Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD)
Abstract
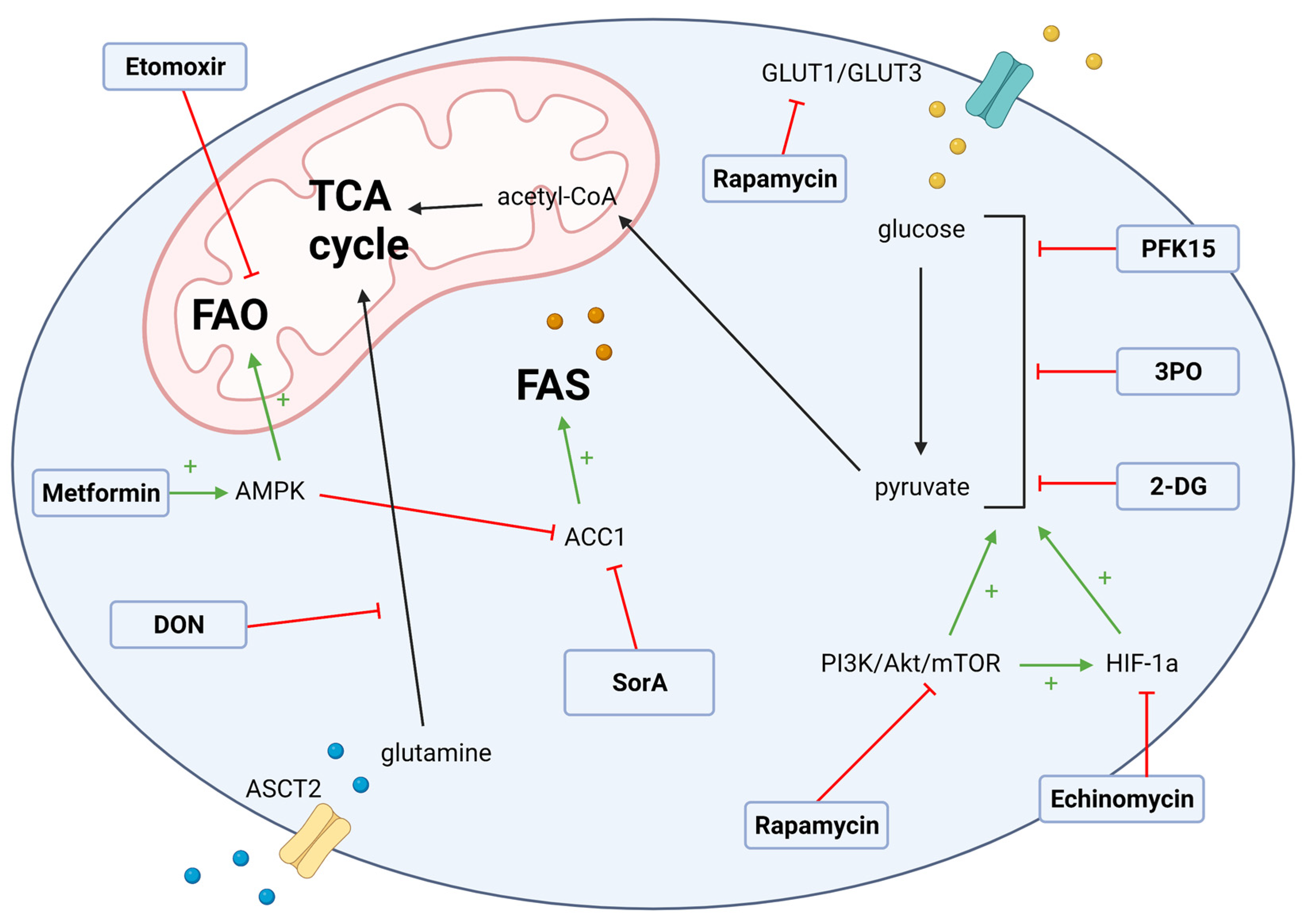
1. Introduction
2. T Cell Metabolism in Allo-HSCT
3. T Cell Metabolic Targets for GvHD
3.1. Glycolysis Targets
3.2. Fatty Acid Metabolism Targets in GvHD
3.3. Glutamate Metabolism Targets
4. Antigen-Presenting Cell Metabolism in GvHD
4.1. Dendritic Cell Targets
4.2. Macrophage Targets
4.3. Myeloid-Derived Suppressor Cell Targets
4.4. B Cell Ttargets
5. Potential Caveats in Metabolic Targeting of Immune Cells in GvHD
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelan R, A.M.; Chen, M. Current Use and Outcome of Hematopoietic Stem Cell Transplantation. 2021. Available online: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx (accessed on 22 October 2021).
