Metabolomics-Based Study of the Effect of Raw Materials to the End Product of Tempe—An Indonesian Fermented Soybean
Abstract
1. Introduction
2. Results and Discussion
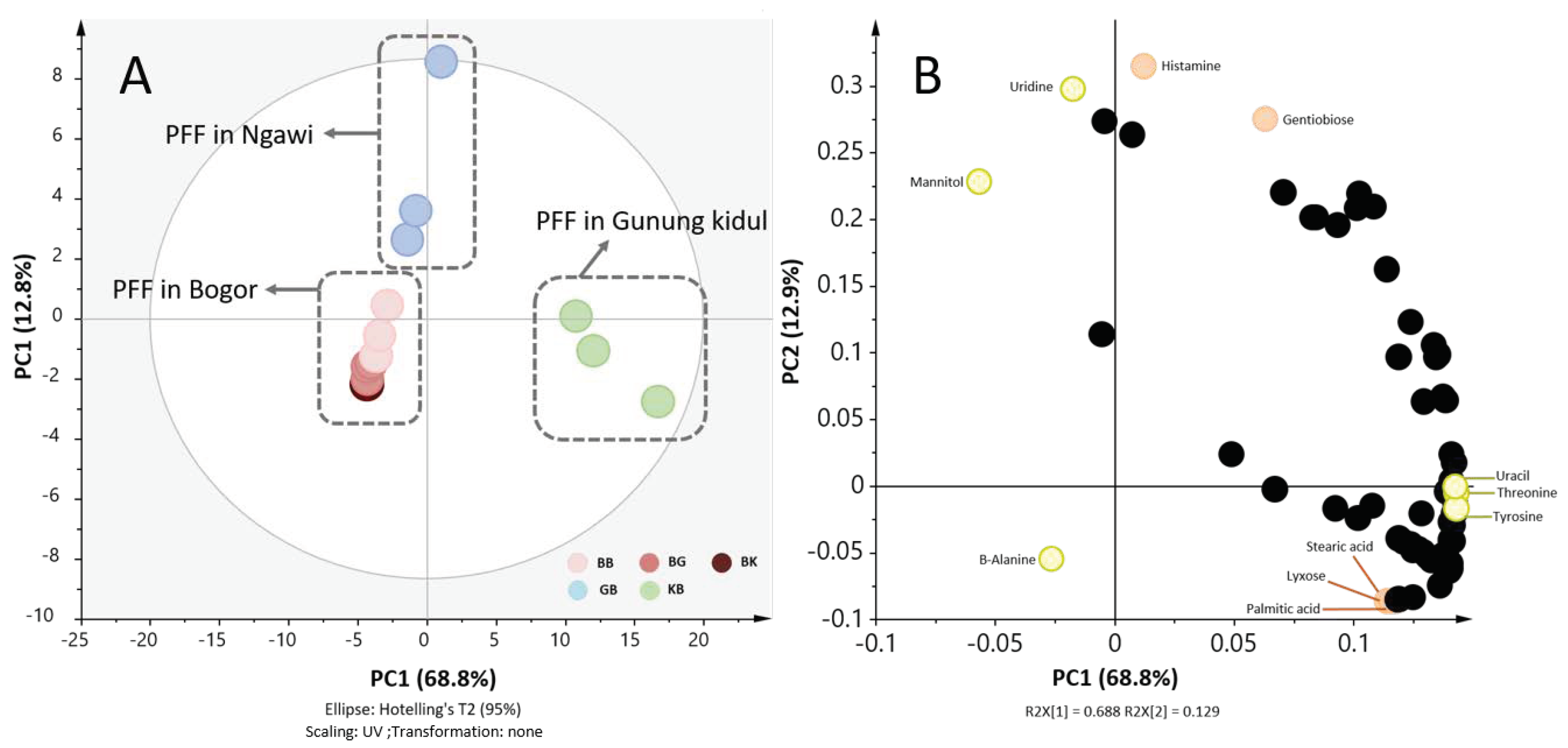
2.1. The Place of Production Affected Metabolite Profiles of Tempe More Than Fermentation Place
2.2. The Soybean, Water and Starter Cultures Affected Metabolite Profiles of Tempe
2.3. Different Brands of Commercial Starter Culture of Tempe Affect Metabolite Profiles of Tempe
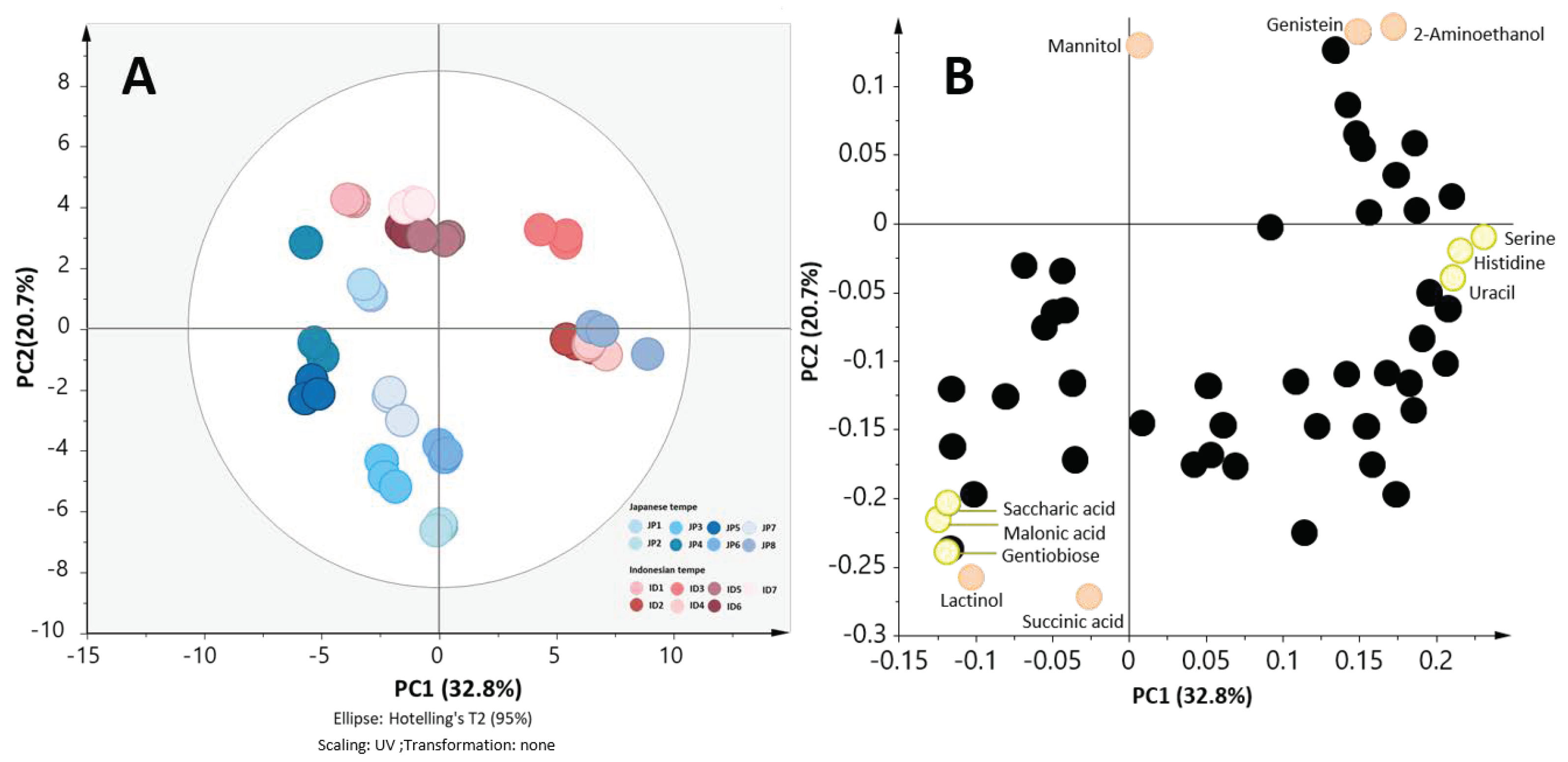
2.4. Japanese Tempe and Indonesian Tempe were Clustered Separately in the Validation Set
2.5. Conclusions
3. Materials and Methods
3.1. Tempe Production in Laboratory
3.2. Cross Fermentation Experiment
3.3. Validation Sample Collection
3.4. Sample Sets
3.5. Extraction and Derivatization
3.6. GC/MS-Based Metabolite Profiling
3.7. Metabolite Annotation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carla, A.; Bavia, F.; Eduardo, C.; Silva, D.; Ferreira, M.P.; Santos Leite, R.; Gontijo Mandarino, J.M.; Concórdia Carrão-Panizzi, M. Chemical composition of tempeh from soybean cultivars specially developed for human consumption. Cienc. Tecnol. Aliment. 2012, 32, 613–620. [Google Scholar]
- Dinesh Babu, P.; Bhakyaraj, R.; Vidhyalakshmi, R. A low cost nutritious food “Tempeh”—A review. World J. Dairy Food Sci. 2009, 4, 22–27. [Google Scholar]
- Nout, M.J.R.; Kiers, J.L. Tempe fermentation, innovation and functionality: Update into the third millenium. J. Appl. Microbiol. 2005, 98, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, T.S.; Kartawidjajaputra, F.; Silo, W.; Yogiara, Y.; Suwanto, A. Tempeh consumption enhanced beneficial bacteria in the human gut. Food Res. 2019, 3, 57–63. [Google Scholar] [CrossRef]
