Growth and Overall Health of Patients with SLC13A5 Citrate Transporter Disorder
Abstract
1. Introduction
2. Results
2.1. SLC13A5 Citrate Transporter Disorder Patient Demographics
2.2. Growth Parameters
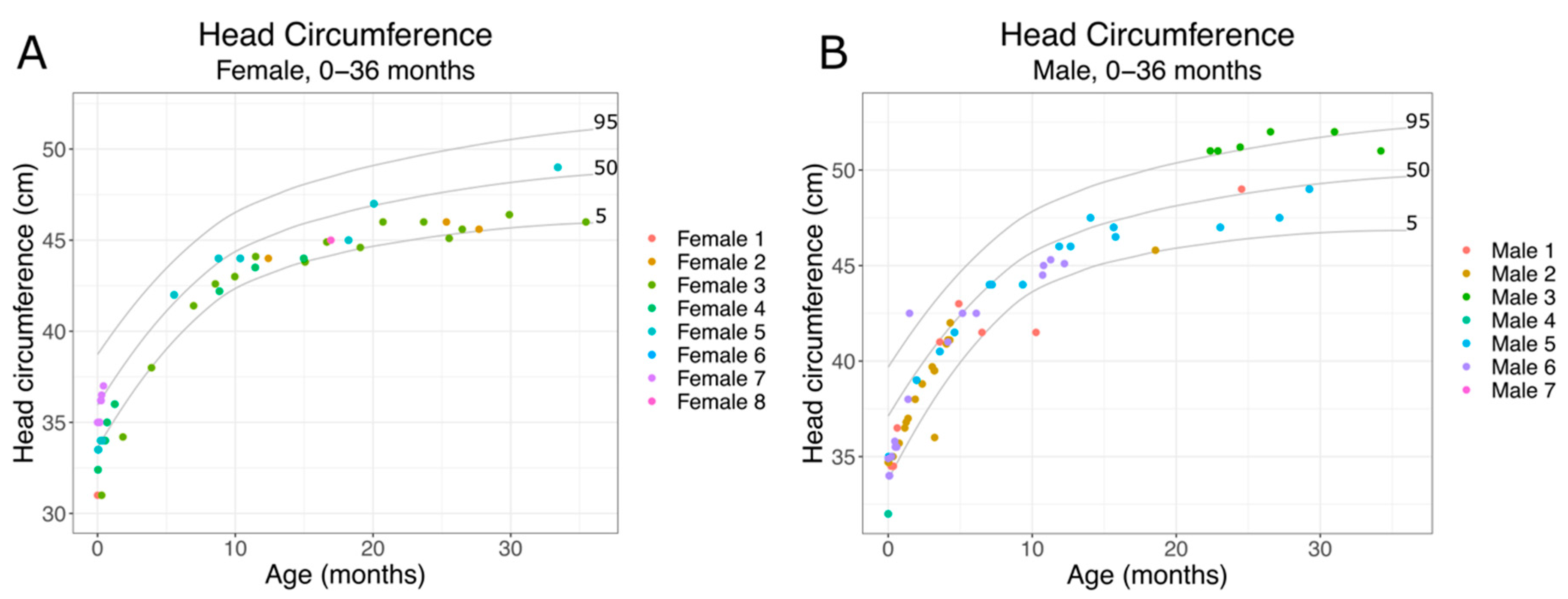
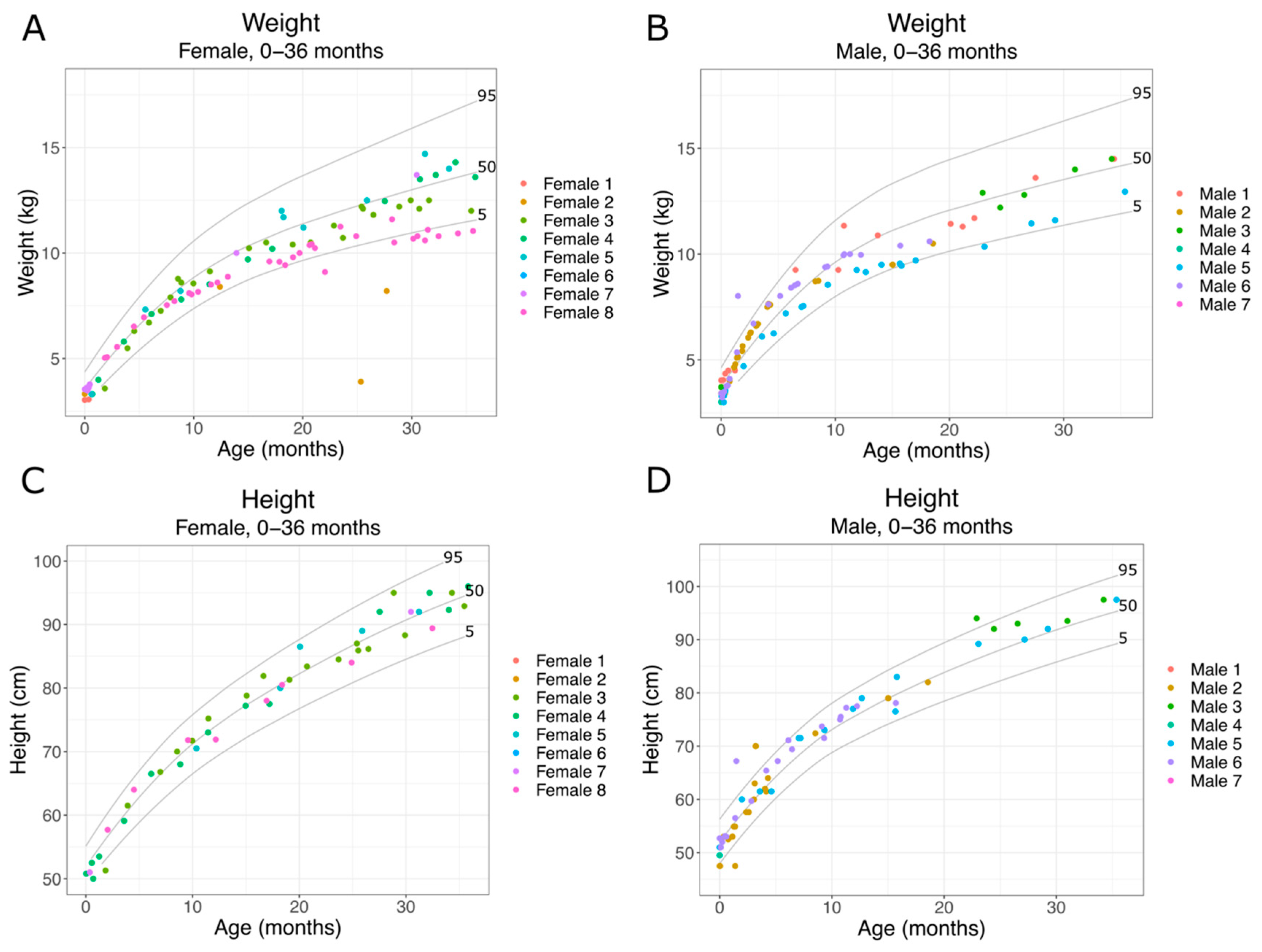
2.2.1. SLC13A5 Citrate Transporter Disorder Patient Physical Development 0–36 Months
2.2.2. SLC13A5 Citrate Transporter Disorder Patient Physical Development 2–20 Years
2.3. Non-Neurologic Diagnoses
2.4. Diagnostic Procedures
2.5. Surgical Procedures
3. Discussion
4. Materials and Methods
4.1. Medical Record Collection and Data Extraction
4.2. Medical Record Analysis: Patient Growth
4.3. Medical Record Analysis: Patient Diagnoses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardies, K.; De Kovel, C.G.F.; Weckhuysen, S.; Asselbergh, B.; Geuens, T.; Deconinck, T.; Abdelkrim, A.; May, P.; Brilstra, E.; Becker, F.; et al. Recessive mutations in SLC13A5 result in a loss of citrate transport and cause neonatal epilepsy, developmental delay and teeth hypoplasia. Brain 2015, 138, 3238–3250. [Google Scholar] [CrossRef]
- Klotz, J.; Porter, B.E.; Colas, C.; Schlessinger, A.; Pajor, A.M. Mutations in the Na+/citrate cotransporter NaCT (SLC13A5) in pediatric patients with epilepsy and developmental delay. Mol. Med. 2016, 22, 310–321. [Google Scholar] [CrossRef]
- Selch, S.; Chafai, A.; Sticht, H.; Birkenfeld, A.L.; Fromm, M.F.; König, J. Analysis of naturally occurring mutations in the human uptake transporter NaCT important for bone and brain development and energy metabolism. Sci. Rep. 2018, 8, 11330. [Google Scholar] [CrossRef]
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 2002, 277, 39469–39476. [Google Scholar] [CrossRef]
- Thevenon, J.; Milh, M.; Feillet, F.; St-Onge, J.; Duffourd, Y.; Jugé, C.; Roubertie, A.; Héron, D.; Mignot, C.; Raffo, E.; et al. Mutations in SLC13A5 Cause Autosomal-Recessive Epileptic Encephalopathy with Seizure Onset in the First Days of Life. Am. J. Hum. Genet. 2014, 95, 113–120. [Google Scholar] [CrossRef]
- Matricardi, S.; De Liso, P.; Freri, E.; Costa, P.; Castellotti, B.; Magri, S.; Gellera, C.; Granata, T.; Musante, L.; Lesca, G.; et al. Neonatal developmental and epileptic encephalopathy due to autosomal recessive variants in SLC13A5 gene. Epilepsia 2020, 61, 2474–2485. [Google Scholar] [CrossRef]
