Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1β and LPS
Abstract
1. Introduction
2. Results
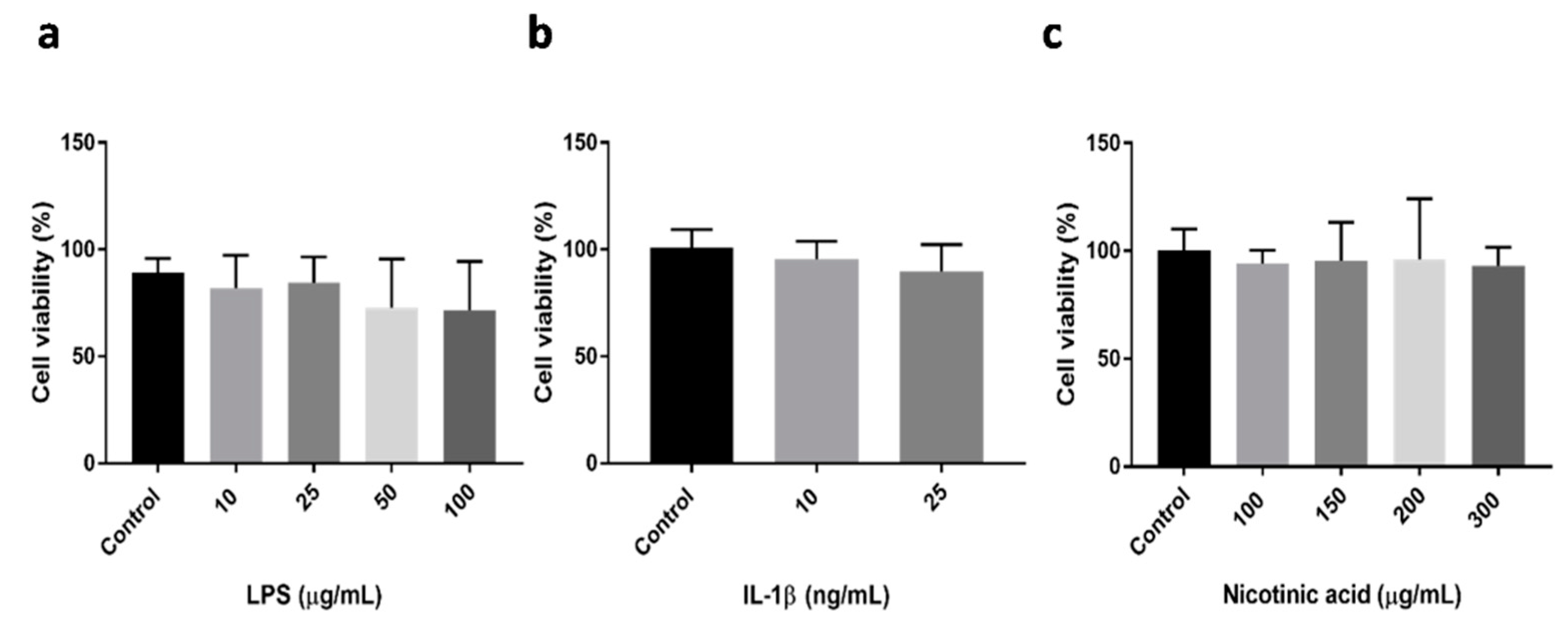
2.1. Evaluation of Cell Viability
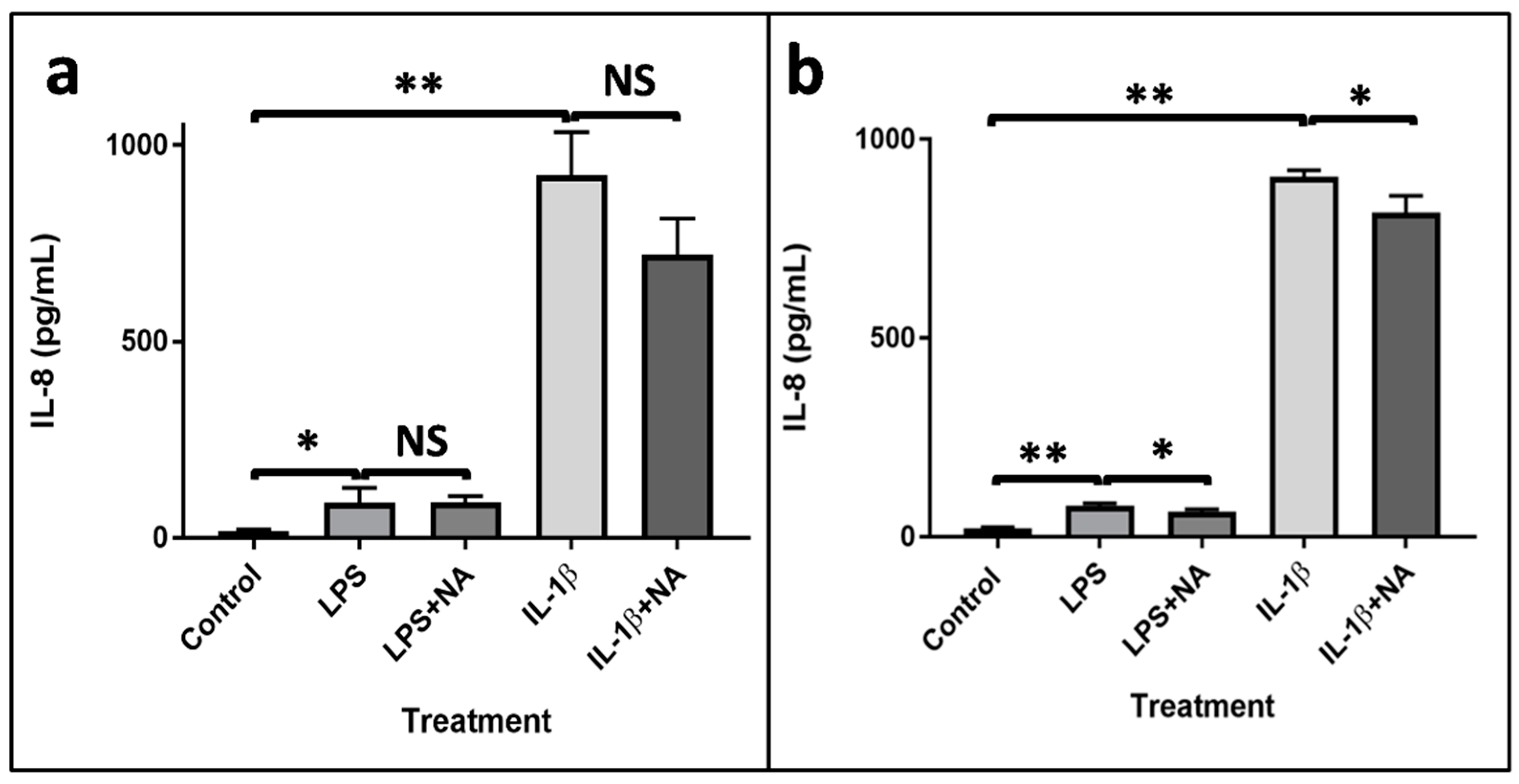
2.2. Evaluation of IL-8 Production
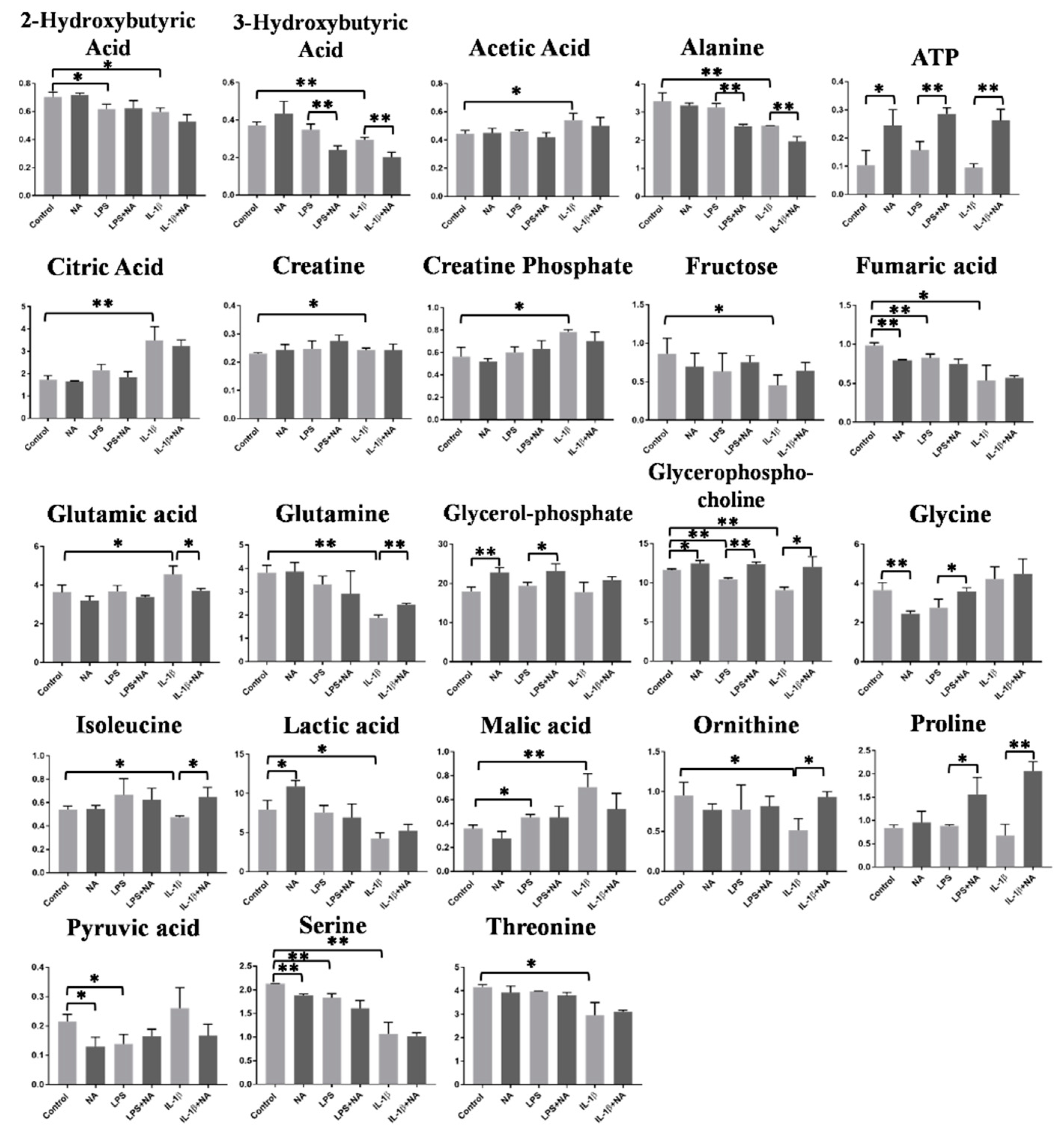
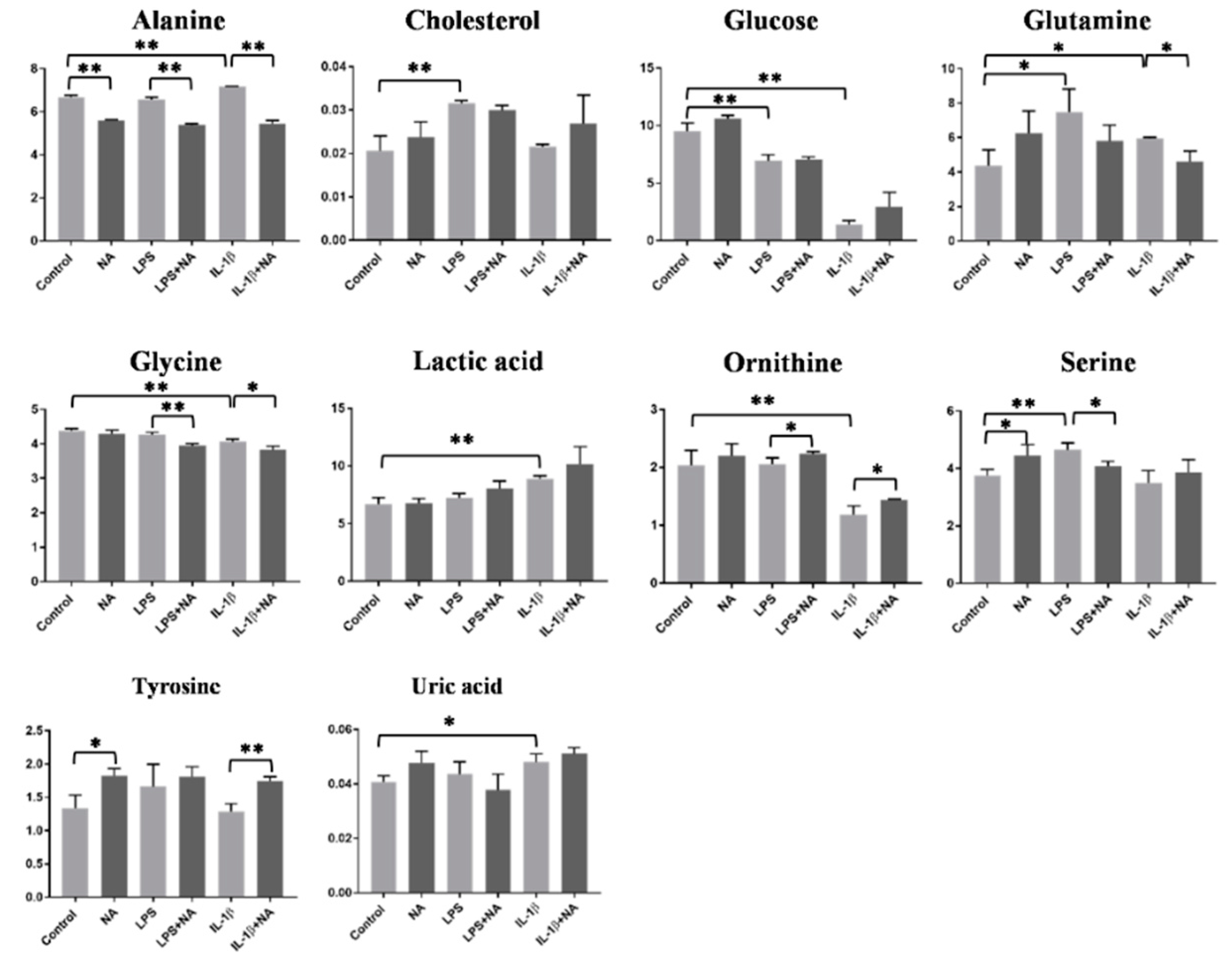
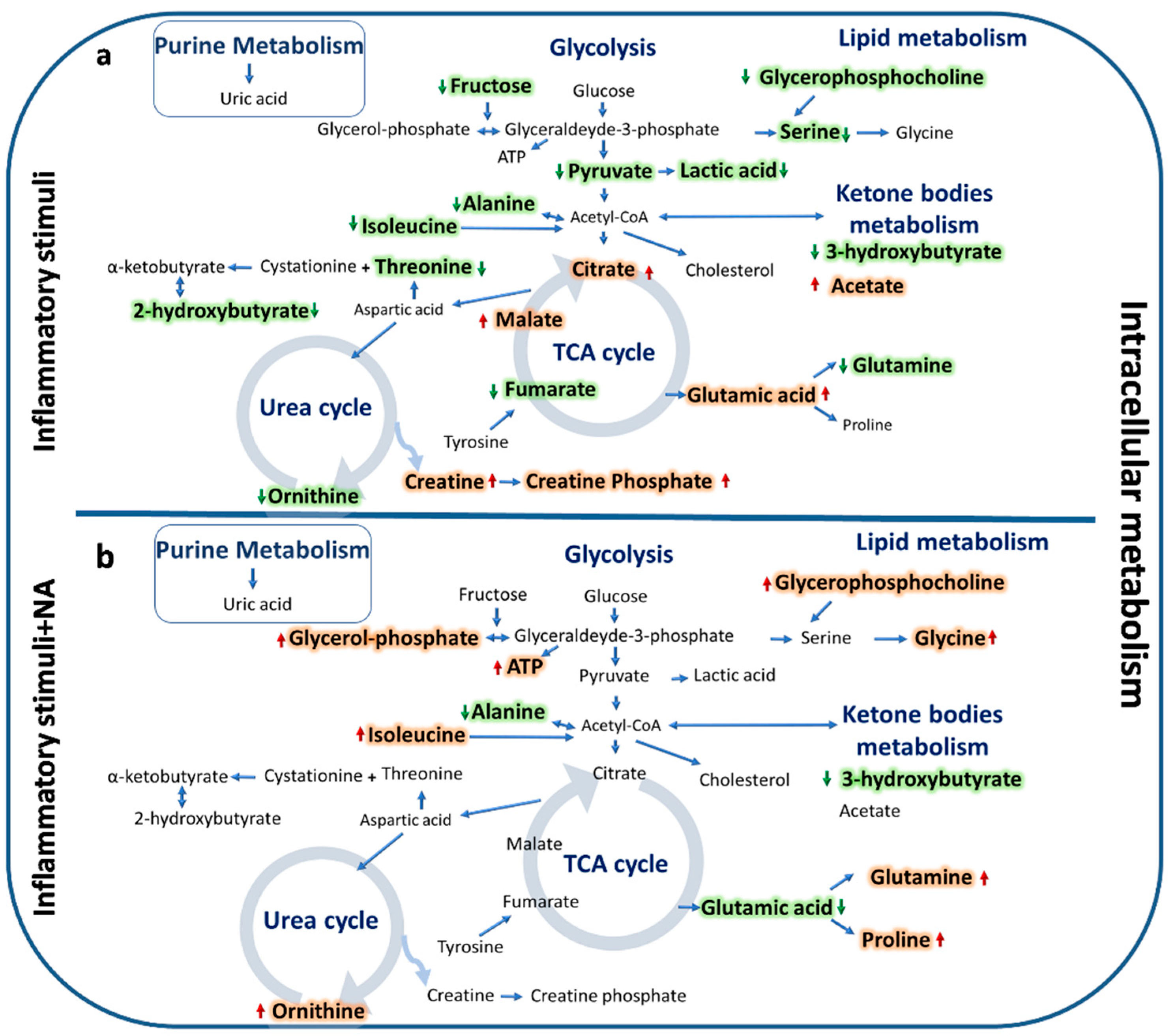
2.3. Metabolomics Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Viability Test