- Astuti, M. Tempe, a nutritious and healthy food FROM indonesia. Asia Pac. J. Clin. Nutr. 2000. [Google Scholar] [CrossRef] [PubMed]
- Puteri, N.E.; Astawan, M.; Palupi, N.S.; Wresdiyati, T.; Takagi, Y. Characterization of biochemical and functional properties of water-soluble tempe flour. Food Sci. Technol. 2018, 38, 147–153. [Google Scholar] [CrossRef]
- Kridawati, A.; Budi Rahardjo, T.W.; Damanik, R.; Hogervorst, E. Comparing the effect of tempe flour and tofu flour consumption on estrogen serum in ovariectomized rats. Heliyon 2017, e01787. [Google Scholar] [CrossRef]
- Keuth, S.; Bisping, B. Formation of vitamins by pure cultures of tempe moulds and bacteria DURING the tempe solid substrate fermentation. J. Appl. Bacteriol. 1993, 75, 427–434. [Google Scholar] [CrossRef]
- Schösler, H.; de Boer, J.; Boersema, J.J. Can we cut out the meat of the dish? constructing consumer-Oriented pathways towards meat substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef]
- Hever, J. Plant-based diets: A physician’s guide. Perm. J. 2016, 20. [Google Scholar] [CrossRef]
- Nurdini, A.L.; Nuraida, L.; Suwanto, S. Microbial growth dynamics during tempe fermentation in two different home industries. Int. Food Res. J. 2015, 22, 1668–1674. [Google Scholar]
- Mulyowidarso, R.K.; Fleet, G.H.; Buckle, K.A. The microbial ecology of soybean soaking for tempe production. Int. J. Food Microbiol. 1989, 8, 35–46. [Google Scholar] [CrossRef]
