Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
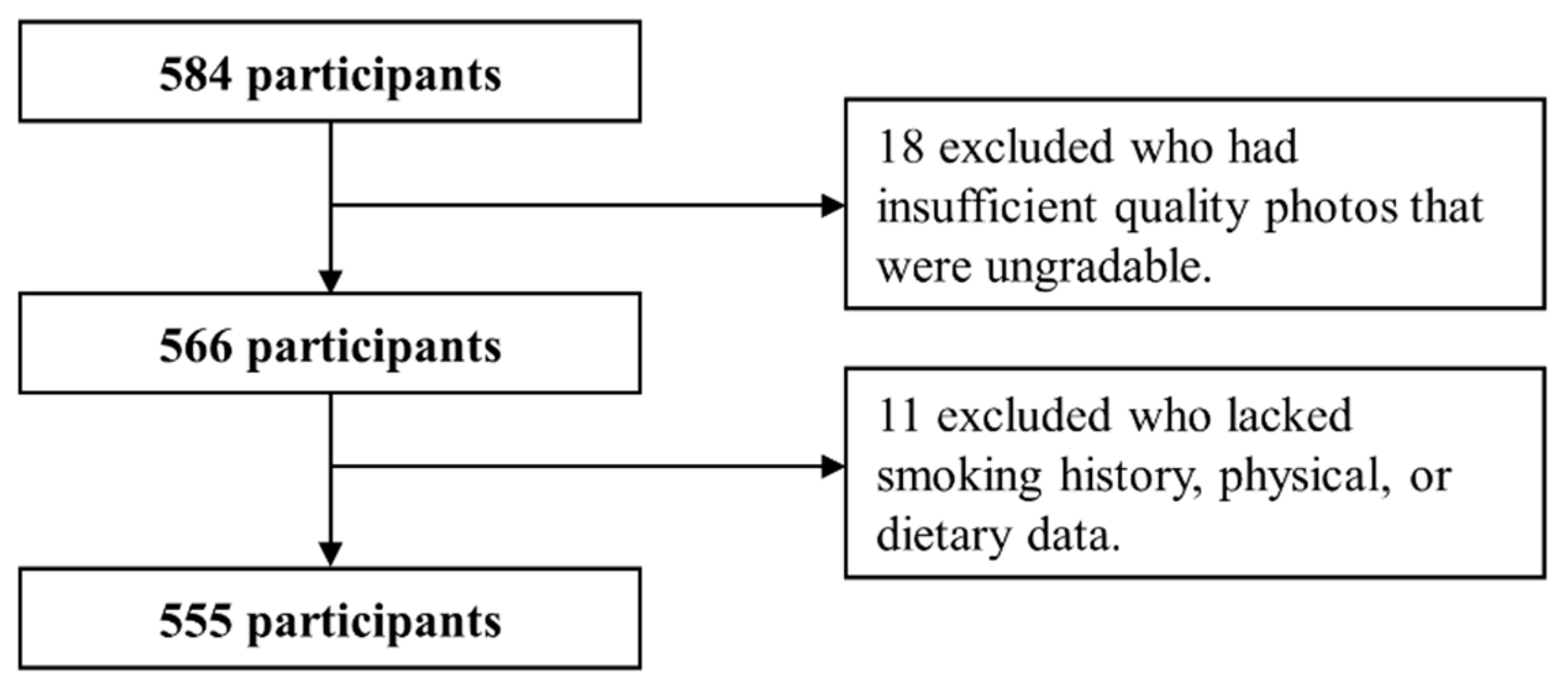
4.1. Study Participants
4.2. Dietary Assessment
4.3. Evaluation of Fundus Photographs
4.4. Assessment of Other Variables
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-Related Macular Degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. The Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Zampatti, S.; Ricci, F.; Cusumano, A.; Marsella, L.T.; Novelli, G.; Giardina, E. Review of nutrient actions on age-related macular degeneration. Nutr. Res. 2014, 34, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Horie-Inoue, K.; Gehlbach, P.L.; Takita, H.; Kabasawa, S.; Kawasaki, I.; Ohkubo, T.; Kurihara, S.; Iizuka, H.; Miyashita, Y.; et al. Phenotype and Genotype Characteristics of Age-related Macular Degeneration in a Japanese Population. Ophthalmology 2010, 117, 928–938. [Google Scholar] [CrossRef]
- Alten, F.; Eter, N. Current knowledge on reticular pseudodrusen in age-related macular degeneration. Br. J. Ophthalmol. 2015, 99, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.I.; Chen, S.J.; Cheng, C.Y.; Yen, M.Y.; Lee, F.L.; Lin, M.W.; Hsu, W.M.; Wei, Y.H. Association of the Y402H Polymorphism in Complement Factor H Gene and Neovascular Age-Related Macular Degeneration in Chinese Patients. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3242–3246. [Google Scholar] [CrossRef] [PubMed]
- Sepp, T.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Moore, A.T.; Bird, A.C.; Yates, J.R.W. Complement Factor H Variant Y402H Is a Major Risk Determinant for Geographic Atrophy and Choroidal Neovascularization in Smokers and Nonsmokers. Invest. Ophthalmol. Vis. Sci. 2006, 47, 536–540. [Google Scholar] [CrossRef]
- Fuse, N.; Miyazawa, A.; Mengkegale, M.; Yoshida, M.; Wakusawa, R.; Abe, T.; Tamai, M. Polymorphisms in Complement Factor H and Hemicentin-1 Genes in a Japanese Population With Dry-type Age-related Macular Degeneration. Am. J. Ophthalmol. 2006, 142, 1074–1076. [Google Scholar] [CrossRef]
- Okamoto, H.; Umeda, S.; Obazawa, M.; Minami, M.; Noda, T.; Mizota, A.; Honda, M.; Tanaka, M.; Koyama, R.; Takagi, I.; et al. Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol. Vis. 2006, 12, 156–158. [Google Scholar]
- Uka, J.; Tamura, H.; Kobayashi, T.; Yamane, K.; Kawakami, H.; Minamoto, A.; Mishima, H.K. No association of complement factor H gene polymorphism and age-related macular degeneration in the Japanese population. Retina 2006, 26, 985–987. [Google Scholar] [CrossRef]
- Ho, L.; van Leeuwen, R.; Witteman, J.C.; van Duijn, C.M.; Uitterlinden, A.G.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Klaver, C.C. Reducing the Genetic Risk of Age-Related Macular Degeneration With Dietary Antioxidants, Zinc, and ω-3 Fatty Acids. Arch. Ophthalmol. 2011, 129, 758–766. [Google Scholar] [CrossRef]