- Dickinson, A.M.; Norden, J.; Li, S.; Hromadnikova, I.; Schmid, C.; Schmetzer, H.; Jochem-Kolb, H. Graft-versus-Leukemia Effect Following Hematopoietic Stem Cell Transplantation for Leukemia. Front. Immunol. 2017, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008, 112, 4371–4383. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Kuril, S.; Bastian, D.; Yu, X.-Z. T-Cell Metabolism in Hematopoietic Cell Transplantation. Front. Immunol. 2018, 9, 176. [Google Scholar] [CrossRef]
- Tijaro-Ovalle, N.M.; Karantanos, T.; Wang, H.-T.; Boussiotis, V.A. Metabolic Targets for Improvement of Allogeneic Hematopoietic Stem Cell Transplantation and Graft-vs.-Host Disease. Front. Immunol. 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Palaniyandi, S.; Hildebrandt, G.C. Metabolic Reprogramming-A New Era How to Prevent and Treat Graft Versus Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation Has Begun. Front. Pharmacol. 2020, 11, 588449. [Google Scholar] [CrossRef]
- Buxbaum, N.P.; Farthing, D.E.; Maglakelidze, N.; Lizak, M.; Merkle, H.; Carpenter, A.C.; Oliver, B.U.; Kapoor, V.; Castro, E.; Swan, G.A.; et al. In vivo kinetics and nonradioactive imaging of rapidly proliferating cells in graft-versus-host disease. JCI Insight 2017, 2, e92851. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Betts, B.C.; Tkachev, V.; Kean, L.S.; Blazar, B.R. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu. Rev. Immunol. 2021, 39, 19–49. [Google Scholar] [CrossRef]
- Teshima, T. Th1 and Th17 join forces for acute GVHD. Blood 2011, 118, 4765–4767. [Google Scholar] [CrossRef] [PubMed]
- Riegel, C.; Boeld, T.J.; Doser, K.; Huber, E.; Hoffmann, P.; Edinger, M. Efficient treatment of murine acute GvHD by in vitro expanded donor regulatory T cells. Leukemia 2020, 34, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Matsuoka, K.-I.; Kim, H.T.; McDonough, S.M.; Bindra, B.; Alyea, E.P.; Armand, P.; Cutler, C.; Ho, V.T.; Treister, N.S.; et al. Interleukin-2 and Regulatory T Cells in Graft-versus-Host Disease. N. Engl. J. Med. 2011, 365, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.; Hoffmann, P.; Ermann, J.; Drago, K.; Fathman, C.G.; Strober, S.; Negrin, R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003, 9, 1144–1150. [Google Scholar] [CrossRef]
- Matsuoka, K.; Koreth, J.; Kim, H.T.; Bascug, G.; McDonough, S.; Kawano, Y.; Murase, K.; Cutler, C.; Ho, V.T.; Alyea, E.P.; et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med. 2013, 5, 179ra143. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Rathmell, J.C. Metabolic pathways in T cell fate and function. Trends Immunol. 2012, 33, 168–173. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Chatterjee, S.; Haarberg, K.M.; Wu, Y.; Bastian, D.; Heinrichs, J.; Fu, J.; Daenthanasanmak, A.; Schutt, S.; Shrestha, S.; et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J. Clin. Investig. 2016, 126, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Gatza, E.; Wahl, D.R.; Opipari, A.W.; Sundberg, T.B.; Reddy, P.; Liu, C.; Glick, G.D.; Ferrara, J.L. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl. Med. 2011, 3, 67ra68. [Google Scholar] [CrossRef] [PubMed]
- Hippen, K.L.; Aguilar, E.G.; Rhee, S.Y.; Bolivar-Wagers, S.; Blazar, B.R. Distinct Regulatory and Effector T Cell Metabolic Demands during Graft-Versus-Host Disease. Trends Immunol. 2020, 41, 77–91. [Google Scholar] [CrossRef]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Kondo, M.; Kojima, S.; Horibe, K.; Kato, K.; Matsuyama, T. Risk factors for chronic graft-versus-host disease after allogeneic stem cell transplantation in children. Bone Marrow Transplant. 2001, 27, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Lazaryan, A.; Weisdorf, D.J.; DeFor, T.; Brunstein, C.G.; MacMillan, M.L.; Bejanyan, N.; Holtan, S.; Blazar, B.R.; Wagner, J.E.; Arora, M. Risk Factors for Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation with Umbilical Cord Blood and Matched Sibling Donors. Biol. Blood Marrow Transplant. 2016, 22, 134–140. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Arora, M.; Klein, J.P.; Hassebroek, A.; Hemmer, M.; Urbano-Ispizua, A.; Antin, J.H.; Bolwell, B.J.; Cahn, J.Y.; Cairo, M.S.; et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin. Cancer Res. 2015, 21, 2020–2028. [Google Scholar] [CrossRef]
- Kato, M.; Kurata, M.; Kanda, J.; Kato, K.; Tomizawa, D.; Kudo, K.; Yoshida, N.; Watanabe, K.; Shimada, H.; Inagaki, J.; et al. Impact of graft-versus-host disease on relapse and survival after allogeneic stem cell transplantation for pediatric leukemia. Bone Marrow Transpl. 2019, 54, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, M.; Antin, J.H. GVHD Prophylaxis 2020. Front. Immunol. 2021, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.K. Current approaches to prevent and treat GVHD after allogeneic stem cell transplantation. Hematology 2018, 2018, 228–235. [Google Scholar] [CrossRef]
- Bertz, H.; Spyridonidis, A.; Wäsch, R.; Grüllich, C.; Egger, M.; Finke, J. A novel GVHD-prophylaxis with low-dose alemtuzumab in allogeneic sibling or unrelated donor hematopoetic cell transplantation: The feasibility of deescalation. Biol. Blood Marrow Transpl. 2009, 15, 1563–1570. [Google Scholar] [CrossRef]
- Saidu, N.E.B.; Bonini, C.; Dickinson, A.; Grce, M.; Inngjerdingen, M.; Koehl, U.; Toubert, A.; Zeiser, R.; Galimberti, S. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front. Immunol. 2020, 11, 2625. [Google Scholar] [CrossRef]
