An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus
Abstract
1. Introduction
2. Results
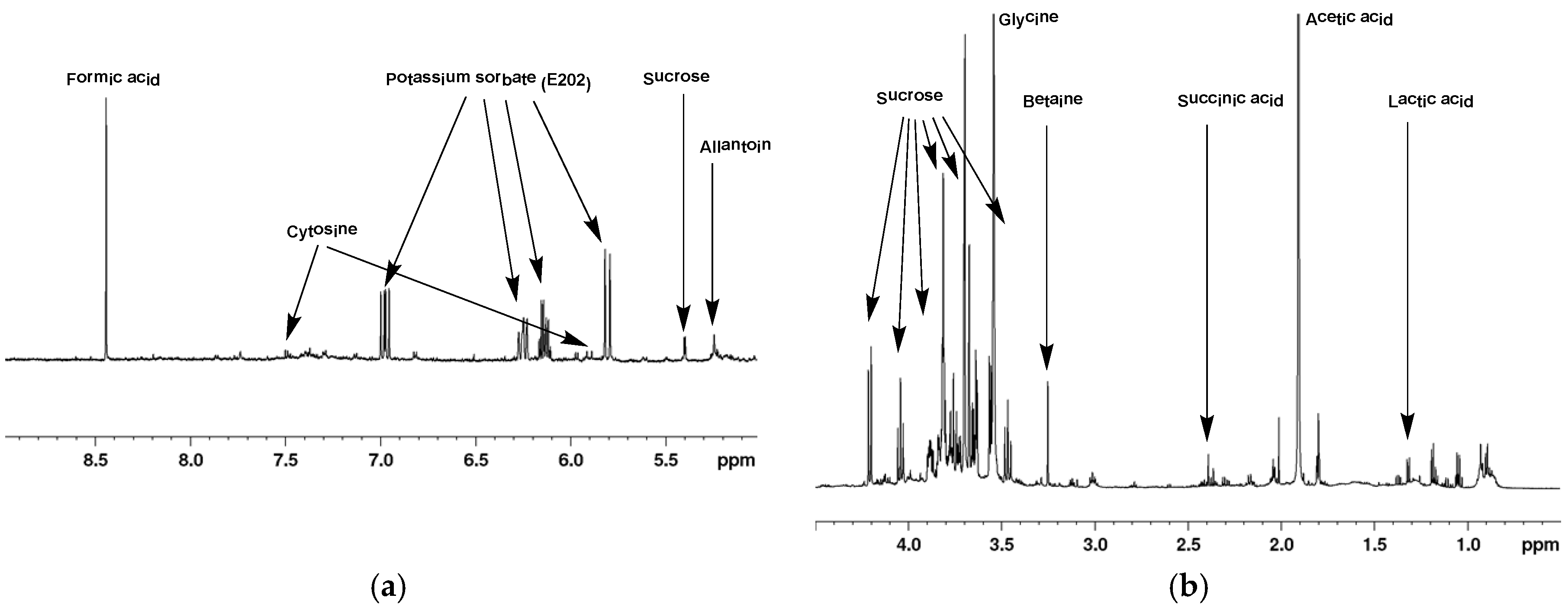
2.1. 1H-NMR of Mucus from Helix aspersa
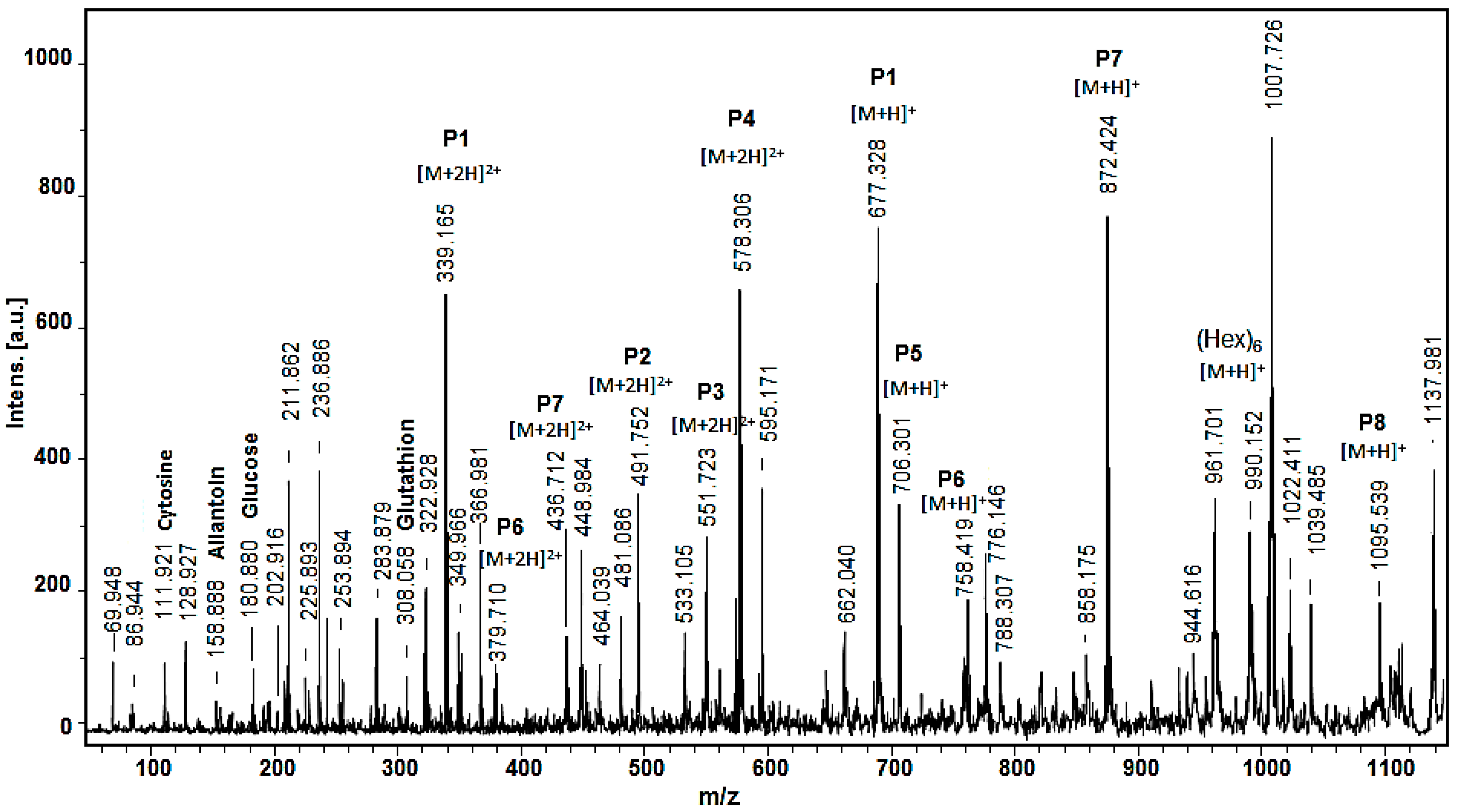
2.2. Mass Spectrometric Analysis
3. Discussion
3.1. Metabolic Pathway and Activity
3.1.1. Antibacterial Metabolic Activity of Acetic, Citric, Lactic, Tartaric, and Isovaleric Acids, and Glycerol
3.1.2. Antimicrobial Activity of Peptides
3.1.3. Antifungal Metabolic Activity of Alpha-Ketoisocaproic Acid, Betaine Derivatives, and Choline
3.2. Antioxidant Metabolic Activity
4. Materials and Methods
4.1. Mucus Collection and Preparation of Different Fractions
4.2. Analysis by Mass Spectrometry
4.3. 1H-NMR Spectroscopy
4.4. Assignment of Metabolites and Database Search
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bortolotti, D.; Trapella, C.; Bernardi, T.; Rizzo, R. Letter to the Editor: Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2016, 73, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Velkova, L.; Stevanovic, S.; Molin, L.; Traldi, P.; Velikova, R.; Voelter, W. Bioactive compounds isolated from garden snails. J. BioSci. Biotechnol. Se/Online 2015, 147–155. [Google Scholar]
- Dolashki, A.; Nissimova, A.; Daskalova, E.; Velkova, L.; Topalova, Y.; Hristova, P.; Traldi, P.; Voelter, W.; Dolashka, P. Structure and antibacterial activity of isolated peptides from the mucus of garden snail Cornu aspersum. Bulg. Chem. Commun. 2018, 50, 195–200. [Google Scholar]
- Kostadinova, N.; Voynikov, Y.; Dolashki, A.; Krumova, E.; Abrashev, R.; Kowalewski, D.; Stevanovic, S.; Velkova, L.; Velikova, R.; Dolashka, P. Antioxidative screening of fractions from the mucus of garden snail Cornu aspersum. Bulg. Chem. Commun. 2018, 50, 176–183. [Google Scholar]
- Dolashka-Angelova, P.; Schick, M.; Stoeva, S.; Voelter, W. Isolation and partial characterization of the N-terminal functional unit of subunit RtH1 from Rapana thomasiana grosse hemocyanin. Int. J. Biochem. Cell Biol. 2000, 32, 529–538. [Google Scholar] [CrossRef]
- De Smet, L.; Dimitrov, I.; Debyser, G.; Dolashka-Angelova, P.; Dolashki, A.; Van Beeumen, J.; Devreese, B. The cDNA sequence of three hemocyanin subunits from the garden snail Helix lucorum. Gene 2011, 487, 118–128. [Google Scholar] [CrossRef]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Velkova, L.; Dimitrov, I.; Schwarz, H.; Stevanovic, S.; Voelter, W.; Salvato, B.; Dolashka-Angelova, P. Structure of hemocyanin from garden snail Helix lucorum. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 16–25. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Stefanova, T.; Livaniou, E.; Velkova, L.; Klimentzou, P.; Stevanovic, S.; Salvato, B.; Neychev, H.; Voelter, W. Immunological potential of Helix vulgaris and Rapana venosa hemocyanins. Immunol. Investig. 2008, 37, 822–840. [Google Scholar] [CrossRef]