- Yang, Q.-Z.; Spelbrink, E.M.; Nye, K.L.; Hsu, E.R.; Porter, B.E. Epilepsy and EEG Phenotype of SLC13A5 Citrate Transporter Disorder. Child. Neurol. Open 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Duan, R.; Saadi, N.W.; Grochowski, C.M.; Bhadila, G.; Faridoun, A.; Mitani, T.; Du, T.; Fatih, J.M.; Jhangiani, S.N.; Akdemir, Z.C.; et al. A novel homozygous SLC13A5 whole-gene deletion generated by Alu/Alu-mediated rearrangement in an Iraqi family with epileptic encephalopathy. Am. J. Med. Genet. A 2021, 7, 1972–1980. [Google Scholar] [CrossRef]
- Schossig, A.; Bloch-Zupan, A.; Lussi, A.; Wolf, N.I.; Raskin, S.; Cohen, M.; Giuliano, F.; Jurgens, J.; Krabichler, B.; Koolen, D.A.; et al. SLC13A5 is the second gene associated with Kohlschütter-Tönz syndrome. J. Med. Genet. 2017, 54, 54–62. [Google Scholar] [CrossRef]
- Bainbridge, M.N.; Cooney, E.; Miller, M.; Kennedy, A.D.; Wulff, J.E.; Donti, T.; Jhangiani, S.N.; Gibbs, R.A.; Elsea, S.H.; Porter, B.E.; et al. Analyses of SLC13A5-epilepsy patients reveal perturbations of TCA cycle. Mol. Genet. Metab. 2017, 121, 314–319. [Google Scholar] [CrossRef]
- Rogina, B.; Reenan, R.A.; Nilsen, S.P.; Helfand, S.L. Extended Life-Span Conferred by Cotransporter Gene Mutations in Drosophila. Science 2021, 290, 2137–2140. [Google Scholar] [CrossRef]
- Schwarz, F.; Karadeniz, Z.; Fischer-Rosinsky, A.; Willmes, D.M.; Spranger, J.; Birkenfeld, A.L. Knockdown of Indy/CeNac2 extends Caenorhabditis elegans life span by inducing AMPK/aak-2. Aging 2015, 7, 553–567. [Google Scholar] [CrossRef][Green Version]
- Fei, Y.J.; Inoue, K.; Ganapathy, V. Structural and functional characteristics of two sodium-coupled dicarboxylate transporters (ceNaDC1 and ceNaDC2) from Caenorhabditis elegans and their relevance to life span. J. Biol. Chem. 2003, 278, 6136–6144. [Google Scholar] [CrossRef] [PubMed]
- Henke, C.; Töllner, K.; van Dijk, R.M.; Miljanovic, N.; Cordes, T.; Twele, F.; Bröer, S.; Ziesak, V.; Rohde, M.; Hauck, S.M.; et al. Disruption of the sodium-dependent citrate transporter SLC13A5 in mice causes alterations in brain citrate levels and neuronal network excitability in the hippocampus. Neurobiol. Dis. 2020, 143, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Lee, H.Y.; Guebre-Egziabher, F.; Alves, T.C.; Jurczak, M.J.; Jornayvaz, F.R.; Zhang, D.; Hsiao, J.J.; Martin-Montalvo, A.; Fischer-Rosinsky, A.; et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011, 14, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Willmes, D.M.; Daniels, M.; Kurzbach, A.; Lieske, S.; Bechmann, N.; Schumann, T.; Henke, C.; El-Agroudy, N.N.; Da Costa Goncalves, A.C.; Peitzsch, M.; et al. The longevity gene mIndy (I’m Not Dead, Yet) affects blood pressure through sympathoadrenal mechanisms. JCI Insight 2021, 6, 1–17. [Google Scholar] [CrossRef]
- Irizarry, A.R.; Yan, G.; Zeng, Q.; Lucchesi, J.; Hamang, M.J.; Ma, Y.L.; Rong, J.X. Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice. PLoS ONE 2017, 12, e0175465. [Google Scholar] [CrossRef]
- Willmes, D.M.; Kurzbach, A.; Henke, C.; Schumann, T.; Zahn, G.; Heifetz, A.; Jordan, J.; Helfand, S.L.; Birkenfeld, A.L. The longevity gene INDY (I’m Not Dead Yet) in metabolic control: Potential as pharmacological target. Pharmacol. Ther. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Knauf, F.; Rogina, B.; Jiang, Z.; Aronson, P.S.; Helfand, S.L. Functional characterization and immunolocalization of the transporter encoded by the life-extending gene Indy. Proc. Natl. Acad. Sci. USA 2002, 29, 14315–14319. [Google Scholar] [CrossRef]
- Huard, K.; Brown, J.; Jones, J.C.; Cabral, S.; Futatsugi, K.; Gorgoglione, M.; Lanba, L.; Vera, N.B.; Zhu, Y.; Yan, Q.; et al. Discovery and characterization of novel inhibitors of the sodium-coupled citrate transporter (NaCT or SLC13A5). Sci. Rep. 2015, 5, 17391. [Google Scholar] [CrossRef]
- Brachs, S.; Winkel, A.F.; Tang, H.; Birkenfeld, A.L.; Brunner, B.; Jahn-Hofmann, K.; Margerie, D.; Ruetten, H.; Schmoll, D.; Spranger, J. Inhibition of citrate cotransporter Slc13a5/mINDY by RNAi improves hepatic insulin sensitivity and prevents diet-induced non-alcoholic fatty liver disease in mice. Mol. Metab. 2016, 5, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Huang, W.; Li, Z.; Kane, M.A.; Zhang, L.; Huang, S.M.; Wang, H. Comparative proteomic analysis of SLC13A5 knockdown reveals elevated ketogenesis and enhanced cellular toxic response to chemotherapeutic agents in HepG2 cells. Toxicol. Appl. Pharmacol. 2020, 402, 115117. [Google Scholar] [CrossRef]