- Wang, J.J.; Buitendijk, G.H.S.; Rochtchina, E.; Lee, K.E.; Klein, B.E.K.; Van Duijn, C.M.; Flood, V.M.; Meuer, S.M.; Attia, J.; Myers, C.; et al. Genetic Susceptibility, Dietary Antioxidants, and Long-Term Incidence of Age-Related Macular Degeneration in Two Populations. Ophthalmology 2014, 121, 667–675. [Google Scholar] [CrossRef]
- Sugihiro, T.; Yoneda, M.; Ohno, H.; Oki, K.; Hattori, N. Associations of nutrient intakes with obesity and diabetes mellitus in the longitudinal medical surveys of Japanese Americans. J. Diabetes Investig. 2019, 10, 1229–1236. [Google Scholar] [CrossRef]
- Yoneda, M.; Kobuke, K. A 50-year history of the health impacts of Westernization on the lifestyle of Japanese Americans: A focus on the Hawaii-Los Angeles-Hiroshima Study. J. Diabetes Investig. 2020, 11, 1382–1387. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; VandenLangenberg, G.M.; Klein, B.E.K.; Palta, M. Dietary Fat and Age-Related Maculopathy. Arch. Ophthalmol. 1995, 113, 743–748. [Google Scholar] [CrossRef]
- Delcourt, C.; Carrière, I.; Cristol, J.P.; Lacroux, A.; Gerber, M. Dietary fat and the risk of age-related maculopathy: The POLANUT Study. Eur. J. Clin. Nutr. 2007, 61, 1341–1344. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Harada, S.; Tsubota, K.; Yasukawa, T.; Takebayashi, T.; Nishiwaki, Y.; Kawasaki, R. Dietary Saturated Fatty Acid Intake and Early Age-Related Macular Degeneration in a Japanese Population. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Stamler, J.; Dennis, B.; Moag-Stahlberg, A.; Okuda, N.; Robertson, C.; Zhao, L.; Chan, Q.; Elliott, P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP Study. J. Hum. Hypertens. 2003, 17, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Kubota, M.; Watanabe, H.; Egusa, G. Westernization of Lifestyle and Atherosclerosis in the Japanese: Lessons from the Hawaii-Los Angeles-Hiroshima Study. J. Atheroscler. Thromb. 2021, 28, 214–222. [Google Scholar] [CrossRef]
- Yanagi, Y.; Foo, V.H.X.; Yoshida, A. Asian age-related macular degeneration: From basic science research perspective. Eye 2019, 33, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-De La Paz, S.; Naranjo, M.C.; Bermudez, B.; Lopez, S.; Moreda, W.; Abia, R.; Muriana, F.J.G. Postprandial dietary fatty acids exert divergent inflammatory responses in retinal-pigmented epithelium cells. Food Funct. 2016, 7, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kawashima, H.; Toda, E.; Homma, K.; Osada, H.; Guzman, N.A.; Shibata, S.; Uchiyama, Y.; Okano, H.; Tsubota, K.; et al. Renin–angiotensin system impairs macrophage lipid metabolism to promote age-related macular degeneration in mouse models. Commun. Biol. 2020, 3, 767. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; De Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Jeffrey, B.G.; Weisinger, H.S.; Neuringer, M.; Mitchell, D.C. The role of docosahexaenoic acid in retinal function. Lipids 2001, 36, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Seddon, J.M.; George, S.; Rosner, B. Cigarette Smoking, Fish Consumption, Omega-3 Fatty Acid Intake, and Associations With Age-Related Macular Degeneration. Arch. Ophthalmol. 2006, 124, 995–1001. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Davis, M.D.; Ferris, F.L.R.; Gensler, G.R.; Kurinij, N.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; et al. The Relationship of Dietary Lipid Intake and Age-Related Macular Degeneration in a Case-Control Study. Arch. Ophthalmol. 2007, 125, 671–679. [Google Scholar]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; De Jong, P.T.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [CrossRef]
- Christen, W.G.; Schaumberg, D.A.; Glynn, R.J.; Buring, J.E. Dietary ω-3 Fatty Acid and Fish Intake and Incident Age-Related Macular Degeneration in Women. Arch. Ophthalmol. 2011, 129, 921–929. [Google Scholar] [CrossRef]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 Fatty Acid, α-Tocopherol, Zinc, vitamin D, vitamin C and β-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef]
- De Lorgeril, M. Essential polyunsaturated fatty acids, inflammation, atherosclerosis and cardiovascular diseases. Subcell. Biochem. 2007, 42, 283–297. [Google Scholar]
- Seddon, J.M.; Rosner, B.; Sperduto, R.D.; Yannuzzi, L.; Haller, J.A.; Blair, N.P.; Walter, W. Dietary Fat and Risk for Advanced Age-Related Macular Degeneration. Arch. Ophthalmol. 2001, 119, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Seddon, J.M. Progression of Age-Related Macular Degeneration. Arch. Ophthalmol. 2003, 121, 1728–1737. [Google Scholar] [CrossRef]
- Cougnard-Grégoire, A.; Merle, B.M.J.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Le Goff, M.; Samieri, C.; Dartigues, J.F.; Delcourt, C. Olive Oil Consumption and Age-Related Macular Degeneration: The Alienor Study. PLoS ONE 2016, 11, e0160240. [Google Scholar] [CrossRef]
- Chiu, C.J.; Hubbard, L.D.; Armstrong, J.; Rogers, G.; Jacques, P.F.; Chylack, L.T.; Hankinson, S.E.; Willett, W.C.; Taylor, A. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am. J. Clin. Nutr. 2006, 83, 880–886. [Google Scholar] [CrossRef]
- Chiu, C.J.; Milton, R.C.; Gensler, G.; Taylor, A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2007, 86, 180–188. [Google Scholar] [CrossRef]
- Chiu, C.J.; Taylor, A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011, 30, 18–53. [Google Scholar] [CrossRef]
- Hu, Y.; Block, G.; Norkus, E.P.; Morrow, J.D.; Dietrich, M.; Hudes, M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am. J. Clin. Nutr. 2006, 84, 70–76. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Bantle, J.P. Is fructose the optimal low glycemic index sweetener? Nestle Nutr. Workshop Ser. Clin. Perform. Program. 2006, 11, 83–95. [Google Scholar]