- Kanakry, C.G.; O’Donnell, P.V.; Furlong, T.; Lima, M.J.d.; Wei, W.; Medeot, M.; Mielcarek, M.; Champlin, R.E.; Jones, R.J.; Thall, P.F.; et al. Multi-Institutional Study of Post-Transplantation Cyclophosphamide As Single-Agent Graft-Versus-Host Disease Prophylaxis After Allogeneic Bone Marrow Transplantation Using Myeloablative Busulfan and Fludarabine Conditioning. J. Clin. Oncol. 2014, 32, 3497–3505. [Google Scholar] [CrossRef]
- Martin, P.J.; Rizzo, J.D.; Wingard, J.R.; Ballen, K.; Curtin, P.T.; Cutler, C.; Litzow, M.R.; Nieto, Y.; Savani, B.N.; Schriber, J.R.; et al. First- and second-line systemic treatment of acute graft-versus-host disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Flowers, M.E.; Lee, S.J.; Carpenter, P.A.; Warren, E.H.; Deeg, H.J.; Storb, R.F.; Appelbaum, F.R.; Storer, B.E.; Martin, P.J. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood 2011, 118, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Axt, L.; Naumann, A.; Toennies, J.; Haen, S.; Vogel, W.; Schneidawind, D.; Wirths, S.; Moehle, R.; Faul, C.; Kanz, L. Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019, 54, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Saliba, R.M.; De Lima, M.; Alousi, A.; Hosing, C.; Qazilbash, M.H.; Khouri, I.F.; Shpall, E.J.; Anderlini, P.; Rondon, G. Steroid-refractory acute GVHD: Predictors and outcomes. Adv. Hematol. 2011, 2011. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Zhao, X.-Y.; Huang, X.-J. Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease. Front. Immunol. 2018, 9, 3041. [Google Scholar] [CrossRef] [PubMed]
- Bantug, G.R.; Galluzzi, L.; Kroemer, G.; Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 2018, 18, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.K. Regulation of glucose metabolism in T cells: New insight into the role of Phosphoinositide 3-kinases. Front. Immunol. 2012, 3, 247. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Weaver, J.; Herbel, C.; Seth, P.; Li, L.; Boussiotis, V.A. The role of metabolic reprogramming in T cell fate and function. Curr. Trends Immunol. 2016, 17, 1–12. [Google Scholar]
- Wang, R.; Green, D.R. Metabolic reprogramming and metabolic dependency in T cells. Immunol. Rev. 2012, 249, 14–26. [Google Scholar] [CrossRef]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.; van der Windt, G.J.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef]
- van der Windt, G.J.W.; Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Tan-Sah, V.P.; Smith, J.M.; Miyamoto, S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J. Biol. Chem. 2013, 288, 23798–23806. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.R.; Herman, C.E.; Maciver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. (Baltim. Md. 1950) 2008, 180, 4476–4486. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; Deoliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014, 20, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.A.; Thangavelu, G.; Rhee, S.Y.; Sage, P.T.; O’Connor, R.S.; Rathmell, J.C.; Blazar, B.R. Recent Metabolic Advances for Preventing and Treating Acute and Chronic Graft Versus Host Disease. Front. Immunol. 2021, 12, 4012. [Google Scholar] [CrossRef]
- Brown, R.A.; Byersdorfer, C.A. Metabolic Pathways in Alloreactive T Cells. Front. Immunol. 2020, 11, 1517. [Google Scholar] [CrossRef]
- Wahl, D.R.; Byersdorfer, C.A.; Ferrara, J.L.M.; Opipari, A.W., Jr.; Glick, G.D. Distinct metabolic programs in activated T cells: Opportunities for selective immunomodulation. Immunol. Rev. 2012, 249, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Zhou, J.; Zhang, X. HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. J. Transl. Med. 2017, 15, 28. [Google Scholar] [CrossRef]
- Assmann, J.C.; Farthing, D.E.; Saito, K.; Maglakelidze, N.; Oliver, B.; Warrick, K.A.; Sourbier, C.; Ricketts, C.J.; Meyer, T.J.; Pavletic, S.Z.; et al. Glycolytic metabolism of pathogenic T cells enables early detection of GVHD by 13C-MRI. Blood 2021, 137, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J. mTOR Regulation of Glycolytic Metabolism in T Cells. Front. Cell Dev. Biol. 2018, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Sánchez, M.C.; Rodríguez-Serrano, C.; Almeida, J.; San Segundo, L.; Inogés, S.; Santos-Briz, Á.; García-Briñón, J.; Corchete, L.A.; San Miguel, J.F.; del Cañizo, C.; et al. Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft-versus-host disease development. J. Hematol. Oncol. 2016, 9, 113. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef]
- Raha, S.; Raud, B.; Oberdörfer, L.; Castro, C.N.; Schreder, A.; Freitag, J.; Longerich, T.; Lochner, M.; Sparwasser, T.; Berod, L.; et al. Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur. J. Immunol. 2016, 46, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 2018, 175, 1780–1795.e1719. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Nian, Y.; Iske, J.; Maenosono, R.; Minami, K.; Heinbokel, T.; Quante, M.; Liu, Y.; Azuma, H.; Yang, J.; Abdi, R.; et al. Targeting age-specific changes in CD4+ T cell metabolism ameliorates alloimmune responses and prolongs graft survival. Aging Cell 2021, 20, e13299. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Yin, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017, 8, e2655. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Basit, F.; Mathan, T.; Sancho, D.; de Vries, I.J.M. Human Dendritic Cell Subsets Undergo Distinct Metabolic Reprogramming for Immune Response. Front. Immunol. 2018, 9, 2489. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Barnes, C.E.; Nichols, A.G.; Cohen, S.; Gerriets, V.A.; Siska, P.J.; Macintyre, A.N.; Goraksha-Hicks, P.; de Cubas, A.A.; Liu, T.; et al. AMPK Is Essential to Balance Glycolysis and Mitochondrial Metabolism to Control T-ALL Cell Stress and Survival. Cell Metab. 2016, 23, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Tang, D.; Zhu, Z.; Li, Y.; Huang, T.; Müller, R.; Yu, W.; Li, P. ACC1 (Acetyl Coenzyme A Carboxylase 1) Is a Potential Immune Modulatory Target of Cerebral Ischemic Stroke. Stroke 2019, 50, 1869–1878. [Google Scholar] [CrossRef]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bähre, H.; et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Cluxton, D.; Petrasca, A.; Moran, B.; Fletcher, J.M. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front. Immunol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, Z.; Zhu, L.; Yang, T.; Wang, P.; Liu, F.; Huang, Y.; Zhang, F.; Zhang, X.; Ding, W.; et al. 2-Deoxy-d-Glucose Treatment Decreases Anti-inflammatory M2 Macrophage Polarization in Mice with Tumor and Allergic Airway Inflammation. Front. Immunol. 2017, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Jalota, A.; Kumar, M.; Das, B.C.; Yadav, A.K.; Chosdol, K.; Sinha, S. Synergistic increase in efficacy of a combination of 2-deoxy-D-glucose and cisplatin in normoxia and hypoxia: Switch from autophagy to apoptosis. Tumor Biol. 2016, 37, 12347–12358. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, M.; Malavašič, P.; Dolinar, K.; Pohar, J.; Pirkmajer, S.; Pavlin, M. Combined treatment with Metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, Y.; Zhu, Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget 2017, 8, 62793–62802. [Google Scholar] [CrossRef] [PubMed]
- Clem, B.; Telang, S.; Clem, A.; Yalcin, A.; Meier, J.; Simmons, A.; Rasku, M.A.; Arumugam, S.; Dean, W.L.; Eaton, J.; et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol. Cancer Ther. 2008, 7, 110–120. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, L. mTOR up-regulation of PFKFB3 is essential for acute myeloid leukemia cell survival. Biochem. Biophys. Res. Commun. 2017, 483, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Clem, B.F.; O’Neal, J.; Tapolsky, G.; Clem, A.L.; Imbert-Fernandez, Y.; Kerr, D.A.; Klarer, A.C.; Redman, R.; Miller, D.M.; Trent, J.O.; et al. Targeting 6-Phosphofructo-2-Kinase (PFKFB3) as a Therapeutic Strategy against Cancer. Mol. Cancer Ther. 2013, 12, 1461–1470. [Google Scholar] [CrossRef]
- Telang, S.; Clem, B.F.; Klarer, A.C.; Clem, A.L.; Trent, J.O.; Bucala, R.; Chesney, J. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J. Transl. Med. 2012, 10, 95. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Sabatini, D.M. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017, 26, 301–309. [Google Scholar] [CrossRef]
- Rolf, J.; Zarrouk, M.; Finlay, D.K.; Foretz, M.; Viollet, B.; Cantrell, D.A. AMPK α1: A glucose sensor that controls CD 8 T-cell memory. Eur. J. Immunol. 2013, 43, 889–896. [Google Scholar] [CrossRef]
- Toschi, A.; Lee, E.; Gadir, N.; Ohh, M.; Foster, D.A. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J. Biol. Chem. 2008, 283, 34495–34499. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 6204. [Google Scholar] [CrossRef]
- Hoda, D.; Pidala, J.; Salgado-Vila, N.; Kim, J.; Perkins, J.; Bookout, R.; Field, T.; Perez, L.; Ayala, E.; Ochoa-Bayona, J.L.; et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010, 45, 1347–1351. [Google Scholar] [CrossRef]
- Blazar, B.R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vallera, D.A. Rapamycin Inhibits the Generation of Graft-Versus-Host Disease- and Graft-Versus-Leukemia-Causing T Cells by Interfering with the Production of Th1 or Th1 Cytotoxic Cytokines. J. Immunol. 1998, 160, 5355–5365. [Google Scholar]
- Chen, B.J.; Morris, R.E.; Chao, N.J. Graft-versus-host disease prevention by rapamycin: Cellular mechanisms. Biol. Blood Marrow Transplant. 2000, 6, 529–536. [Google Scholar] [CrossRef]
- Johnston, L.J.; Brown, J.; Shizuru, J.A.; Stockerl-Goldstein, K.E.; Stuart, M.J.; Blume, K.G.; Negrin, R.S.; Chao, N.J. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2005, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Baker, J.; Leveson-Gower, D.; Sega, E.I.; Negrin, R. Rapamycin and IL-2 Prevents Lethal Acute Graft-Versus Host Disease by Expansion of Donor Type CD4+CD25+Foxp3+ Regulatory T Cells. Blood 2009, 114, 1334. [Google Scholar] [CrossRef]
- Zeiser, R.; Nguyen, V.H.; Beilhack, A.; Buess, M.; Schulz, S.; Baker, J.; Contag, C.H.; Negrin, R.S. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006, 108, 390–399. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; Sainvil, M.-M.; Lange, P.B.; Giardino, A.A.; Bachanova, V.; Devine, S.M.; Waller, E.K.; Jagirdar, N.; Herrera, A.F.; et al. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: A multicentre randomized trial. Br. J. Haematol. 2016, 173, 96–104. [Google Scholar] [CrossRef]
- Hochegger, K.; Wurz, E.; Nachbaur, D.; Rosenkranz, A.R.; Clausen, J. Rapamycin-induced proteinuria following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2009, 44, 63–65. [Google Scholar] [CrossRef]
- Sandmaier, B.M.; Kornblit, B.; Storer, B.E.; Olesen, G.; Maris, M.B.; Langston, A.A.; Gutman, J.A.; Petersen, S.L.; Chauncey, T.R.; Bethge, W.A.; et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: A multicentre, randomised, phase 3 trial. Lancet Haematol 2019, 6, e409–e418. [Google Scholar] [CrossRef]