- Iliev, I.; Toshkova, R.; Dolashka-Angelova, P.; Yossifova, L.; Hristova, R.; Yaneva, J.; Zacharieva, S. Haemocyanins from Rapana venosa and Helix vulgaris display an antitumour activity via specific activation of spleen lymphocytes. C. R. Acad. Bulg. Sci. 2008, 61, 203–210. [Google Scholar]
- Salazar, M.L.; Jiménez, J.M.; Villar, J.; Rivera, M.; Báez, M.; Manubens, A.; Becker, M.I. N-glycosylation of mollusk hemocyanins contributes to their structural stability and immunomodulatory properties in mammals. J. Biol. Chem. 2019, 294, 19546–19564. [Google Scholar] [CrossRef]
- Guncheva, M.; Idakieva, K.; Todinova, S.; Stoyanova, E.; Yancheva, D. Folate-conjugated Helix lucorum hemocyanin-preparation, stability, and cytotoxicity. Zeitschrift für Naturforschung C 2020, 75, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Dolashka, P.; Stenzl, A.; Stevanovic, S.; Aicher, W.K.; Velkova, L.; Velikova, R.; Voelter, W. Antitumour activity of Helix hemocyanin against bladder carcinoma permanent cell lines. Biotechnol. Biotechnol. Equip. 2019, 33, 20–32. [Google Scholar] [CrossRef]
- Stenzl, A.; Dolashki, A.; Stevanovic, S.; Voelter, W.; Aicher, W.; Dolashka, P. Cytotoxic effects of Rapana venosa hemocyanin on bladder cancer permanent cell lines. J. US–China Med. Sci. 2016, 13, 179–188. [Google Scholar]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial activity of molluscan hemocyanins from Helix and Rapana snails. Curr. Pharm. Biotechnol. 2016, 17, 263–270. [Google Scholar] [CrossRef]
- Digilio, G.; Sforzini, S.; Cassino, C.; Robotti, E.; Oliveri, C.; Marengo, E.; Musso, D.; Osella, D.; Viarengo, A. Haemolymph from Mytilus galloprovincialis: Response to copper and temperature challenges studied by 1H-NMR metabonomics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 183, 61–71. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Shao, Z.-M.; Zhang, Y.-J.; Vu, T.T.; Wu, Y.-C.; Xu, J.-P.; Deng, M.-J. A 1H NMR based study of hemolymph metabonomics in different resistant silkworms, Bombyx mori (Lepidotera), after BmNPV inoculation. J. Insect Physiol. 2019, 117, 103911. [Google Scholar] [CrossRef]