- Flood, V.; Smith, W.; Wang, J.J.; Manzi, F.; Webb, K.; Mitchell, P. Dietary antioxidant intake and incidence of early age-related maculopathy. Ophthalmology 2002, 109, 2272–2278. [Google Scholar] [CrossRef]
- Cho, E.; Seddon, J.M.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Prospective Study of Intake of Fruits, Vegetables, Vitamins, and Carotenoidsand Risk of Age-Related Maculopathy. Arch. Ophthalmol. 2004, 122, 883–892. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Russell, J.; Cosatto, V.; Burlutsky, G.; Mitchell, P. Intake of key micronutrients and food groups in patients with late-stage age-related macular degeneration compared with age–sex-matched controls. Br. J. Ophthalmol. 2017, 101, 1027–1031. [Google Scholar] [CrossRef]
- Chong, E.W.T.; Wong, T.Y.; Kreis, A.J.; Simpson, J.A.; Guymer, R.H. Dietary antioxidants and primary prevention of age related macular degeneration: Systematic review and meta-analysis. BMJ 2007, 335, 755. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D.; Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.M.; Klaver, C.C.W.; Hofman, A.; De Jong, P.T.V.M. Dietary Intake of Antioxidants and Risk of Age-Related Macular Degeneration. JAMA 2005, 294, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Yoserizal, M.; Hirooka, K.; Yoneda, M.; Ohno, H.; Kobuke, K.; Kawano, R.; Kiuchi, Y. Associations of nutrient intakes with glaucoma among Japanese Americans. Medicine 2019, 98, e18314. [Google Scholar] [CrossRef]
- Adams, C.F. The Agricultural Research Service. Nutritive Value of American Foods in Common Units; Agriculture Handbook; Agricultural Research Service, U.S. Dept. of Agriculture: Washington, DC, USA, 1975; No. 456. Available online: https://books.google.co.jp/books?hl=ja&lr=lang_ja%7Clang_en&id=VLBd7WNEfWQC&oi=fnd&pg=PA2&dq=The+Agricultur-al+Research+Service.+Nutritive+Value+of+American+Foods+in+Common+Units,+Agriculture+Handbook&ots=7y1N0jGYqY&sig=rJih9ZJodIPxrap7up10h6TTi_E&redir_esc=y#v=onepage&q=The%20Agricultural%20Research%20Service.%20Nutritive%20Value%20of%20American%20Foods%20in%20Common%20Units%2C%20Agriculture%20Handbook&f=false (accessed on 9 March 2021).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Klein, R.; Davis, M.D.; Magli, Y.L.; Segal, P.; Klein, B.E.; Hubbard, L. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991, 98, 1128–1134. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Attebo, K.; Wang, J.J. Prevalence of Age-related Maculopathy in Australia. Ophthalmology 1995, 102, 1450–1460. [Google Scholar] [CrossRef]
- Itakura, K.; Takahashi, I.; Nakashima, E.; Yanagi, M.; Kawasaki, R.; Neriishi, K.; Wang, J.J.; Wong, T.Y.; Hida, A.; Ohishi, W.; et al. Exposure to Atomic Bomb Radiation and Age-Related Macular Degeneration in Later Life: The Hiroshima-Nagasaki Atomic Bomb Survivor Study. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5401–5406. [Google Scholar] [CrossRef][Green Version]