- Bejanyan, N.; Rogosheske, J.; DeFor, T.E.; Lazaryan, A.; Arora, M.; Holtan, S.G.; Jacobson, P.A.; MacMillan, M.L.; Verneris, M.R.; Blazar, B.R.; et al. Sirolimus and Mycophenolate Mofetil as Calcineurin Inhibitor-Free Graft-versus-Host Disease Prophylaxis for Reduced-Intensity Conditioning Umbilical Cord Blood Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 2025–2030. [Google Scholar] [CrossRef]
- Paz, K.; Flynn, R.; Du, J.; Tannheimer, S.; Johnson, A.J.; Dong, S.; Stark, A.K.; Okkenhaug, K.; Panoskaltsis-Mortari, A.; Sage, P.T.; et al. Targeting PI3Kδ function for amelioration of murine chronic graft-versus-host disease. Am. J. Transplant. 2019, 19, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Palaniyandi, S.; Kumari, R.; Venniyil Radhakrishnan, S.; Strattan, E.; Hakim, N.; Munker, R.; Kesler, M.V.; Hildebrandt, G.C. The Prolyl Hydroxylase Inhibitor Dimethyl Oxalyl Glycine Decreases Early Gastrointestinal GVHD in Experimental Allogeneic Hematopoietic Cell Transplantation. Transplantation 2020, 104, 2507–2515. [Google Scholar] [CrossRef]
- Byersdorfer, C.A.; Tkachev, V.; Opipari, A.W.; Goodell, S.; Swanson, J.; Sandquist, S.; Glick, G.D.; Ferrara, J.L. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood 2013, 122, 3230–3237. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, S.Y.; Moon, S.J.; Son, H.J.; Lee, S.H.; Kim, E.K.; Byun, J.K.; Shin, D.Y.; Park, S.H.; Yang, C.W.; et al. Metformin attenuates graft-versus-host disease via restricting mammalian target of rapamycin/signal transducer and activator of transcription 3 and promoting adenosine monophosphate-activated protein kinase-autophagy for the balance between T helper 17 and Tregs. Transl. Res. 2016, 173, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Park, M.-J.; Jhun, J.Y.; Beak, J.-A.; Choi, J.W.; Rye, J.-Y.; Jang, J.W.; Bae, S.H.; Yoon, S.K.; Choi, H.J.; et al. Combination Treatment With Metformin and Tacrolimus Improves Systemic Immune Cellular Homeostasis by Modulating Treg and Th17 Imbalance. Front. Immunol. 2021, 11, 581728. [Google Scholar] [CrossRef]
- Gualdoni, G.A.; Mayer, K.A.; Göschl, L.; Boucheron, N.; Ellmeier, W.; Zlabinger, G.J. The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. Faseb. J. 2016, 30, 3800–3809. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, S.Y.; Choi, J.W.; Lee, A.R.; Yoo, J.H.; Moon, S.J.; Park, S.H.; Cho, M.L. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med. 2021, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.H.; Yang, E.J.; Kim, E.K.; Kim, J.K.; Shin, D.Y.; Cho, M.L. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS ONE 2015, 10, e0135858. [Google Scholar] [CrossRef]
- Yin, Y.; Choi, S.C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra218. [Google Scholar] [CrossRef] [PubMed]
- Lepez, A.; Pirnay, T.; Denanglaire, S.; Perez-Morga, D.; Vermeersch, M.; Leo, O.; Andris, F. Long-term T cell fitness and proliferation is driven by AMPK-dependent regulation of reactive oxygen species. Sci. Rep. 2020, 10, 21673. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Blagih, J.; Saucillo, D.C.; Tonelli, L.; Griss, T.; Rathmell, J.C.; Jones, R.G. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 2011, 187, 4187–4198. [Google Scholar] [CrossRef] [PubMed]
- Monlish, D.A.; Beezhold, K.J.; Chiaranunt, P.; Paz, K.; Moore, N.J.; Dobbs, A.K.; Brown, R.A.; Ozolek, J.A.; Blazar, B.R.; Byersdorfer, C.A. Deletion of AMPK minimizes graft-versus-host disease through an early impact on effector donor T cells. JCI Insight 2021, 6, e143811. [Google Scholar] [CrossRef]
- Raud, B.; Roy, D.G.; Divakaruni, A.S.; Tarasenko, T.N.; Franke, R.; Ma, E.H.; Samborska, B.; Hsieh, W.Y.; Wong, A.H.; Stüve, P.; et al. Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab. 2018, 28, 504–515.e507. [Google Scholar] [CrossRef]
- Glick, G.D.; Rossignol, R.; Lyssiotis, C.A.; Wahl, D.; Lesch, C.; Sanchez, B.; Liu, X.; Hao, L.-Y.; Taylor, C.; Hurd, A.; et al. Anaplerotic Metabolism of Alloreactive T Cells Provides a Metabolic Approach To Treat Graft-Versus-Host Disease. J. Pharmacol. Exp. Ther. 2014, 351, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Min, W.S.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Min, C.K.; Lee, S.; Cho, S.G.; Jin, J.Y.; Lee, J.W.; et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol. Blood Marrow Transplant. 2009, 15, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Saegusa, J.; Okano, T.; Sendo, S.; Yamada, H.; Nishimura, K.; Morinobu, A. Additive effects of inhibiting both mTOR and glutamine metabolism on the arthritis in SKG mice. Sci. Rep. 2019, 9, 6374. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jun, S.A.; Tsukamoto, T.; Fathi, A.T.; Minden, M.D.; Dang, C.V. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp. Hematol. 2014, 42, 247–251. [Google Scholar] [CrossRef]
- Zhou, X.; Curbo, S.; Li, F.; Krishnan, S.; Karlsson, A. Inhibition of glutamate oxaloacetate transaminase 1 in cancer cell lines results in altered metabolism with increased dependency of glucose. BMC Cancer 2018, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.I.; Furlong, T.; Martin, P.J.; Anasetti, C.; Appelbaum, F.R.; Doney, K.; Nash, R.A.; Papayannopoulou, T.; Storb, R.; Sullivan, K.M.; et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease1. Transplantation 2001, 72, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Song, E.K.; Yim, J.M.; Yim, J.Y.; Song, M.Y.; Rho, H.W.; Yim, S.K.; Han, Y.H.; Jeon, S.Y.; Kim, H.S.; Yhim, H.Y.; et al. Glutamine protects mice from acute graft-versus-host disease (aGVHD). Biochem. Biophys. Res. Commun. 2013, 435, 94–99. [Google Scholar] [CrossRef]