- Mulyowidarso, R.K.; Fleet, G.H.; Buckle, K.A. Association of bacteria with the fungal fermentation of soybean tempe. J. Appl. Bacteriol. 1990, 68, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.; Anwar, M.; Mubeen, H.; Raza, S.; Chiang, J.; Claire Juico, M.W. Evaluation of physicochemical and nutritional contents in soybean fermented food tempeh by rhizopus oligosporus. J. Adv. Biol. Biotechnol. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, R.M.; Rombouts, F.M. In vitro digestibility of processed and fermented Soya Bean, Cowpea and Maize. J. Sci. Food Agric. 2000, 80, 1325–1331. [Google Scholar] [CrossRef]
- Lukito, W. Candidate foods in the Asia-Pacific region for cardiovascular protection: Nuts, soy, lentils and tempe. Asia Pac. J. Clin. Nutr. 2001, 10, 128–133. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wu, B.-H.; Chu, Y.-L.; Chang, W.-C.; Wu, M.-C. Effects of tempeh fermentation with lactobacillus plantarum and rhizopus oligosporus on streptozotocin-induced type II diabetes mellitus in Rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef]
- Kadar, A.D.; Aditiawati, P.; Astawan, M.; Putri, S.P.; Fukusaki, E. Gas chromatography coupled with mass spectrometry-based metabolomics for the classification of tempe from different regions and production processes in indonesia. J. Biosci. Bioeng. 2018, 126, 411–416. [Google Scholar] [CrossRef]
- Barus, T.; Maya, F.; Hartanti, A.T. The role of some strains of rhizopus microsporus originating from “laru tradisional” in determining tempe quality. J. Apl. Teknol. Pangan 2019, 8, 17–22. [Google Scholar]
- SNI 3144:2009. Available online: http://www.forumtempe.org/images/resep/SNI-Tempe.pdf (accessed on 4 July 2020).