- Oshima, Y.; Ishibashi, T.; Murata, T.; Tahara, Y.; Kiyohara, Y.; Kubota, T. Prevalence of age related maculopathy in a representative Japanese population: The Hisayama study. Br. J. Ophthalmol. 2001, 85, 1153–1157. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R. Prevalence of Age-Related Macular Degeneration in 4 Racial/Ethnic Groups in the Multi-ethnic Study of Atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef]
- Nath Kundu, R.; Biswas, S.; Das, M. Mean Arterial Pressure Classification: A Better Tool for Statistical Interpretation of Blood Pressure Related Risk Covariates. Cardiol. Angiol. Int. J. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef]
| Characteristics | Early AMD (n = 111) | Control (n = 444) | p Value 1 |
|---|---|---|---|
| Age (years) | 66.5 ± 10.3 | 60.4 ± 13.7 | <0.001 |
| Sex (M/F) | 41/70 | 176/268 | 0.60 |
| BMI (kg/m2) | 23.2 ± 3.7 | 23.3 ± 3.7 | 0.73 |
| Smoking, n (%) | 0.46 | ||
| Never | 71 (64.0%) | 256 (57.7%) | |
| Former | 30 (27.0%) | 136 (30.6%) | |
| Current | 10 (9.0%) | 52 (11.7%) | |
| Hypertension, n (%) | 24 (21.6%) | 71 (16.0%) | 0.16 |
| Diabetes, n (%) | 15 (13.5%) | 35 (7.9%) | 0.06 |
| Generation, n (%) | 0.75 | ||
| 1st | 97 (87.4%) | 381 (85.8%) | |
| 2nd | 5 (4.5%) | 30 (6.8%) | |
| 3rd | 8 (7.2%) | 24 (5.4%) | |
| 4th | 1 (0.9%) | 8 (1.8%) | |
| 5th | 0 (0%) | 1 (0.2%) |
| Nutrient | Early AMD (n = 111) | Control (n = 444) | p Value 1 |
|---|---|---|---|
| Total energy (kcal) | 2249.5 ± 684.8 | 2220.2 ± 607.1 | 0.86 |
| Animal protein | 36.7 ± 11.3 | 37.8 ± 10.9 | 0.31 |
| Vegetable protein | 34.6 ± 7.8 | 35.1 ± 7.0 | 0.57 |
| Animal fat (g) | 44.5 ± 36.2 | 35.9 ± 26.2 | 0.03 |
| Vegetable fat (g) | 34.0 ± 10.5 | 35.9 ± 8.1 | 0.03 |
| SFA (g) | 23.3 ± 10.3 | 21.0 ± 7.7 | 0.06 |
| PUFA (g) | 13.7 ± 3.5 | 14.4 ± 2.8 | 0.007 |
| Cholesterol (mg) | 274.8 ± 91.2 | 279.6 ± 95.9 | 0.91 |
| Total carbohydrate (g) | 280.1 ± 70.6 | 293.1 ± 58.4 | 0.08 |
| Simple carbohydrate (g) | 255.0 ± 62.0 | 267.1 ± 51.0 | 0.07 |
| Complex carbohydrate (g) | 25.1 ± 11.1 | 26.0 ± 10.6 | 0.75 |
| Sugar (g) | 252.2 ± 62.7 | 263.4 ± 51.5 | 0.09 |
| Fructose (g) | 2.8 ± 3.6 | 3.7 ± 4.2 | 0.23 |
| Fiber (g) | 4.3 ± 1.8 | 4.3 ± 1.8 | 0.62 |
| Vitamin A (μgRAE) | 597.6 ± 255.6 | 599.3 ± 257.7 | 0.79 |
| Vitamin B1 (mg) | 0.91 ± 0.27 | 0.92 ± 0.28 | 0.89 |
| Vitamin B2 (mg) | 1.23 ± 0.37 | 1.23 ± 0.34 | 0.74 |
| Vitamin C (mg) | 167.8 ± 71.7 | 169.6 ± 69.0 | 0.90 |
| Calcium (mg) | 636.2 ± 201.9 | 602.7 ± 176.3 | 0.10 |
| Iron (mg) | 6.9 ± 1.9 | 7.0 ± 2.1 | 0.92 |
| Potassium (mg) | 2924.5 ± 748.8 | 2949.3 ± 729.9 | 0.89 |
| Salt (g) | 5.4 ± 2.5 | 5.7 ± 2.3 | 0.30 |
| Nutrient | Odds Ratio (95% CI) | p for Trend | |||
|---|---|---|---|---|---|
| Q1 (Lowest) | Q2 | Q3 | Q4 (Highest) | ||
| Animal protein (g) | ≤30.3 | 30.3–36.8 | 36.8–42.9 | >42.9 | |
