Characterizing Marathon-Induced Metabolic Changes Using 1H-NMR Metabolomics
Abstract
:1. Introduction
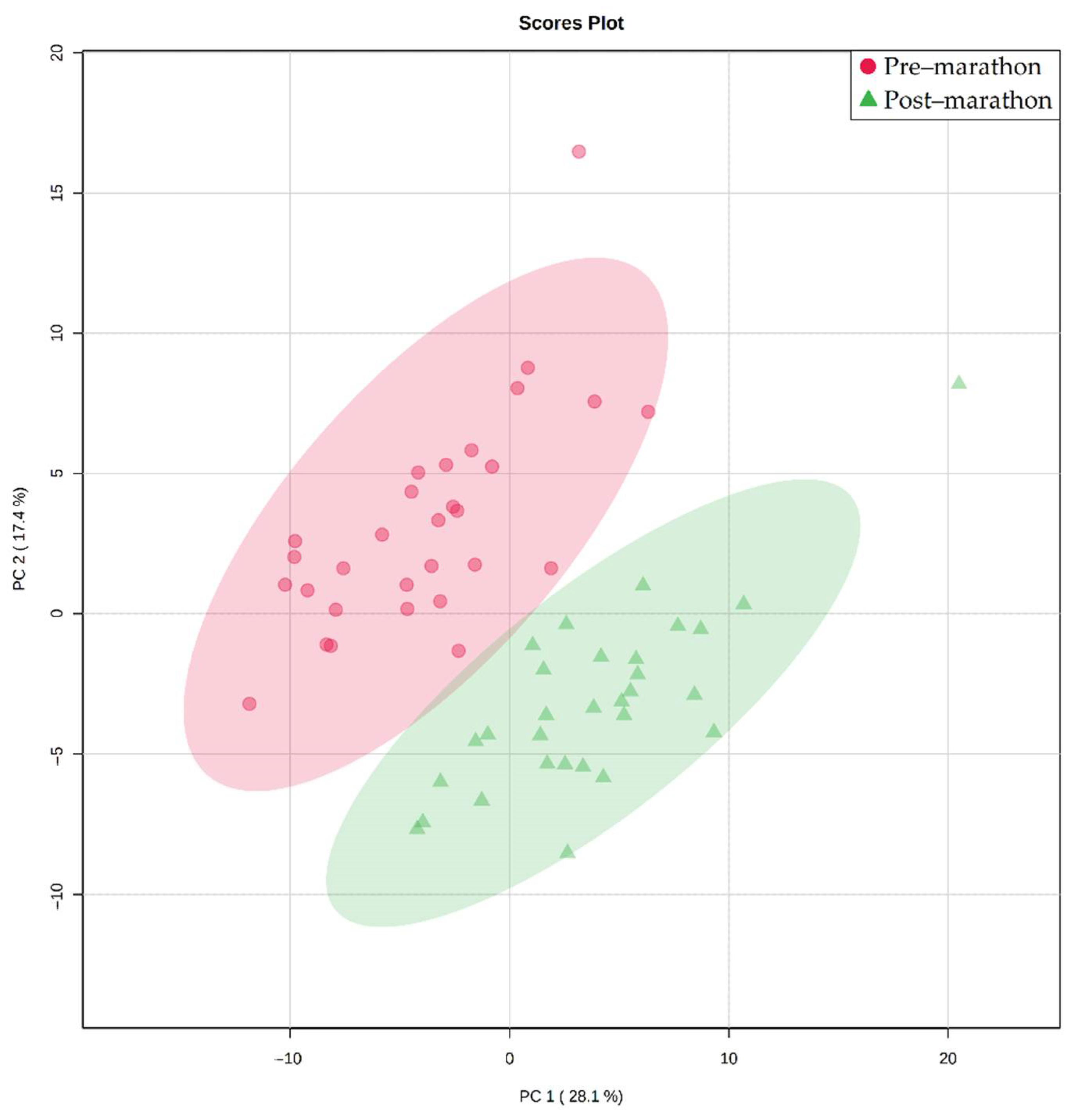
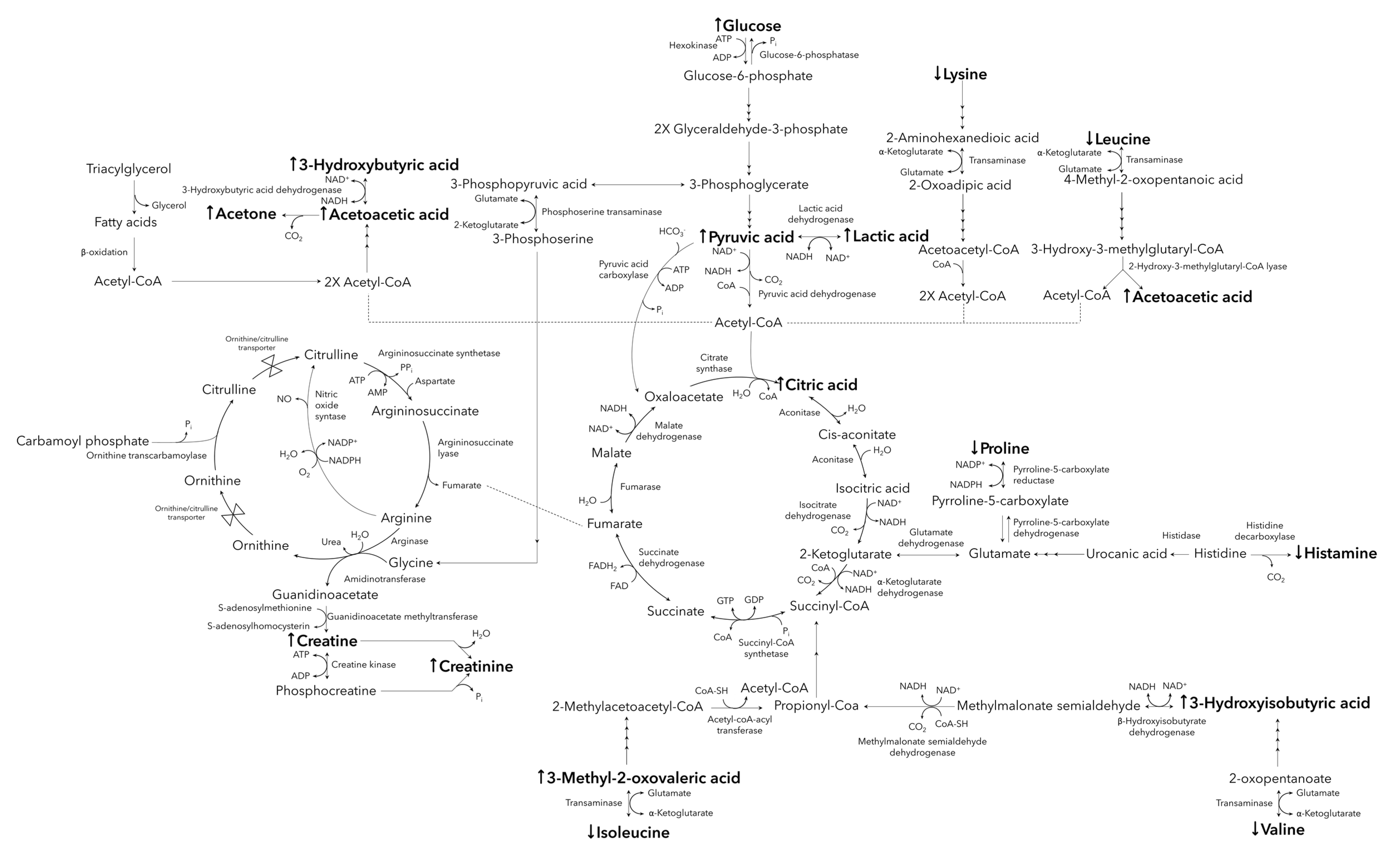
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Druridge Bay Marathon
4.3. Sample Collection and Storage
4.4. 1H-NMR Serum Buffer Solution
4.5. Sample Preparation and Randomization
4.6. 1H-NMR Analysis
4.7. Data Processing and Clean-Up
4.8. Bins/Metabolite Marker Selection and Statistical Analysis
4.9. 2D-NMR Analysis and Identification
4.10. Absolute Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambrozic, G.; Udovc, G.; Krusic, P. IAAF Competition Rules 2018–2019; Athletic Association of Slovenia: Ljubljana, Slovenia, 2018. [Google Scholar]
- Febbraio, M.A. Exercise metabolism in 2016: Health benefits of exercise—more than meets the eye! Nat. Rev. Endocrinol. 2017, 13, 72–74. [Google Scholar] [CrossRef]
- Lee, D.C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Morici, G.; Gruttad’Auria, C.I.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Bonsignore, M.R. Endurance training: Is it bad for you? Breathe 2016, 12, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.S.; Nascimento, D.C.; Prestes, J.; Nobrega, O.T.; Cordova, C.; Sousa, F.; Boullosa, D.A. Acute and Chronic Effects of Endurance Running on Inflammatory Markers: A Systematic Review. Front. Physiol. 2017, 8, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimenti, L.; Morici, G.; Paterno, A.; Santagata, R.; Bonanno, A.; Profita, M.; Riccobono, L.; Bellia, V.; Bonsignore, M.R. Bronchial epithelial damage after a half-marathon in nonasthmatic amateur runners. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L857–L862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper Respiratory Symptoms, Gut Health and Mucosal Immunity in Athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, A.P.R.; Lima, G.H.O.; da Silva, E.D.; Maciel, J.F.S.; Benetti, M.P.; de Oliveira, R.A.; Martins, P.F.O.; Kiss, M.A.P.; Ghorayeb, N.; Newsholme, P.; et al. Angiotensin-Converting Enzyme Related-Polymorphisms on Inflammation, Muscle and Myocardial Damage After a Marathon Race. Front. Genet. 2019, 10, 984. [Google Scholar] [CrossRef] [Green Version]