- Olsson, T.; MacMillan, H.A.; Nyberg, N.; Staerk, D.; Malmendal, A.; Overgaard, J. Hemolymph metabolites and osmolality are tightly linked to cold tolerance of Drosophila species: A comparative study. J. Exp. Biol. 2016, 219, 2504–2513. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef]
- Aude, D.; Marion, B.; Raphaël, C.; Colet, J.-M. Proton Nuclear Magnetic Resonance (1H NMR) profiling of isolated organs in the snail Helix aspersa maxima. Ecol. Indic. 2019, 105, 177–187. [Google Scholar] [CrossRef]
- Kostidis, S.; Addie, R.D.; Morreau, H.; Mayboroda, O.A.; Giera, M. Quantitative NMR analysis of intra- and extracellular metabolism of mammalian cells: A tutorial. Anal. Chim. Acta 2017, 980, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.J. Recent developments in our understanding of glycogen structure. Carbohydr. Polym. 1991, 16, 37–82. [Google Scholar] [CrossRef]
- Greistorfer, S.; Klepal, W.; Cyran, N.; Gugumuck, A.; Rudoll, L.; Suppan, J.; von Byern, J. Snail mucus-glandular origin and composition in Helix pomatia. Zoology 2017, 122, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Biosynthesis and Functions of Glutathione, an Essential Biofactor. J. Nutr. Sci. Vitaminol. 1992, 38, 1–6. [Google Scholar] [CrossRef]
- Gupta, A.; Dwivedi, M.; Mahdi, A.A.; Khetrapal, C.L.; Bhandari, M. Broad Identification of Bacterial Type in Urinary Tract Infection Using 1H NMR Spectroscopy. J. Proteome Res. 2012, 11, 1844–1854. [Google Scholar] [CrossRef]
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the in Vivo Metabolic Potential of Two Human Gut Acetogens. J. Biol. Chem. 2010, 285, 22082–22090. [Google Scholar] [CrossRef]
- Gibb, J.O.T.; Holmes, E.; Nicholson, J.K.; Weeks, J.M. Proton NMR Spectroscopic Studies on Tissue Extracts of Invertebrate Species with Pollution Indicator Potential. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 587–598. [Google Scholar] [CrossRef]
- Cimino, G.; Sodano, G. Biosynthesis of secondary metabolites in marine molluscs. In Proceedings of the Marine Natural Products—Diversity and Biosynthesis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 77–115. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sautin, Y.Y.; Oliver, W.J.; Roncal, C.; Mu, W.; Gabriela Sanchez-Lozada, L.; Rodriguez-Iturbe, B.; Nakagawa, T.; Benner, S.A. Lessons from comparative physiology: Could uric acid represent a physiologic alarm signal gone awry in western society? J. Comp. Physiol. B 2009, 179, 67–76. [Google Scholar] [CrossRef] [PubMed]