- Noth, R.; Häsler, R.; Stüber, E.; Ellrichmann, M.; Schäfer, H.; Geismann, C.; Hampe, J.; Bewig, B.; Wedel, T.; Böttner, M.; et al. Oral glutamine supplementation improves intestinal permeability dysfunction in a murine acute graft-vs.-host disease model. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G646–G654. [Google Scholar] [CrossRef]
- Hong, Y.Q.; Wan, B.; Li, X.F. Macrophage regulation of graft-vs-host disease. World J. Clin. Cases 2020, 8, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Chakraverty, R.; Sykes, M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood 2007, 110, 9–17. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Reddy, P.; Maeda, Y.; Liu, C.; Krijanovski, O.I.; Korngold, R.; Ferrara, J.L. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat. Med. 2005, 11, 1244–1249. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Foerster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Cerboni, S.; Gentili, M.; Manel, N. Diversity of pathogen sensors in dendritic cells. Adv. Immunol. 2013, 120, 211–237. [Google Scholar] [PubMed]
- Amsen, D.; Blander, J.M.; Lee, G.R.; Tanigaki, K.; Honjo, T.; Flavell, R.A. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 2004, 117, 515–526. [Google Scholar] [CrossRef]
- Kadowaki, N. Dendritic Cells—A Conductor of T Cell Differentiation—. Allergol. Int. 2007, 56, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Louboutin, J.P.; Zhu, J.; Rivera, A.J.; Emerson, S.G. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J. Clin. Investig. 2002, 109, 1335–1344. [Google Scholar] [CrossRef]
- Du, X.; Chapman, N.M.; Chi, H. Emerging Roles of Cellular Metabolism in Regulating Dendritic Cell Subsets and Function. Front. Cell Dev. Biol. 2018, 6, 152. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef]
- Lawless, S.J.; Kedia-Mehta, N.; Walls, J.F.; McGarrigle, R.; Convery, O.; Sinclair, L.V.; Navarro, M.N.; Murray, J.; Finlay, D.K. Glucose represses dendritic cell-induced T cell responses. Nat. Commun. 2017, 8, 15620. [Google Scholar] [CrossRef] [PubMed]
- Thwe, P.M.; Pelgrom, L.R.; Cooper, R.; Beauchamp, S.; Reisz, J.A.; D’Alessandro, A.; Everts, B.; Amiel, E. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab. 2017, 26, 558–567.e555. [Google Scholar] [CrossRef] [PubMed]
- Elze, M.C.; Ciocarlie, O.; Heinze, A.; Kloess, S.; Gardlowski, T.; Esser, R.; Klingebiel, T.; Bader, P.; Huenecke, S.; Serban, M.; et al. Dendritic cell reconstitution is associated with relapse-free survival and acute GVHD severity in children after allogeneic stem cell transplantation. Bone Marrow Transplant. 2015, 50, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.S.; Stokes, J.; Hoffman, E.A.; Eremija, J.; Zeng, Y.; Simpson, R.J.; Katsanis, E. Bendamustine Conditioning Skews Murine Host DCs Toward Pre-cDC1s and Reduces GvHD Independently of Batf3. Front. Immunol. 2020, 11, 1410. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, X.; Bolli, E.; Fendt, S.M.; Van Ginderachter, J.A. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front. Immunol. 2017, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.M.; Gonzalez-Cotto, M.; Baseler, W.A.; Davies, L.C.; Ghesquière, B.; Maio, N.; Rice, C.M.; Rouault, T.A.; Cassel, T.; Higashi, R.M.; et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Singh, A.R.; Zulcic, M.; Durden, D.L. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α and HIF2α stability and tumor growth, angiogenesis, and metastasis. Mol. Cancer Res. 2014, 12, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Qualls, J.E.; Subramanian, C.; Rafi, W.; Smith, A.M.; Balouzian, L.; DeFreitas, A.A.; Shirey, K.A.; Reutterer, B.; Kernbauer, E.; Stockinger, S. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe 2012, 12, 313–323. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Terakura, S.; Martin, P.; Shulman, H.; Storer, B. Cutaneous macrophage infiltration in acute GvHD. Bone Marrow Transplant. 2015, 50, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Piérard, G.; Hermanns-Lê, T.; Paquet, P.; Rousseau, A.-F.; Delvenne, P.; Piérard-Franchimont, C. Toxic epidermal necrolysis and graft-versus-host reaction: Revisiting a puzzling similarity. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Su, Y.; Sun, X.; Fu, H.; Huang, Q.; Chen, Q.; Mo, X.; Lv, M.; Kong, Y.; Xu, L.; et al. Arsenic trioxide alleviates acute graft-versus-host disease by modulating macrophage polarization. Sci. China Life Sci. 2020, 63, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Seno, K.; Yasunaga, M.; Kajiya, H.; Izaki-Hagio, K.; Morita, H.; Yoneda, M.; Hirofuji, T.; Ohno, J. Dynamics of M1 macrophages in oral mucosal lesions during the development of acute graft-versus-host disease in rats. Clin. Exp. Immunol. 2017, 190, 315–327. [Google Scholar] [CrossRef]
- Sundarasetty, B.; Volk, V.; Theobald, S.J.; Rittinghausen, S.; Schaudien, D.; Neuhaus, V.; Figueiredo, C.; Schneider, A.; Gerasch, L.; Mucci, A. Human effector memory T helper cells engage with mouse macrophages and cause graft-versus-host–like pathology in skin of humanized mice used in a nonclinical immunization study. Am. J. Pathol. 2017, 187, 1380–1398. [Google Scholar] [CrossRef]
- Hanaki, R.; Toyoda, H.; Iwamoto, S.; Morimoto, M.; Nakato, D.; Ito, T.; Niwa, K.; Amano, K.; Hashizume, R.; Tawara, I.; et al. Donor-derived M2 macrophages attenuate GVHD after allogeneic hematopoietic stem cell transplantation. Immun. Inflamm. Dis. 2021. [Google Scholar] [CrossRef]