- Newman, P.; Waddington, G.; Adams, R. Shockwave treatment for medial tibial stress syndrome: A randomized double blind sham-controlled pilot trial. J. Sci. Med. Sport 2017, 20, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; O’Hanlon, R.; Prasad, S.; Deighan, A.; Macmillan, P.; Oxborough, D.; Godfrey, R.; Smith, G.; Maceira, A.; Sharma, S.; et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J. Appl. Physiol. 2011, 110, 1622–1626. [Google Scholar] [CrossRef]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.H.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective-An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef]
- Nescolarde, L.; Roca, E.; Bogonez-Franco, P.; Hernandez-Hermoso, J.; Bayes-Genis, A.; Ara, J. Relationship Between Bioimpedance Vector Displacement and Renal Function After a Marathon in Non-elite Runners. Front. Physiol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Deda, O.; Gika, H.; Panagoulis, T.; Taitzoglou, I.; Raikos, N.; Theodoridis, G. Impact of exercise on fecal and cecal metabolome over aging: A longitudinal study in rats. Bioanalysis 2017, 9, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef]
- Schader, J.F.; Haid, M.; Cecil, A.; Schoenfeld, J.; Halle, M.; Pfeufer, A.; Prehn, C.; Adamski, J.; Nieman, D.C.; Scherr, J. Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Shanely, R.A.; Gillitt, N.D.; Pappan, K.L.; Lila, M.A. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J. Proteome Res. 2013, 12, 4577–4584. [Google Scholar] [CrossRef]
- Stander, Z.; Luies, L.; Mienie, L.J.; Keane, K.M.; Howatson, G.; Clifford, T.; Stevenson, E.J.; Loots, D.T. The altered human serum metabolome induced by a marathon. Metabolomics 2018, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerf, T. Case study: Nutrition and training periodization in three elite marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Leckey, J.J. Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med. 2015, 45, S5–S12. [Google Scholar] [CrossRef] [Green Version]
- Engelking, L.R. Exercise (Substrate Utilization and Endocrine Parameters). In Textbook of Veterinary Physiological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 498–502. [Google Scholar] [CrossRef]
- Waskiewicz, Z.; Klapcinska, B.; Sadowska-Krepa, E.; Czuba, M.; Kempa, K.; Kimsa, E.; Gerasimuk, D. Acute metabolic responses to a 24-h ultra-marathon race in male amateur runners. Eur. J. Appl. Physiol. 2012, 112, 1679–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schranner, D.; Kastenmuller, G.; Schonfelder, M.; Romisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterhuizen, K.; van der Westhuizen, F.H.; Louw, R. Metabolomics of mitochondrial disease. Mitochondrion 2017, 35, 97–110. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrol. 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Beserra, J.B.; de Oiveira, A.R.S.; Cruz, K.J.C.; de Sousa Melo, S.R.; do Nascimento, G.V.R.; de Macedo, G.F.S.; do Nascimento Marreiro, D. Association Between Cortisol, Insulin Resistance and Zinc in Obesity: A Mini-Review. Biol. Trace Elem. Res. 2019, 191, 323–330. [Google Scholar] [CrossRef]