- El Mubarak, M.A.S.; Lamari, F.N.; Kontoyannis, C. Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection. J. Chromatogr. A 2013, 1322, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2017, 46, D608–D617. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Norton, R.S. Identification of mollusc metabolites by natural-abundance 13C NMR studies of whole tissue and tissue homogenates. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 63, 67–72. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 1–25. [Google Scholar] [CrossRef]
- Bacca, H. Étude des voies Métaboliques des Sucres chez l’huître creuse Crassostrea Gigas. Implication dans les Mortalités Estivales. Ph.D. Thesis, Université de Rennes, Rennes, France, 2007. [Google Scholar]
- Livingstone, D.R. Invertebrate and vertebrate pathways of anaerobic metabolism: Evolutionary considerations. J. Geol. Soc. 1983, 140, 27–37. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Zahli, R.; Abrini, J. Evaluation of Antimicrobial Activity of Four Organic Acids Used in Chicks Feed to Control Salmonella typhimurium: Suggestion of Amendment in the Search Standard. Int. J. Microbiol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Ryssel, H.; Kloeters, O.; Germann, G.; Schäfer, T.; Wiedemann, G.; Oehlbauer, M. The antimicrobial effect of acetic acid—An alternative to common local antiseptics? Burns 2009, 35, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Mohan, A. Antimicrobial effects of pyruvic and succinic acids on Salmonella survival in ground chicken. LWT 2019, 116, 108596. [Google Scholar] [CrossRef]
- Oh, D.-H.; Marshall, D.L. Antimicrobial activity of ethanol, glycerol monolaurate or lactic acid against Listeria monocytogenes. Int. J. Food Microbiol. 1993, 20, 239–246. [Google Scholar] [CrossRef]
- Velkova, L.; Nissimova, A.; Dolashki, A.; Daskalova, E.; Topalova, P.D.Y. Glycine-rich peptides from Cornu aspersum snail with antibacterial activity. Bulg. Chem. Commun. 2018, 50, 169–175. [Google Scholar]
- Li, M.; Wang, J.; Lu, Z.; Wei, D.; Yang, M.; Kong, L. NMR-based metabolomics approach to study the toxicity of lambda-cyhalothrin to goldfish (Carassius auratus). Aquat. Toxicol. 2014, 146, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; Wang, J.S.; Li, M.H.; Liu, Y.; Chen, T.; Jia, A.Q. 1H NMR based metabolomics approach to study the toxic effects of herbicide butachlor on goldfish (Carassius auratus). Aquat. Toxicol. 2015, 159, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379. [Google Scholar] [CrossRef]
- Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Asp. Med. 2009, 30, 60–76. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and analogs in antiviral therapy. Mol. Asp. Med. 2009, 30, 99–110. [Google Scholar] [CrossRef]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free radical scavenging and antioxidant effects of lactate ion: An In Vitro study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sawa, K.; Uematsu, T.; Korenaga, Y.; Hirasawa, R.; Kikuchi, M.; Murata, K.; Zhang, J.; Gai, X.; Sakamoto, K.; Koyama, T.; et al. Krebs cycle intermediates protective against oxidative stress by modulating the level of reactive oxygen species in neuronal HT22 cells. Antioxidants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P. Device for collecting garden snail extract. BG Useful Model 2015, 2097. [Google Scholar]
- Bax, A. A spatially selective composite 90 radiofrequency pulse. J. Magn. Reson. 1985, 65, 142–145. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Water Suppression That Works. Excitation Sculpting Using Arbitrary Wave-Forms and Pulsed-Field Gradients. J. Magn. Reson. Ser. A 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Adams, R.W.; Holroyd, C.M.; Aguilar, J.A.; Nilsson, M.; Morris, G.A. “Perfecting” WATERGATE: Clean proton NMR spectra from aqueous solution. Chem. Commun. 2013, 49, 358–360. [Google Scholar] [CrossRef]
- Ravanbakhsh, S.; Liu, P.; Bjordahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, Fully-Automated NMR Spectral Profiling for Metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res 2008, 36, D402–D408. [Google Scholar] [CrossRef]


| Metabolite | Mass | 13C Chemical Shift a,b | 1H Chemical Shift (Multiplicity, Coupling Constants) a,c | Concentrations in (<1 kDa) Sample (mM) | Concentrations in (<3 kDa) Sample (mM) |
|---|---|---|---|---|---|
| 3-Hydroxybutyrate | 103.1 | 1.19(d, J = 6.2), 2.29(dd, J = 6.4, 14.3), 2.40(dd, J = 7.3, 14.3), 4.14(sextet, J = 6.4) | 0.32 | 0.06 | |
| 4-Methyl-2-oxovaleric Acid | 130.1 | 0.93(d, J = 6.5), 2.08(m), 2.60(d, J = 7.0) | 0.41 | 0.49 | |
| Acetic acid | 60.052 | 23.45 | 1.92(s) | 16.14 | 3.51 |
| Alanine | 89.09 | 1.47(d, J = 7.3), 3.77(q, J = 7.3) | 0.06 | ||
| Allantoin | 158.121 | 5.38(s) | 0.09 | 0.04 | |
| Betaine | 117.148 | 53.31 | 3.27(s) | 0.15 | 0.03 |
| Choline | 104.1708 | 3.23(s) | 0.02 | 0.01 | |
| Cytosine | 111.10 | 7.51(d, J = 7.4), 5.98(d, J = 7.4) | 0.08 | 0.05 | |
| Ethanol | 46.07 | 62.80 | 3.67(q, J = 7.1), 1.19(t, J = 7.1) | 0.33 | 0.30 |
| Formic acid/formate | 46.03 | 8.46(s) | 0.30 | 0.15 | |