| No. with outcome/at risk | 34/139 (24.5%) | 28/139 (20.1%) | 18/139 (13.0%) | 31/138 (22.5%) | |
| Model 1 1 | 1 (Reference) | 0.70 (0.39–1.26) | 0.47 (0.25–0.89) | 0.91 (0.51–1.61) | 0.42 |
| Model 2 2 | 1 (Reference) | 0.69 (0.39–1.24) | 0.47 (0.25–0.89) | 0.92 (0.52–1.65) | 0.22 |
| Vegetable protein (g) | ≤30.6 | 30.6–34.9 | 34.9–39.6 | >39.6 | |
| No. with outcome/at risk | 34/139 (24.5%) | 24/139 (17.3%) | 24/139 (17.3%) | 29/138 (21.0%) | |
| Model 1 1 | 1 (Reference) | 0.55 (0.30–1.00) | 0.55 (0.30–1.00) | 0.69 (0.39–1.24) | 0.25 |
| Model 2 2 | 1 (Reference) | 0.54 (0.29–0.99) | 0.54 (0.29–0.99) | 0.68 (0.38–1.23) | 0.27 |
| Animal fat (g) | ≤22.3 | 22.3–33.2 | 33.2–46.2 | >46.2 | |
| No. with outcome/at risk | 22/139 (15.8%) | 27/139 (19.4%) | 23/139 (16.6%) | 39/138 (28.3%) | |
| Model 1 1 | 1 (Reference) | 1.12 (0.59–2.11) | 0.93 (0.48–1.78) | 1.88 (1.03–3.42) | 0.01 |
| Model 2 2 | 1 (Reference) | 1.12 (0.59–2.13) | 0.88 (0.45–1.72) | 1.86 (1.01–3.42) | 0.01 |
| Vegetable fat (g) | ≤29.7 | 29.7–35.5 | 35.5–40.6 | >40.6 | |
| No. with outcome/at risk | 35/139 (25.2%) | 30/139 (21.6%) | 24/139 (17.3%) | 22/138 (15.9%) | |
| Model 1 1 | 1 (Reference) | 0.74 (0.42–1.31) | 0.56 (0.31–1.03) | 0.55 (0.30–1.01) | 0.03 |
| Model 2 2 | 1 (Reference) | 0.71 (0.39–1.26) | 0.53 (0.29–0.99) | 0.54 (0.29–1.01) | 0.04 |
| SFA (g) | ≤17.0 | 17.0–20.7 | 20.7–24.5 | >24.5 | |
| No. with outcome/at risk | 20/139 (14.4%) | 29/139 (20.9%) | 27/139 (19.4%) | 35/138 (25.4%) | |
| Model 1 1 | 1 (Reference) | 1.31 (0.69–2.49) | 1.19 (0.62–2.29) | 1.85 (0.99–3.44) | 0.02 |
| Model 2 2 | 1 (Reference) | 1.27 (0.66–2.44) | 1.14 (0.58–2.23) | 1.80 (0.96–3.40) | 0.02 |
| PUFA (g) | ≤12.4 | 12.4–13.9 | 13.9–15.9 | >15.9 | |
| No. with outcome/at risk | 36/139 (25.9%) | 32/140 (22.9%) | 21/138 (15.2%) | 22/138 (15.9%) | |
| Model 1 1 | 1 (Reference) | 0.81 (0.46–1.42) | 0.49 (0.27–0.91) | 0.56 (0.31–1.04) | 0.07 |
| Model 2 2 | 1 (Reference) | 0.78 (0.44–1.37) | 0.48 (0.26–0.89) | 0.56 (0.30–1.04) | 0.08 |
| Cholesterol (mg) | ≤218.7 | 218.7–267.0 | 267.0–322.3 | >322.3 | |
| No. with outcome/at risk | 23/139 (16.5%) | 31/139 (22.3%) | 31/139 (22.3%) | 26/138 (18.8%) | |
| Model 1 1 | 1 (Reference) | 1.59 (0.86–2.93) | 1.52 (0.82–2.81) | 1.17 (0.62–2.20) | 0.55 |
| Model 2 2 | 1 (Reference) | 1.52 (0.82–2.83) | 1.52 (0.82–2.83) | 1.14 (0.61–2.16) | 0.55 |
| Total carbohydrate (g) | ≤266.4 | 266.4–295.3 | 295.3–323.4 | >323.4 | |
| No. with outcome/at risk | 36/139 (25.9%) | 27/139 (19.4%) | 25/139 (18.0%) | 23/138 (16.7%) | |
| Model 1 1 | 1 (Reference) | 0.68 (0.38–1.21) | 0.61 (0.34–1.10) | 0.56 (0.21–1.03) | 0.04 |
| Model 2 2 | 1 (Reference) | 0.68 (0.38–1.23) | 0.61 (0.34–1.11) | 0.58 (0.31–1.05) | 0.046 |
| Simple carbohydrate (g) | ≤244.0 | 244.0–268.8 | 268.8–293.7 | >293.7 | |
