Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure
Abstract
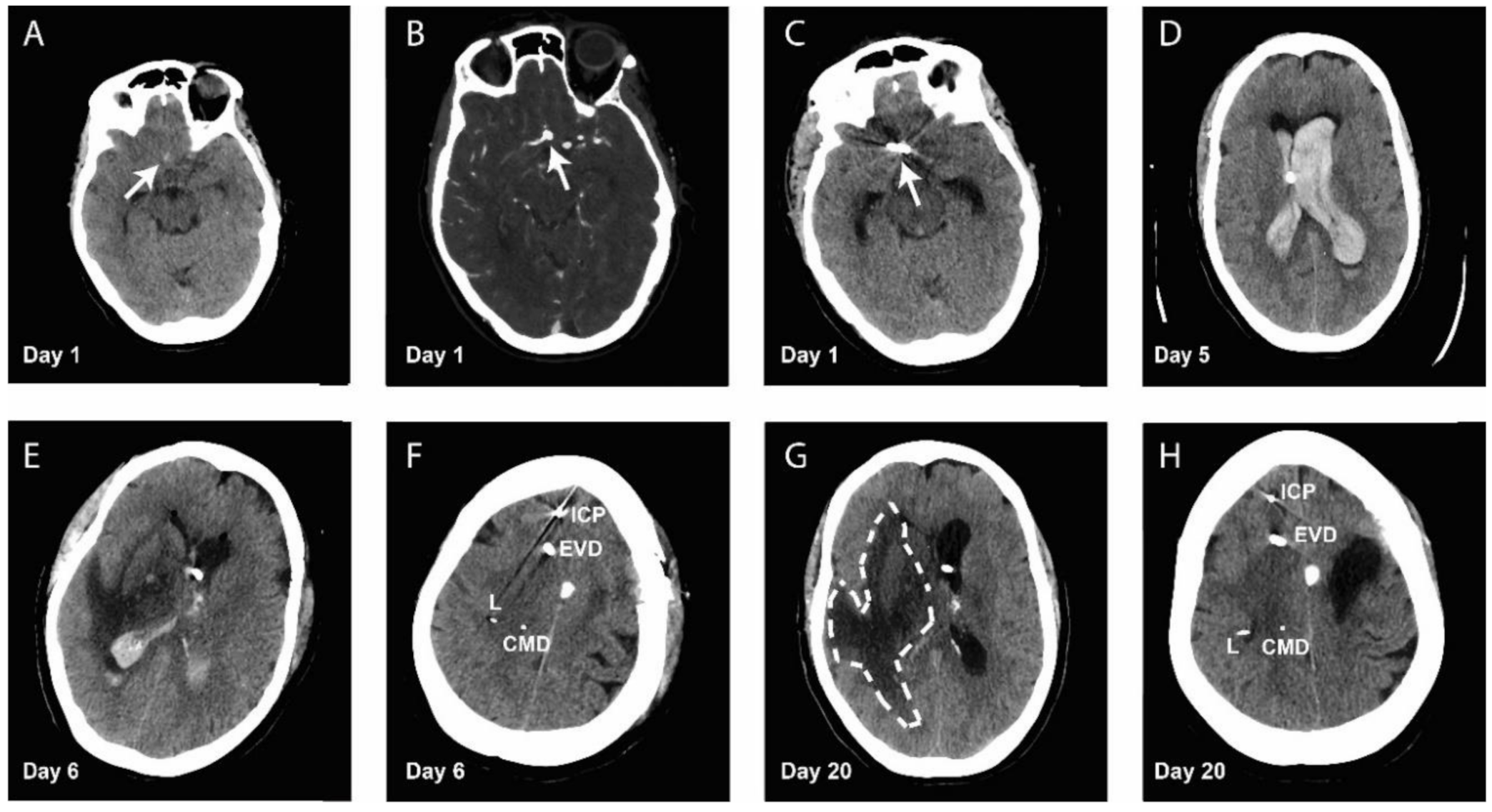
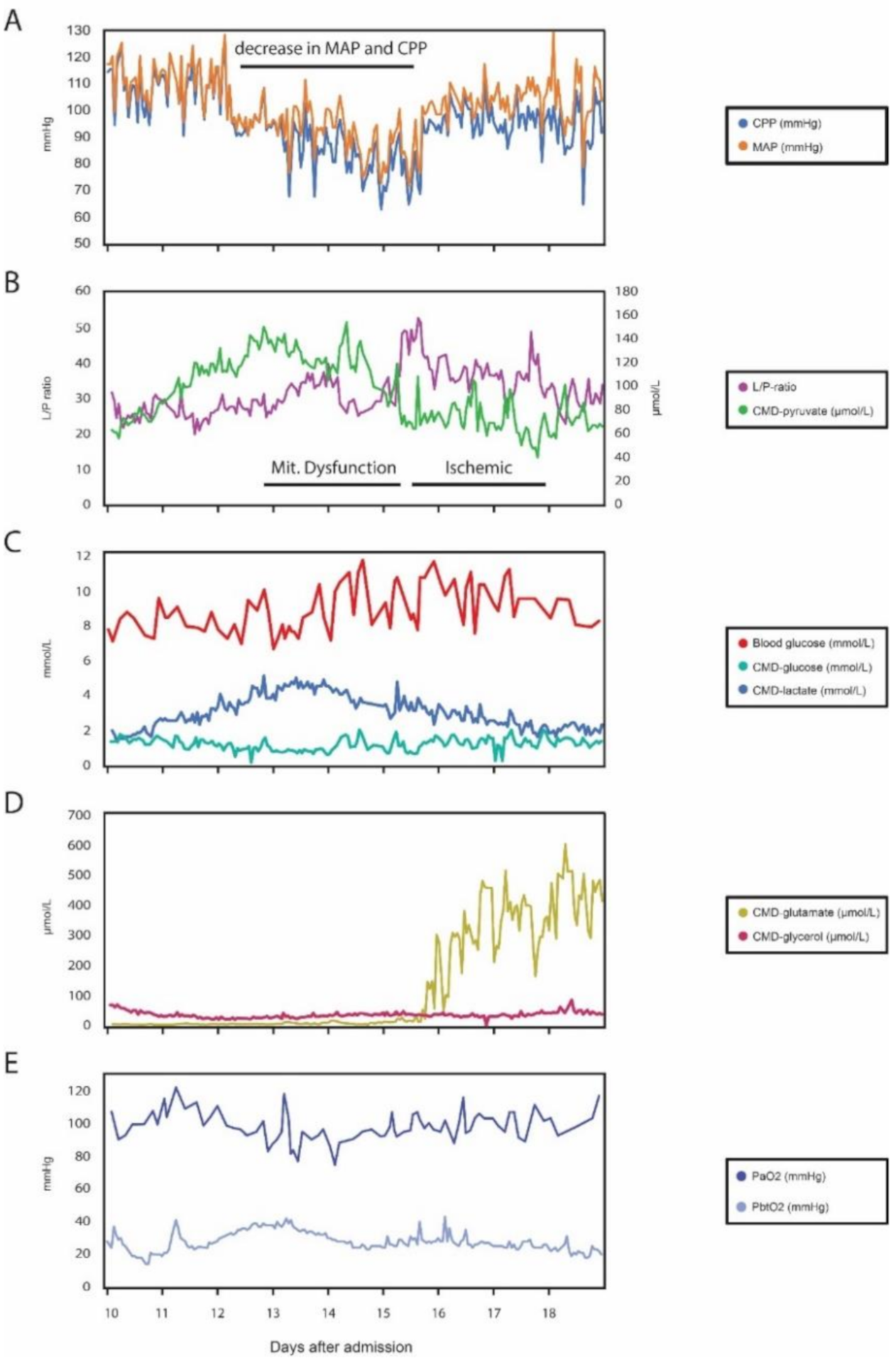
Patient Case: Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure
Author Contributions
Funding
Conflicts of Interest
References
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T. AHA/ASA Guideline Guidelines for the Management of Aneurysmal Subarachnoid Haemorrhage a Guideline for Healthcare Professionals from the American Heart Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Johnson, U.; Engquist, H.; Lewén, A.; Howells, T.; Nilsson, P.; Ronne-Engström, E.; Rostami, E.; Enblad, P. Increased risk of critical CBF levels in SAH patients with actual CPP below calculated optimal CPP. Acta Neurochir. 2017, 159, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Yousef, K.M.; Balzer, J.R.; Bender, C.M.; Hoffman, L.A.; Poloyac, S.M.; Ye, F.; Sherwood, P.R. Cerebral Perfusion Pressure and Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Am. J. Crit. Care 2015, 24, 65–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmidt, J.M.; Ko, S.-B.; Helbok, R.; Kurtz, P.; Stuart, R.M.; Presciutti, M.; Fernandez, L.; Lee, K.; Badjatia, N.; Connolly, E.S.; et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 2011, 42, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fang, W.; Chen, F.; Lin, Z.; Lin, Y.; Yu, L.; Yao, P.; Kang, D. Cerebral perfusion pressure threshold to prevent delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2018, 54, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Dankbaar, J.W.; De Rooij, N.K.; Rijsdijk, M.; Velthuis, B.K.; Frijns, C.J.; E Rinkel, G.J.; Van Der Schaaf, I.C. Diagnostic threshold values of cerebral perfusion measured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2010, 41, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Nordström, C.