| Glucose | 180.156 | 4.64(d) | 0.26 | 0.08 | |
| Glycine | 75.07 | 62.73 | 3.56(s) | 5.53 | |
| Glycerol | 92.09382 | 3.55(dd, J = 6.5,11.7), 3.64(dd, J = 4.3,11.7), 3.77(m) | 27.66 | ||
| Isobutyric acid | 88.11 | 1.05(d, J = 7.2) | 0.02 | 0.08 | |
| Isovaleric acid | 102.1317 | 0.90(d, J = 6.5), 1.95(m), 2.04(d, J = 7.4) | 0.14 | 0.23 | |
| Lactic acid | 90.08 | 20.23 | 4.12(q, J = 7.0), 1.33(d, J = 6.9) | 0.42 | 0.87 |
| Phenylalanine | 165.19 | 7.33(d, J = 7.9), 7.38(t, J = 7.4), 7.43(t, J = 7.6) | 0.04 | ||
| L-Tartaric Acid | 150.087 | 4.34(s) | 0.03 | 0.05 | |
| Succinic Acid | 118.09 | 2.40(s) | 0.07 | 0.07 | |
| Sucrose | 342.3 | 5.415(d, J = 3.9), 4.22(d,), 4.06(t,), 3.48(t,) | 1.25 | 0.21 | |
| Valine | 117.151 | 0.99(d, J = 7.0), 1.02(d, J = 7.0), 3.57(dt) | 0.04 | 0.02 | |
| TSPA | −0.0159 | 0.186 | 0.186 |
| № | Amino Acid Sequence of Peptides | Experimental m/z (Da) | [M+H]+ (Da) | Calculated Monoisotopic Mass (Da) | pI |
|---|---|---|---|---|---|
| P1 | LGHDVH | 339.165 [M+2H]2+ | 677.328 | 676.33 | 5.97 |
| P2 | LFSNQLFN | 491.752 [M+2H]2+ | 982.504 | 981.49 | 5.52 |
| P3 | DQDSHPYSGP | 551.725 [M+2H]2+ | 1102.450 | 1101.44 | 4.20 |
| P4 | LGLGNGGAGGGLVGG | 578.306 [M+H]2+ | 1155.612 | 1154.60 | 5.52 |
| P5 | NNTVCGV | 706.322 [M+2H]+ | 706.322 | 705.31 | 5.52 |
| P6 | LLMGPEV | 379.710 [M+2H]2+ | 758.412 | 757.40 | 4.00 |
| P7 | AAGLAGAGNGGG | 436.712 [M+2H]2+ | 872.424 | 871.41 | 5.57 |
| P8 | ACAATCGVEDGV | 1095.539 [M+H]+ | 1095.539 | 1094.44 | 3.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilev, N.G.; Simova, S.D.; Dangalov, M.; Velkova, L.; Atanasov, V.; Dolashki, A.; Dolashka, P. An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus. Metabolites 2020, 10, 360. https://doi.org/10.3390/metabo10090360
Vassilev NG, Simova SD, Dangalov M, Velkova L, Atanasov V, Dolashki A, Dolashka P. An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus. Metabolites. 2020; 10(9):360. https://doi.org/10.3390/metabo10090360
Chicago/Turabian StyleVassilev, Nikolay G., Svetlana D. Simova, Miroslav Dangalov, Lyudmila Velkova, Venceslav Atanasov, Aleksandar Dolashki, and Pavlinka Dolashka. 2020. "An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus" Metabolites 10, no. 9: 360. https://doi.org/10.3390/metabo10090360
APA StyleVassilev, N. G., Simova, S. D., Dangalov, M., Velkova, L., Atanasov, V., Dolashki, A., & Dolashka, P. (2020). An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus. Metabolites, 10(9), 360. https://doi.org/10.3390/metabo10090360