| No. with outcome/at risk | 38/139 (27.3%) | 23/139 (16.6%) | 26/139 (18.7%) | 24/138 (17.4%) | |
| Model 1 1 | 1 (Reference) | 0.51 (0.28–0.92) | 0.59 (0.33–1.06) | 0.53 (0.29–0.96) | 0.03 |
| Model 2 2 | 1 (Reference) | 0.50 (0.27–0.91) | 0.60 (0.34–1.07) | 0.54 (0.30–0.97) | 0.03 |
| Complex carbohydrate (g) | ≤18.8 | 18.8–25.7 | 25.7–32.1 | >32.1 | |
| No. with outcome/at risk | 29/139 (20.9%) | 26/139 (18.7%) | 30/139 (21.6%) | 26/138 (18.8%) | |
| Model 1 1 | 1 (Reference) | 0.88 (0.48–1.61) | 1.10 (0.61–1.97) | 0.96 (0.53–1.76) | 0.68 |
| Model 2 2 | 1 (Reference) | 0.89 (0.49–1.63) | 1.12 (0.62–2.03) | 0.99 (0.54–1.82) | 0.73 |
| Sugar (g) | ≤239.3 | 239.3–265.8 | 265.8–291.6 | >291.6 | |
| No. with outcome/at risk | 37/139 (26.6%) | 24/140 (17.1%) | 27/139 (19.4%) | 24/137 (16.8%) | |
| Model 1 1 | 1 (Reference) | 0.56 (0.31–1.00) | 0.65 (0.36–1.16) | 0.54 (0.29–0.98) | 0.04 |
| Model 2 2 | 1 (Reference) | 0.56 (0.31–1.01) | 0.66 (0.37–1.17) | 0.54 (0.30–0.99) | 0.046 |
| Fructose (g) | ≤0.1 | 0.1–1.3 | 1.3–6.8 | >6.8 | |
| No. with outcome/at risk | 28/142 (19.7%) | 36/136 (26.5%) | 26/139 (18.7%) | 21/138 (15.2%) | |
| Model 1 1 | 1 (Reference) | 1.30 (0.73–2.32) | 0.85 (0.46–1.56) | 0.60 (0.32–1.14) | 0.02 |
| Model 2 2 | 1 (Reference) | 1.27 (0.70–2.32) | 0.83 (0.45–1.54) | 0.59 (0.31–1.13) | 0.02 |
| Fiber (g) | ≤3.2 | 3.2–4.0 | 4.0–5.1 | >5.1 | |
| No. with outcome/at risk | 30/139 (21.6%) | 27/139 (19.4%) | 30/139 (21.6%) | 24/138 (17.4%) | |
| Model 1 1 | 1 (Reference) | 0.86 (0.47–1.56) | 0.92 (0.51–1.66) | 0.75 (0.41–1.38) | 0.63 |
| Model 2 2 | 1 (Reference) | 0.89 (0.49–1.63) | 0.94 (0.52–1.70) | 0.73 (0.40–1.36) | 0.62 |
| Nutrient | Odds Ratio (95% CI) | p for Trend | |||
|---|---|---|---|---|---|
| Q1 (Lowest) | Q2 | Q3 | Q4 (Highest) | ||
| Vitamin A (μgRAE) | ≤423.4 | 423.4–525.2 | 525.2–729.1 | >729.1 | |
| No. with outcome/at risk | 32/139 (23.0%) | 21/139 (15.1%) | 27/139 (19.4%) | 31/138 (22.4%) | |
| Model 1 1 | 1 (Reference) | 0.54 (0.29–1.00) | 0.73 (0.40–1.31) | 0.89 (0.50–1.59) | 0.82 |
| Model 2 2 | 1 (Reference) | 0.55 (0.29–1.04) | 0.74 (0.41–1.35) | 0.88 (0.49–1.58) | 0.77 |
| Vitamin B1 (g) | ≤0.73 | 0.73–0.88 | 0.88–1.1 | >1.1 | |
| No. with outcome/at risk | 28/141 (19.9%) | 27/140 (19.3%) | 25/141 (17.7%) | 31/133 (23.3%) | |
| Model 1 1 | 1 (Reference) | 0.92 (0.51–1.68) | 0.90 (0.49–1.67) | 1.32 (0.73–2.39) | 0.83 |
| Model 2 2 | 1 (Reference) | 0.94 (0.51–1.72) | 0.95 (0.51–1.77) | 1.39 (0.76–2.53) | 0.94 |
| Vitamin B2 (g) | ≤1.0 | 1.0–1.2 | 1.2–1.5 | >1.5 | |
| No. with outcome/at risk | 29/139 (20.9%) | 23/143 (16.1%) | 24/136 (17.7%) | 35/137 (25.6%) | |
| Model 1 1 | 1 (Reference) | 0.66 (0.36–1.23) | 0.71 (0.38–1.32) | 1.23 (0.69–2.19) | 0.97 |
| Model 2 2 | 1 (Reference) | 0.67 (0.36–1.25) | 0.72 (0.39–1.34) | 1.27 (0.70–2.28) | 0.96 |
| Vitamin C (mg) | ≤119.3 | 119.3–155.6 | 155.6–206.0 | >206.0 | |