- Garcia, J.; Wical, B.; Wical, W.; Schaffer, L.; Wical, T.; Wendorf, H.; Roiko, S. Obstructive sleep apnea in children with cerebral palsy and epilepsy. Dev. Med. Child Neurol. 2016, 58, 1057–1062. [Google Scholar] [CrossRef]
- McLellan, A.; Cipparone, C.; Giancola, D.; Armstrong, D.; Bartlett, D. Medical and surgical procedures experienced by young children with cerebral palsy. Pediatr. Phys. Ther. 2012, 24, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Kroner, B.; Grinspan, Z.; Fureman, B.; Farrell, K.; Zhang, J.; Buelow, J.; Hesdorffer, D.C. Comorbidities of Rare Epilepsies: Results from the Rare Epilepsy Network. J. Pediatr. 2018, 203, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.P.; Rogina, B. The role of INDY in metabolism, health and longevity. Front. Genet. 2015, 6, 1–5. [Google Scholar] [CrossRef]

| ID | DNA Variant | Protein Variant | Variant Classification | Clinical Significance (ClinVar) | Age at Diagnosis (Years) | Birth Year | Medical Records Collection |
|---|---|---|---|---|---|---|---|
| Female 1 | c.1599C > A | p.Asn533Lys | missense | VUS | <1 | 2020 | 2020–2021 |
| c.650G > A | p.Ser217Asn | missense | VUS | ||||
| Female 2 a | c.655G > A | p.Gly219Arg | missense | P/LP | 4 | 2010 | 2010–2020 |
| c.245A > G | p.Tyr82Cys | missense | N | ||||
| Female 3 b | c.511delG | p.Glu171Serfs*16 | frameshift | P | 7 | 2006 | 2006–2015 |
| c.511delG | p.Glu171Serfs*16 | frameshift | P | ||||
| Female 4 c | c.368 + 1G > A | splice site | N | 2013 | 2013–2020 | ||
| c.368 + 1G > A | splice site | N | |||||
| Female 5 c | c.368 + 1G > A | splice site | N | 5 | 2008 | 2008–2020 | |
| c.368 + 1G > A | splice site | N | |||||
| Female 6 d | c.1475T > C | p.Leu492Pro | missense | LP | 10 | 2003 | 2007–2020 |
| c.655G > A | p.Gly219Arg | missense | P/LP | ||||
| Female 7 | Gene Deletion | gene deletion | N | <1 | 2018 | 2018–2021 | |
| c.425C > T | p.Thr142Met | missense | LP | ||||
| Female 8 | chr17:6606,915-6611,132 × 1 | partial gene deletion | N | <1 | 2016 | 2016–2020 | |
| c.389G > A | p.Gly130Asp | missense | N | ||||
| Male 1 | c.997C > T | p.Arg333Ter | missense | P | 6 | 2013 | 2013–2020 |
| c.997C > T | p.Arg333Ter | missense | P | ||||
| Male 2 | c.232-2A > G | splice site | LP | <1 | 2019 | 2019–2020 | |
| c.1460C > T | p.Pro487Leu | missense | VUS | ||||