- MacDonald, K.P.A.; Hill, G.R.; Blazar, B.R. Chronic graft-versus-host disease: Biological insights from preclinical and clinical studies. Blood 2017, 129, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Foley, J.E.; Erdmann, A.A.; Toda, Y.; Borenstein, T.; Mariotti, J.; Fowler, D.H. Ex Vivo Rapamycin Generates Th1/Tc1 or Th2/Tc2 Effector T Cells With Enhanced In Vivo Function and Differential Sensitivity to Post-transplant Rapamycin Therapy. Biol. Blood Marrow Transplant. 2006, 12, 905–918. [Google Scholar] [CrossRef]
- Mercalli, A.; Calavita, I.; Dugnani, E.; Citro, A.; Cantarelli, E.; Nano, R.; Melzi, R.; Maffi, P.; Secchi, A.; Sordi, V.; et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology 2013, 140, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.; Laoui, D.; Wenes, M.; Rizzolio, S.; Bassani, N.; Mambretti, M.; Deschoemaeker, S.; Van Ginderachter, J.A.; Tamagnone, L.; Mazzone, M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013, 24, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.; Colombo, M.; Frey, A.; Greten, T.; Mandruzzato, S.; Murray, P.; Ochoa, A.; Ostrand-Rosenberg, S. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Koehn, B.H.; Apostolova, P.; Haverkamp, J.M.; Miller, J.S.; McCullar, V.; Tolar, J.; Munn, D.H.; Murphy, W.J.; Brickey, W.J.; Serody, J.S. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood J. Am. Soc. Hematol. 2015, 126, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Koehn, B.H.; Blazar, B.R. Role of myeloid-derived suppressor cells in allogeneic hematopoietic cell transplantation. J. Leukoc. Biol. 2017, 102, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Koehn, B.H.; Saha, A.; McDonald-Hyman, C.; Loschi, M.; Thangavelu, G.; Ma, L.; Zaiken, M.; Dysthe, J.; Krepps, W.; Panthera, J.; et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood 2019, 134, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Allen, J.L.; Fore, M.S.; Wooten, J.; Roehrs, P.A.; Bhuiya, N.S.; Hoffert, T.; Sharf, A.; Deal, A.M.; Armistead, P.; Coghill, J.; et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood 2012, 120, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- McManigle, W.; Youssef, A.; Sarantopoulos, S. B cells in chronic graft-versus-host disease. Hum. Immunol. 2019, 80, 393–399. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Hallek, M.J.; Storb, R.F.; von Bergwelt-Baildon, M.S. The role of B cells in the pathogenesis of graft-versus-host disease. Blood 2009, 114, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Edry, E.; Melamed, D. Receptor editing in positive and negative selection of B lymphopoiesis. J. Immunol. 2004, 173, 4265–4271. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Lesley, R.; Xu, Y.; Kalled, S.L.; Hess, D.M.; Schwab, S.R.; Shu, H.-B.; Cyster, J.G. Reduced Competitiveness of Autoantigen-Engaged B Cells due to Increased Dependence on BAFF. Immunity 2004, 20, 441–453. [Google Scholar] [CrossRef]
- Thien, M.; Phan, T.G.; Gardam, S.; Amesbury, M.; Basten, A.; Mackay, F.; Brink, R. Excess BAFF Rescues Self-Reactive B Cells from Peripheral Deletion and Allows Them to Enter Forbidden Follicular and Marginal Zone Niches. Immunity 2004, 20, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, S.; Stevenson, K.E.; Kim, H.T.; Cutler, C.S.; Bhuiya, N.S.; Schowalter, M.; Ho, V.T.; Alyea, E.P.; Koreth, J.; Blazar, B.R.; et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood 2009, 113, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Poe, J.C.; Su, H.; Anand, S.; Matsushima, G.K.; Rathmell, J.C.; Maillard, I.; Radojcic, V.; Imai, K.; Reyes, N.J.; et al. BAFF promotes heightened BCR responsiveness and manifestations of chronic GVHD after allogeneic stem cell transplantation. Blood 2021, 137, 2544–2557. [Google Scholar] [CrossRef]
- Zhao, D.; Young, J.S.; Chen, Y.-H.; Shen, E.; Yi, T.; Todorov, I.; Chu, P.G.; Forman, S.J.; Zeng, D. Alloimmune Response Results in Expansion of Autoreactive Donor CD4 T Cells in Transplants That Can Mediate Chronic Graft-versus-Host Disease. J. Immunol. 2011, 186, 856. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, Z.; Gounden, V.; Curtis, L.; Avila, D.; Rnp, T.T.; Baruffaldi, J.; Cowen, E.W.; Naik, H.B.; Hasni, S.A.; Mays, J.W.; et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am. J. Hematol. 2015, 90, 114–119. [Google Scholar] [CrossRef]
- Sarantopoulos, S.; Stevenson, K.E.; Kim, H.T.; Bhuiya, N.S.; Cutler, C.S.; Soiffer, R.J.; Antin, J.H.; Ritz, J. High Levels of B-Cell Activating Factor in Patients with Active Chronic Graft-Versus-Host Disease. Clin. Cancer Res. 2007, 13, 6107. [Google Scholar] [CrossRef] [PubMed]
- Dufort, F.J.; Bleiman, B.F.; Gumina, M.R.; Blair, D.; Wagner, D.J.; Roberts, M.F.; Abu-Amer, Y.; Chiles, T.C. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J. Immunol. 2007, 179, 4953–4957. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.A.; Bleiman, B.F.; Wagner, D.J.; Dufort, F.J.; Mataraza, J.M.; Roberts, M.F.; Chiles, T.C. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: Role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 2006, 107, 4458–4465. [Google Scholar] [CrossRef]
- Caro-Maldonado, A.; Wang, R.; Nichols, A.G.; Kuraoka, M.; Milasta, S.; Sun, L.D.; Gavin, A.L.; Abel, E.D.; Kelsoe, G.; Green, D.R. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J. Immunol. 2014, 192, 3626–3636. [Google Scholar] [CrossRef]