| No. with outcome/at risk | 29/139 (20.9%) | 26/139 (18.7%) | 32/139 (23.0%) | 24/138 (17.4%) | |
| Model 1 1 | 1 (Reference) | 0.75 (0.41–1.38) | 0.98 (0.55–1.76) | 0.70 (0.38–1.30) | 0.58 |
| Model 2 2 | 1 (Reference) | 0.78 (0.42–1.46) | 1.00 (0.55–1.82) | 0.69 (0.37–1.29) | 0.54 |
| Calcium (g) | ≤473.7 | 473.7–605.1 | 605.1–729.5 | >729.5 | |
| No. with outcome/at risk | 29/139 (20.9%) | 20/139 (14.4%) | 23/139 (16.6%) | 39/138 (28.3%) | |
| Model 1 1 | 1 (Reference) | 0.55 (0.29–1.05) | 0.64 (0.34–1.20) | 1.20 (0.67–2.12) | 0.34 |
| Model 2 2 | 1 (Reference) | 0.54 (0.28–1.03) | 0.61 (0.32–1.15) | 1.21 (0.68–2.17) | 0.32 |
| Iron (g) | ≤5.4 | 5.4–6.7 | 6.7–8.2 | >8.2 | |
| No. with outcome/at risk | 26/140 (18.6%) | 25/138 (18.1%) | 34/139 (24.5%) | 26/138 (18.8%) | |
| Model 1 1 | 1 (Reference) | 0.95 (0.51–1.77) | 1.32 (0.73–2.38) | 1.00 (0.54–1.83) | 0.66 |
| Model 2 2 | 1 (Reference) | 0.97 (0.52–1.82) | 1.36 (0.75–2.47) | 1.00 (0.54–1.85) | 0.67 |
| Potassium (mg) | ≤2413.2 | 2413.2–2822.2 | 2822.2–3341.8 | >3341.8 | |
| No. with outcome/at risk | 33/139 (23.7%) | 21/139 (15.1%) | 28/139 (20.1%) | 29/138 (21.0%) | |
| Model 1 1 | 1 (Reference) | 0.51 (0.27–0.94) | 0.72 (0.40–1.28) | 0.76 (0.43–1.37) | 0.57 |
| Model 2 2 | 1 (Reference) | 0.52 (0.28–0.97) | 0.73 (0.40–1.33) | 0.75 (0.42–1.35) | 0.57 |
| Salt (g) | ≤3.9 | 3.9–5.4 | 5.4–7.2 | >7.2 | |
| No. with outcome/at risk | 31/140 (22.1%) | 27/138 (19.6%) | 27/140 (19.3%) | 26/137 (19.0%) | |
| Model 1 1 | 1 (Reference) | 0.97 (0.53–1.75) | 0.86 (0.48–1.55) | 0.89 (0.49–1.62) | 0.36 |
| Model 2 2 | 1 (Reference) | 0.98 (0.54–1.79) | 0.86 (0.47–1.56) | 0.93 (0.51–1.71) | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edo, A.; Pertiwi, Y.D.; Hirooka, K.; Masuda, S.; Kamaruddin, M.I.; Yanagi, M.; Nagao, A.; Ohno, H.; Yoneda, M.; Kiuchi, Y. Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans. Metabolites 2021, 11, 673. https://doi.org/10.3390/metabo11100673
Edo A, Pertiwi YD, Hirooka K, Masuda S, Kamaruddin MI, Yanagi M, Nagao A, Ohno H, Yoneda M, Kiuchi Y. Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans. Metabolites. 2021; 11(10):673. https://doi.org/10.3390/metabo11100673
Chicago/Turabian StyleEdo, Ayaka, Yunialthy Dwia Pertiwi, Kazuyuki Hirooka, Shun Masuda, Muhammad Irfan Kamaruddin, Masahide Yanagi, Akiko Nagao, Haruya Ohno, Masayasu Yoneda, and Yoshiaki Kiuchi. 2021. "Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans" Metabolites 11, no. 10: 673. https://doi.org/10.3390/metabo11100673
APA StyleEdo, A., Pertiwi, Y. D., Hirooka, K., Masuda, S., Kamaruddin, M. I., Yanagi, M., Nagao, A., Ohno, H., Yoneda, M., & Kiuchi, Y. (2021). Association of Dietary Nutrient Intake with Early Age-Related Macular Degeneration in Japanese-Americans. Metabolites, 11(10), 673. https://doi.org/10.3390/metabo11100673






