± 1.61 | 1.94 ± 0.711 | 2.72 ± 2.65 | 0.915 | >0.05 | 1.28 | >0.05 |
| C24:0 | 3.16 ± 2.92 | 3.49 ± 1.92 | 8.52 ± 8.44 | 1.102 | >0.05 | 2.70 | 0.015 |
| C26:0 | 0.556 ± 0.686 | 0.631 ± 0.621 | 1.68 ± 1.98 | 1.133 | >0.05 | 3.02 | >0.05 |
| C13:0 | 1.17 ± 1.186 | 1.23 ± 1.21 | 2.47 ± 1.61 | 1.054 | >0.05 | 2.11 | 0.002 |
| C15:0 | 26.7 ± 28.9 | 29.7 ± 24.8 | 65.3 ± 51.6 | 1.112 | >0.05 | 2.45 | 0.003 |
| C17:0 | 8.07 ± 7.16 | 8.72 ± 6.95 | 14.3 ± 9.45 | 1.081 | >0.05 | 1.77 | 0.007 |
| C19:0 | 0.989 ± 0.824 | 1.01 ± 0.686 | 2.07 ± 1.45 | 1.016 | >0.05 | 2.09 | 0.004 |
| C21:0 | 0.483 ± 0.677 | 0.329 ± 0.137 | 0.722 ± 0.824 | 0.682 | >0.05 | 1.49 | 0.048 |
| C23:0 | 0.516 ± 0.443 | 0.496 ± 0.233 | 1.14 ± 0.862 | 0.961 | >0.05 | 2.21 | 0.002 |
| C25:0 | 0.437 ± 0.424 | 0.576 ± 0.471 | 1.202 ± 2.176 | 1.316 | >0.05 | 2.75 | 0.054 |
| C14:1 | 0.934 ± 1.164 | 0.901 ± 1.012 | 2.75 ± 2.74 | 0.964 | >0.05 | 2.94 | 0.003 |
| C16:1 | 21.6 ± 25.8 | 24.1 ± 19.5 | 67.01 ± 67.2 | 1.117 | >0.05 | 3.10 | 0.001 |
| C18:1 | 38.7 ± 35.9 | 45.9 ± 27.3 | 92.2 ± 60.5 | 1.189 | >0.05 | 2.38 | 0.000 |
| C20:1 | 1.33 ± 1.22 | 1.39 ± 1.19 | 3.07 ± 2.14 | 1.052 | >0.05 | 2.31 | 0.002 |
| C22:1 | 0.144 ± 0.150 | 0.101 ± 0.0702 | 0.326 ± 0.351 | 0.700 | >0.05 | 2.26 | 0.054 |
| C24:1 | 0.283 ± 0.238 | 0.241 ± 0.210 | 0.641 ± 0.523 | 0.850 | >0.05 | 2.26 | 0.018 |
| C15:1 | 1.06 ± 1.31 | 1.09 ± 1.149 | 2.82 ± 2.95 | 1.032 | >0.05 | 2.66 | 0.004 |
| C17:1 | 4.19 ± 4.22 | 4.75 ± 3.96 | 10.4 ± 7.56 | 1.135 | >0.05 | 2.48 | 0.001 |
| 15Me-C17:1 | 0.881 ±1.76 | 0.462 ± 0.277 | 1.26 ± 1.62 | 0.524 | >0.05 | 1.43 | 0.019 |
| 14Me-C17:1 | 1.05 ± 1.19 | 1.02 ± 0.767 | 2.19 ± 1.37 | 0.968 | >0.05 | 2.09 | 0.003 |
| C18:2 | 3.19 ± 1.53 | 3.53 ± 1.59 | 4.33 ± 1.65 | 1.109 | >0.05 | 1.36 | 0.030 |
| C20:2 | 0.471 ± 0.501 | 0.451 ± 0.452 | 1.11 ± 1.04 | 0.956 | >0.05 | 2.36 | 0.048 |
| FOHC14:0 | 1.37 ± 0.908 | 3.91 ± 5.85 | 1.83 ± 1.31 | 2.859 | >0.05 | 1.34 | >0.05 |
| FOHC16:0 | 10.4 ± 15.4 | 15.1 ± 23.9 | 7.17 ± 19.2 | 1.439 | >0.05 | 0.69 | >0.05 |
| FOHC18:0 | 23.7 ± 26.6 | 23.8 ± 30.1 | 16.2 ± 26.3 | 1.007 | >0.05 | 0.68 | >0.05 |
| FOHC20:0 | 7.29 ± 12.1 | 3.63 ± 3.19 | 4.21 ± 1.59 | 0.499 | >0.05 | 0.58 | >0.05 |
| FOHC22:0 | 7.74 ± 13.3 | 5.95 ± 13.4 | 3.76 ± 1.71 | 0.769 | >0.05 | 0.49 | >0.05 |
| FOHC24:0 | 1.82 ± 0.992 | 1.57 ± 0.568 | 2.59 ± 1.19 | 0.861 | >0.05 | 1.42 | 0.026 |
| FOHC26:0 | 0.919 ± 0.998 | 1.05 ± 0.872 | 2.67 ± 3.149 | 1.144 | >0.05 | 2.91 | 0.030 |
| Vitamin E | 0.0271 ± 0.0776 | 0.0031 ± 0.0049 | 0.0062 ± 0.014 | 0.115 | >0.05 | 0.23 | >0.05 |
| Cholesterol | 17.1 ± 11.03 | 17.7 ± 7.85 | 22.1 ± 5.36 | 1.033 | >0.05 | 1.29 | 0.008 |
| Squalene | 276.4 ± 252.9 | 202.1 ± 165.9 | 481.9 ± 241.8 | 0.731 | >0.05 | 1.74 | 0.010 |
| TGs | 539.8 ± 315.7 | 415.9 ± 286.9 | 620.4 ± 299.6 | 0.771 | >0.05 | 0.11 | >0.05 |
| WEs | 361.2 ± 182.1 | 278.5 ± 132.9 | 496.7 ± 217.6 | 0.771 | >0.05 | 1.38 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briganti, S.; Truglio, M.; Angiolillo, A.; Lombardo, S.; Leccese, D.; Camera, E.; Picardo, M.; Di Costanzo, A. Application of Sebum Lipidomics to Biomarkers Discovery in Neurodegenerative Diseases. Metabolites 2021, 11, 819. https://doi.org/10.3390/metabo11120819
Briganti S, Truglio M, Angiolillo A, Lombardo S, Leccese D, Camera E, Picardo M, Di Costanzo A. Application of Sebum Lipidomics to Biomarkers Discovery in Neurodegenerative Diseases. Metabolites. 2021; 11(12):819. https://doi.org/10.3390/metabo11120819
Chicago/Turabian StyleBriganti, Stefania, Mauro Truglio, Antonella Angiolillo, Salvatore Lombardo, Deborah Leccese, Emanuela Camera, Mauro Picardo, and Alfonso Di Costanzo. 2021. "Application of Sebum Lipidomics to Biomarkers Discovery in Neurodegenerative Diseases" Metabolites 11, no. 12: 819. https://doi.org/10.3390/metabo11120819
APA StyleBriganti, S., Truglio, M., Angiolillo, A., Lombardo, S., Leccese, D., Camera, E., Picardo, M., & Di Costanzo, A. (2021). Application of Sebum Lipidomics to Biomarkers Discovery in Neurodegenerative Diseases. Metabolites, 11(12), 819. https://doi.org/10.3390/metabo11120819