| Male 3 | c.1511delT | p.Leu504Cysfs*23 | frameshift | N | 4 | 2012 | 2012–2020 |
| c.1511delT | p.Leu504Cysfs*23 | frameshift | N | ||||
| Male 4 b | c.511delG | p.Glu171Serfs*16 | frameshift | P | 6 | 2007 | 2007–2016 |
| c.511delG | p.Glu171Serfs*16 | frameshift | P | ||||
| Male 5 d | c.655G > A | p.Gly219Arg | missense | P/LP | <1 | 2013 | 2013–2020 |
| c.1475T > C | p.Leu492Pro | missense | LP | ||||
| Male 6 | c.1514C > T | p.Pro505Leu | missense | VUS | <1 | 2019 | 2019–2020 |
| c.997C > T | p.Arg333Ter | missense | P | ||||
| Male 7 a | c.655G > A | p.Gly219Arg | missense | P/LP | 7 | 2007 | 2012–2021 |
| c.245A > G | p.Tyr82Cys | missense | N |
| Gastrointestinal | Subjects |
|---|---|
| Feeding difficulty | F8; M1,3,6 |
| Gastroesophageal reflux disease (GERD) | F1,5,8; M1,7 |
| Abdominal pain | F2 |
| Vomiting | M3,5,6 |
| Projectile Vomiting | M2 |
| Oropharyngeal dysphagia | F2; M1 |
| Incontinence of feces | M3 |
| Urinary | |
| Urinary incontinence | M3 |
| Vesicoureteral reflux | F2 |
| Recurrent urinary tract infections | F8; M4,6 |
| Retention of urine | F8; |
| Musculoskelatal | |
| Talipes planovalgus | F3; M4 |
| Trigonocephaly/Simple craniosynostosis | M1 |
| Fracture of radius | F5 |
| Calcaneovalgus deformity of foot | F3 |
| Joint hypermobility | F3; M4 |
| Varus deformity | F3 |
| Simple craniosynostosis | M1 |
| Nutrition and growth | |
| Slow weight gain | F2; M7 |
| Short stature | F2,6; M7 |
| Failure to thrive | F3 |
| Respiratory | |
| Ineffective airway clearance | M3 |
| Breathing-related sleep disorder | M1 |
| Pulmonary aspiration | M1 |
| Reactive airway disease | F2; M3 |
| Respiratory distress | F2 |
| Respiratory failure | M1 |
| Respiratory insufficiency | F5 |
| Restrictive lung disease | M1 |
| Ineffective airway clearance | M3 |
| Apnea | F3,5; M3,6 |
| Acute bronchitis | M1 |
| Acute respiratory failure | M6 |
| Snoring | F8; M1,3 |
| Chronic cough | M1 |
| Atelectasis | M6 |
| Vocal cord paralysis | F2 |
| Chronic pulmonary aspiration | M3 |
| Cardiovascular | |
| Patent ductus arteriosus | F3 |
| Patent foramen ovale | F3 |
| Mitral valve regurgitation | F2 |
| Heart murmur | M1 |
| Hematologic | |
| Anemia | F3 |
| Integument | |
| Eczema | F8 |
| Dental Health | |
| Impaired dentition | F3 |