- Regional Standard for Tempe (Codex Stan 313r-2013). Available online: https://www.maff.go.jp/e/ccasia/about_ccasia/pdf/cxs_313re.pdf (accessed on 4 July 2020).
- Nissen, L.; Bordoni, A.; Gianotti, A. Shift of volatile organic compounds (VOCs) in gluten-free hemp-enriched sourdough bread: A metabolomic approach. Nutrients 2020, 12, 1050. [Google Scholar] [CrossRef]
- Li, P.; Tang, H.; Shi, C.; Xie, Y.; Zhou, H.; Xia, B.; Zhang, C.; Chen, L.; Jiang, L. Untargeted metabolomics analysis of mucor racemosus douchi fermentation process by gas chromatography with time-of-flight mass spectrometry. Food Sci. Nutr. 2019, 7, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Park, S.E.; Kim, E.J.; Lee, K.I.; Na, C.S.; Son, H.S. A GC-MS based metabolomics approach to determine the effect of salinity on kimchi. Food Res. Int. 2018, 105, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ochi, H.; Naito, H.; Iwatsuki, K.; Bamba, T.; Fukusaki, E. Metabolomics-based component profiling of hard and semi-hard natural cheeses with gas chromatography/time-of-flight-mass spectrometry, and its application to sensory predictive modeling. J. Biosci. Bioeng. 2012, 113, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 6–23. [Google Scholar] [CrossRef]


| Data Set | Code | Denotation |
|---|---|---|
| Cross fermentation | BB | PFF in Bogor, FF in Bogor |
| BK | PFF in Bogor, FF in Gunung Kidul | |
| BG | PFF in Bogor, FF in Ngawi | |
| GB | PFF in Ngawi, FF in Bogor | |
| KB | PFF in Gunung Kidul, FF in Bogor | |
| Soybean-water effect | AX | Soybean A, water X |
| BX | Soybean B, water X | |
| CX | Soybean C, water X | |
| AY | Soybean A, water Y | |
| BY | Soybean B, water Y | |
| CY | Soybean C, water Y | |
| Starter culture effect | M | Starter culture M |
| N | Starter culture N | |
| O | Starter culture O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadar, A.D.; Astawan, M.; Putri, S.P.; Fukusaki, E. Metabolomics-Based Study of the Effect of Raw Materials to the End Product of Tempe—An Indonesian Fermented Soybean. Metabolites 2020, 10, 367. https://doi.org/10.3390/metabo10090367
Kadar AD, Astawan M, Putri SP, Fukusaki E. Metabolomics-Based Study of the Effect of Raw Materials to the End Product of Tempe—An Indonesian Fermented Soybean. Metabolites. 2020; 10(9):367. https://doi.org/10.3390/metabo10090367
Chicago/Turabian StyleKadar, Adinda Darwati, Made Astawan, Sastia Prama Putri, and Eiichiro Fukusaki. 2020. "Metabolomics-Based Study of the Effect of Raw Materials to the End Product of Tempe—An Indonesian Fermented Soybean" Metabolites 10, no. 9: 367. https://doi.org/10.3390/metabo10090367
APA StyleKadar, A. D., Astawan, M., Putri, S. P., & Fukusaki, E. (2020). Metabolomics-Based Study of the Effect of Raw Materials to the End Product of Tempe—An Indonesian Fermented Soybean. Metabolites, 10(9), 367. https://doi.org/10.3390/metabo10090367