- Patgiri, A.; Skinner, O.S.; Miyazaki, Y.; Schleifer, G.; Marutani, E.; Shah, H.; Sharma, R.; Goodman, R.P.; To, T.L.; Robert Bao, X.; et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD(+) imbalance. Nat. Biotechnol. 2020, 38, 309–313. [Google Scholar] [CrossRef]
- McKenzie, S.; Phillips, S.M.; Carter, S.L.; Lowther, S.; Gibala, M.J.; Tarnopolsky, M.A. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E580–E587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocito, L.; Kleckner, A.S.; Yoo, E.J.; Jones Iv, A.R.; Liesa, M.; Corkey, B.E. The extracellular redox state modulates mitochondrial function, gluconeogenesis, and glycogen synthesis in murine hepatocytes. PLoS ONE 2015, 10, e0122818. [Google Scholar] [CrossRef] [Green Version]
- Philippou, A.; Chryssanthopoulos, C.; Maridaki, M.; Dimitriadis, G.; Koutsilieris, M. Exercise Metabolism in Health and Disease. In Cardiorespiratory Fitness in Cardiometabolic Diseases; Springer International Publishing: Cham, Switzerland, 2019; pp. 57–96. [Google Scholar] [CrossRef]
- Holecek, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luttrell, M.J.; Halliwill, J.R. The Intriguing Role of Histamine in Exercise Responses. Exerc. Sport Sci. Rev. 2017, 45, 16–23. [Google Scholar] [CrossRef]
- Poncet, N.; Taylor, P.M. The role of amino acid transporters in nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 57–65. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, J.D.; Jeon, H.S.; Kim, A.R.; Kim, S.; Lee, H.S.; Kim, K.B. Metabolic Profiling of Eccentric Exercise-Induced Muscle Damage in Human Urine. Toxicol. Res. 2018, 34, 199–210. [Google Scholar] [CrossRef]
- McDermott, B.P.; Smith, C.R.; Butts, C.L.; Caldwell, A.R.; Lee, E.C.; Vingren, J.L.; Munoz, C.X.; Kunces, L.J.; Williamson, K.; Ganio, M.S.; et al. Renal stress and kidney injury biomarkers in response to endurance cycling in the heat with and without ibuprofen. J. Sci. Med. Sport 2018, 21, 1180–1184. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lichtenauer, M.; Wernly, B.; Paar, V.; Pistulli, R.; Rohm, I.; Jung, C.; Figulla, H.R.; Yilmaz, A.; Cadamuro, J.; et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2017, 47, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The plasma bioavailability of nitrate and betanin from Beta vulgaris rubra in humans. Eur. J. Nutr. 2016, 56, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stander, Z.; Luies, L.; Mienie, L.J.; Van Reenen, M.; Howatson, G.; Keane, K.M.; Clifford, T.; Stevenson, E.J.; Loots, D.T. The unaided recovery of marathon-induced serum metabolome alterations. Sci. Rep. 2020, 10, 11060. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Terburgh, K.; Louw, R. Miniaturized (1)H-NMR method for analyzing limited-quantity samples applied to a mouse model of Leigh disease. Metabolomics 2018, 14, 74. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Fuks, L.F.; Huang, F.S.C.; Carter, C.M.; Edelstein, W.A.; Roemer, P.B. Susceptibility, lineshape, and shimming in high-resolution NMR. J. Magn. Reson. 1992, 100, 229–242. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Halouska, S.; Powers, R. Negative impact of noise on the principal component analysis of NMR data. J. Magn. Reson. 2006, 178, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Ialongo, C. Understanding the effect size and its measures. Biochem. Med. 2016, 26, 150–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling The False Discovery Rate—A Practical And Powerful Approach To Multiple Testing. J. R. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ludwig, C.; Viant, M.R. Two-dimensional J-resolved NMR spectroscopy: Review of a key methodology in the metabolomics toolbox. Phytochem. Anal. 2010, 21, 22–32. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
| Metabolite (PubChem ID) | Pre-Marathon | Post-Marathon | Pre- vs. Post-Marathon | |
|---|---|---|---|---|
| Average Concentration in µM (Standard Deviation) | p-Value (<0.05) | d-Value (≥0.5) | ||
| 3-Hydroxybutyric acid (441) c | 56.7 (32.4) | 424.8 (268.8) | 1.0 | 3.7 |
| 3-Hydroxyisobutyric acid (87) a * | 19.4 (5.9) | 38.5 (9.4) | 9.1 | 1.9 |
| 3-Methyl-2-oxovaleric acid (47) b | 35.7 (16.3) | 70.5 (18.2) | 7.7 | 1.4 |
| Acetoacetic acid (96) b | 21.3 (6.2) | 55.0 (26.2) | 2.4 | 2.5 |
| Acetone (180) b | 6.7 (2.1) | 17.2 (11.2) | 7.7 | 2.3 |
| Citric acid (311) c | 137.5 (33.6) | 221.9 (55.0) | 2.9 | 2.0 |
| Creatine (586) b | 67.9 (21.9) | 100.2 (51.8) | 9.7 | 1.1 |
| Creatinine (588) b | 50.7 (9.5) | 70.1 (18.5) | 2.5 | 1.3 |
| Glucose (5793) | 1426.1 (382.1) | 1927.3 (469.6) | 1.4 | 1.1 |
| Histamine (774) a * | 93.9 (26.6) | 68.9 (26.8) | 2.5 | 1.5 |
| Isoleucine (6306) | 72.3 (20.4) | 49.5 (10.9) | 1.7 | 1.1 |
| Lactic acid (612) | 2472.0 (851.5) | 4423.3 (1182.7) | 2.0 | 1.9 |
| Leucine (6106) | 159.0 (36.8) | 119.0 (22.0) | 1.7 | 1.2 |