-H.; Koskinen, L.-O.; Olivecrona, M. Aspects on the Physiological and Biochemical Foundations of Neurocritical Care. Front. Neurol. 2017, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.; Nielsen, T.H.; Nilsson, O.; Schalén, W.; Nordström, C.-H. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 2014, 130, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Jalloh, I.; Helmy, A.; Carpenter, K.; Rostami, E.; Bellander, B.-M.; Boutelle, M.G.; Chen, J.W.; Claassen, J.; Dahyot-Fizelier, C.; et al. Consensus statement from the 2014 International Microdialysis Forum. Intensiv. Care Med. 2015, 41, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Stiefel, M.F.; Oddo, M.; Milby, A.H.; Maloney-Wilensky, E.; Frangos, S.; Levine, J.M.; Kofke, W.A.; Leroux, P.D. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery 2011, 69, 53–63. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, F.; Haure, P.; Madsen, J.; Steenfeldt Nielsen, B.; Reides Bjarkam, C. Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure. Metabolites 2020, 10, 341. https://doi.org/10.3390/metabo10090341
Nielsen F, Haure P, Madsen J, Steenfeldt Nielsen B, Reides Bjarkam C. Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure. Metabolites. 2020; 10(9):341. https://doi.org/10.3390/metabo10090341
Chicago/Turabian StyleNielsen, Frederik, Pernille Haure, Jacob Madsen, Birgitte Steenfeldt Nielsen, and Carsten Reides Bjarkam. 2020. "Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure" Metabolites 10, no. 9: 341. https://doi.org/10.3390/metabo10090341
APA StyleNielsen, F., Haure, P., Madsen, J., Steenfeldt Nielsen, B., & Reides Bjarkam, C. (2020). Cerebral Microdialysis in Aneurismal Subarachnoid Hemorrhage Patient Reveals a Detrimental Shift in Brain Energy Metabolism, Despite Normal Perfusion Pressure. Metabolites, 10(9), 341. https://doi.org/10.3390/metabo10090341