- Hobeika, E.; Levit-Zerdoun, E.; Anastasopoulou, V.; Pohlmeyer, R.; Altmeier, S.; Alsadeq, A.; Dobenecker, M.W.; Pelanda, R.; Reth, M. CD19 and BAFF-R can signal to promote B-cell survival in the absence of Syk. Embo J. 2015, 34, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.D.; Gaudette, B.T.; Wilmore, J.R.; Chernova, I.; Bortnick, A.; Weiss, B.M.; Allman, D. mTOR has distinct functions in generating versus sustaining humoral immunity. J. Clin. Investig. 2016, 126, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Boothby, M.; Rickert, R.C. Metabolic Regulation of the Immune Humoral Response. Immunity 2017, 46, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.; Browne, C.D.; Ruppert, R.; Féral, C.C.; Fässler, R.; Rickert, R.C.; Ginsberg, M.H. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 2009, 10, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.; Miklos, D.; Kim, H.T.; Treister, N.; Woo, S.B.; Bienfang, D.; Klickstein, L.B.; Levin, J.; Miller, K.; Reynolds, C.; et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006, 108, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.R.; Sizemore, C.A.; Ridgeway, M.; Zhang, X.; Brown, S.; Holland, H.K.; Morris, L.E.; Solh, M.; Bashey, A. Safety and efficacy of rituximab-based first line treatment of chronic GVHD. Bone Marrow Transplant. 2019, 54, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Labopin, M.; Yakoub-Agha, I.; Chantepie, S.; Guillaume, T.; Blaise, D.; Tabrizi, R.; Magro, L.; Vanhove, B.; Blancho, G.; et al. Rituximab-based first-line treatment of cGVHD after allogeneic SCT: Results of a phase 2 study. Blood 2017, 130, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.H.; Pavletic, S.Z. Syk and tired of current chronic GVHD therapies. Blood 2015, 125, 3974–3975. [Google Scholar] [CrossRef]
- Jaglowski, S.M.; Blazar, B.R. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv. 2018, 2, 2012–2019. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Flynn, R.; Du, J.; Harrington, B.K.; Zhong, Y.; Kaffenberger, B.; Yang, C.; Towns, W.H.; Lehman, A.; Johnson, A.J.; et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J. Clin. Investig. 2014, 124, 4867–4876. [Google Scholar] [CrossRef]
- Schutt, S.D.; Fu, J.; Nguyen, H.; Bastian, D.; Heinrichs, J.; Wu, Y.; Liu, C.; McDonald, D.G.; Pidala, J.; Yu, X.Z. Inhibition of BTK and ITK with Ibrutinib Is Effective in the Prevention of Chronic Graft-versus-Host Disease in Mice. PLoS ONE 2015, 10, e0137641. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.M.; Zeng, H.; Nguyen, T.-L.M.; Wang, Y.; Vogel, P.; Dhungana, Y.; Liu, X.; Neale, G.; Locasale, J.W.; Chi, H. mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat. Commun. 2018, 9, 2095. [Google Scholar] [CrossRef]
- Zhang, L.; Tschumi, B.O.; Lopez-Mejia, I.C.; Oberle, S.G.; Meyer, M.; Samson, G.; Rüegg, M.A.; Hall, M.N.; Fajas, L.; Zehn, D.; et al. Mammalian Target of Rapamycin Complex 2 Controls CD8 T Cell Memory Differentiation in a Foxo1-Dependent Manner. Cell Rep. 2016, 14, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Yang, K.; Cloer, C.; Neale, G.; Vogel, P.; Chi, H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 2013, 499, 485–490. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.S.; Guo, L.; Ghassemi, S.; Snyder, N.W.; Worth, A.J.; Weng, L.; Kam, Y.; Philipson, B.; Trefely, S.; Nunez-Cruz, S. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Feinman, R.; Colorado, I.; Wang, K.; Dziopa, E.; Flynn, M.A.; Peters, K.; Pecora, A.L.; Korngold, R. Inhibition of HIF Prolyl Hydroxylases Mitigate Gut Graft-Versus-Host Disease. Blood 2016, 128, 3349. [Google Scholar] [CrossRef]
- Lucarelli, G.; Isgrò, A.; Sodani, P.; Gaziev, J. Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb. Perspect. Med. 2012, 2, a011825. [Google Scholar] [CrossRef]
- Zaidman, I.; Rowe, J.M.; Khalil, A.; Ben-Arush, M.; Elhasid, R. Allogeneic Stem Cell Transplantation in Congenital Hemoglobinopathies Using a Tailored Busulfan-Based Conditioning Regimen: Single-Center Experience. Biol. Blood Marrow Transplant. 2016, 22, 1043–1048. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, B.; Zhu, Z.; Sun, C.; Xu, J.; Long, H.; Huang, Y.; Xu, J.; Song, C. Allogeneic Hematopoietic Stem Cell Transplant for Severe Aplastic Anemia: Current State and Future Directions. Curr. Stem. Cell Res. Ther. 2018, 13, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, H.J.; Liu, Z.; Geng, S.; Wang, H.T.; Wang, X.; Li, T.; Morel, L.; Wan, W.; Lu, L. Safety and efficacy of metformin in systemic lupus erythematosus: A multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2020, 2, e210–e216. [Google Scholar] [CrossRef]
- Piranavan, P.; Bhamra, M.; Perl, A. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2020, 2, e200012. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhandire, K.; Saggu, K.; Buxbaum, N.P. Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD). Metabolites 2021, 11, 736. https://doi.org/10.3390/metabo11110736
Mhandire K, Saggu K, Buxbaum NP. Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD). Metabolites. 2021; 11(11):736. https://doi.org/10.3390/metabo11110736
Chicago/Turabian StyleMhandire, Kudakwashe, Komalpreet Saggu, and Nataliya Prokopenko Buxbaum. 2021. "Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD)" Metabolites 11, no. 11: 736. https://doi.org/10.3390/metabo11110736
APA StyleMhandire, K., Saggu, K., & Buxbaum, N. P. (2021). Immunometabolic Therapeutic Targets of Graft-versus-Host Disease (GvHD). Metabolites, 11(11), 736. https://doi.org/10.3390/metabo11110736