| Lysine (5962) | 161.4 (42.0) | 127.6 (30.4) | 1.4 | 0.9 |
| Proline (145742) c | 284.1 (73.3) | 219.2 (59.0) | 5.8 | 1.0 |
| Pyruvic acid (1060) b | 60.9 (28.1) | 112.5 (38.5) | 6.3 | 1.4 |
| Valine (6287) | 267.0 (53.3) | 200.3 (35.1) | 1.8 | 1.3 |
| Participant Characteristics | Average ± Standard Deviation |
|---|---|
| Age (years) | 41 ± 12 |
| Gender (M/F) | 18/12 |
| Height (m) | 1.7 ± 0.1 |
| Mass change (kg) | −1.3 ± 1.0 |
| Experience (years) | 9.6 ± 8.4 |
| Finishing time (hh:mm:ss) | 04:16:13 ± 00:47:01 |
| Peak | Metabolite | Chemical Shift (ppm) | Protons (n) | Multiplicity | Chemical Moiety |
|---|---|---|---|---|---|
| 1 | 3-Hydroxybutyric acid c | 1.21 | 3 | d | CH3 |
| 2 | 3-Hydroxyisobutyric acid a * | 1.08 | 3 | d | CH3 |
| 3 | 3-Methyl-2-oxovaleric acid b | 1.10 | 3 | d | CH3 |
| 4 | Acetoacetic acid b | 2.28 | 3 | s | CH3 |
| 5 | Acetone b | 2.24 | 6 | s | CH3 |
| 6 | Citric acid c | 2.60 | 2 | d | CH2 |
| 7 | Creatine b | 3.93 | 2 | s | CH2 |
| 8 | Creatinine b | 4.06 | 2 | s | CH2 |
| 9 | α-Glucose | 5.24 | 1 | d | CH |
| 10 | β-Glucose | 4.66 | 1 | d | CH |
| 11 | Histamine a * | 7.06 | 1 | s | CH |
| 12 | Isoleucine | 1.01 | 3 | d | CH3 |
| 13 | Lactic acid | 1.33 | 3 | d | CH3 |
| 14 | Leucine | 0.96 | 6 | dd | (CH3)2 |
| 15 | Lysine | 3.02 | 2 | t | CH2 |
| 16 | Proline | 2.01 | 2 | m | CH2 |
| 17 | Pyruvic acid b | 2.38 | 3 | s | CH3 |
| 18 | Valine | 1.04 | 3 | d | CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bester, R.; Stander, Z.; Mason, S.; Keane, K.M.; Howatson, G.; Clifford, T.; Stevenson, E.J.; Loots, D.T. Characterizing Marathon-Induced Metabolic Changes Using 1H-NMR Metabolomics. Metabolites 2021, 11, 656. https://doi.org/10.3390/metabo11100656
Bester R, Stander Z, Mason S, Keane KM, Howatson G, Clifford T, Stevenson EJ, Loots DT. Characterizing Marathon-Induced Metabolic Changes Using 1H-NMR Metabolomics. Metabolites. 2021; 11(10):656. https://doi.org/10.3390/metabo11100656
Chicago/Turabian StyleBester, Rachelle, Zinandré Stander, Shayne Mason, Karen M. Keane, Glyn Howatson, Tom Clifford, Emma J. Stevenson, and Du Toit Loots. 2021. "Characterizing Marathon-Induced Metabolic Changes Using 1H-NMR Metabolomics" Metabolites 11, no. 10: 656. https://doi.org/10.3390/metabo11100656
APA StyleBester, R., Stander, Z., Mason, S., Keane, K. M., Howatson, G., Clifford, T., Stevenson, E. J., & Loots, D. T. (2021). Characterizing Marathon-Induced Metabolic Changes Using 1H-NMR Metabolomics. Metabolites, 11(10), 656. https://doi.org/10.3390/metabo11100656