| Partial congenital absence of teeth | F2 |
| Congenital anomaly in number of teeth | F5; M6 |
| Cardiac | Subjects |
|---|---|
| Echocardiography (Normal) | F3; M1,4 |
| Echocardiography (Patent Foramen Ovale) | F3,5; M2 |
| Electrocardiographic monitoring (Normal) | M1,4 |
| Bone health | |
| Radiography for bone age studies (Normal) | F3; M4 |
| Gastrointestinal | |
| Videofluoroscopic swallow study (Oropharyngeal dysphagia) | M1 |
| Videofluoroscopic swallow study (Pulmonary aspiration) | M1 |
| Videofluoroscopic swallow study (Laryngeal penetration) | M5 |
| Ultrasonography of abdomen (Normal) | F3; M2,4 |
| Upper gastrointestinal tract series (Normal) | M3 |
| Upper gastrointestinal tract series (GERD) | F5 |
| Diagnostic radiography of abdomen (Normal) | M2 |
| Renal | |
| Ultrasonography of bilateral kidneys (Normal) | F8,F8,F8 |
| Ultrasonography of bilateral kidneys (Bladder distention) | F8 |
| Ultrasonography of bilateral kidneys (pyelectasia) | M6 |
| Video urodynamic study (Normal) | F8 |
| Voiding urethrocystography (Normal) | F3; M4 |
| Sleep | |
| Polysomnogram (Obstructive sleep apnea of child) | F3; M1 |
| Polysomnogram (Normal) | M1 |
| Polysomnogram (abnormal) | F8 |
| Procedure | Subjects |
|---|---|
| Gastrostomy tube placement | F2; M3,6 |
| Myringotomy tube insertion | M3 |
| Tonsil and adenoidectomy/adenoidectomy | F7; M3 |
| Release of trigger thumb | F5 |
| Incision of lingual frenum | M6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, T.L.; Nye, K.L.; Porter, B.E. Growth and Overall Health of Patients with SLC13A5 Citrate Transporter Disorder. Metabolites 2021, 11, 746. https://doi.org/10.3390/metabo11110746
Brown TL, Nye KL, Porter BE. Growth and Overall Health of Patients with SLC13A5 Citrate Transporter Disorder. Metabolites. 2021; 11(11):746. https://doi.org/10.3390/metabo11110746
Chicago/Turabian StyleBrown, Tanya L., Kimberly L. Nye, and Brenda E. Porter. 2021. "Growth and Overall Health of Patients with SLC13A5 Citrate Transporter Disorder" Metabolites 11, no. 11: 746. https://doi.org/10.3390/metabo11110746
APA StyleBrown, T. L., Nye, K. L., & Porter, B. E. (2021). Growth and Overall Health of Patients with SLC13A5 Citrate Transporter Disorder. Metabolites, 11(11), 746. https://doi.org/10.3390/metabo11110746