4.3. Treatments
4.4. Evaluation of IL-8 Protein Levels
4.5. Cell Culture Samples Preparation for Metabolomics Analysis
4.6. Protein Extraction and Quantification
4.7. Gas Chromatography–Mass Spectrometry Analysis
4.8. 1H-NMR Measurements
4.9. Univariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kominsky, U.J.; Campbell, E.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.A.; Dʼmello, S.; Jurickova, I.I.; Han, X.; Willson, T.; Flick, L.; Petiniot, L.; Uozumi, N.; Divanovic, S.; Traurnicht, A.; et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohnʼs disease and murine colitis. Inflamm. Bowel Dis. 2010, 16, 856–869. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.M.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 2012, 9. [Google Scholar] [CrossRef]
- Norouzinia, M.; Chaleshi, V.; Alizadeh, A.H.M.; Zali, M.R. Biomarkers in inflammatory bowel diseases: Insight into diagnosis, prognosis and treatment. Gastroenterol. Hepatol. Bed Bench 2017, 10, 155–167. [Google Scholar]
- Lanis, J.M.; Kao, D.J.; Alexeev, E.E.; Colgan, S.P. Tissue metabolism and the inflammatory bowel diseases. J. Mol. Med. 2017, 95, 905–913. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Li, J.; Kong, D.; Wang, Q.; Wu, W.; Tang, Y.; Bai, T.; Guo, L.; Wei, L.; Zhang, Q.; Yu, Y.; et al. Niacin ameliorates ulcerative colitis via prostaglandin D 2 -mediated D prostanoid receptor 1 activation. EMBO Mol. Med. 2017, 9, 571–588. [Google Scholar] [CrossRef]
- Salem, H.A.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 7139. [Google Scholar] [CrossRef] [PubMed]
- Anzola, A.; González, R.; Gámez-Belmonte, R.; Ocón, B.; Aranda, C.J.; Martínez-Moya, P.; López-Posadas, R.; Chirlaque, C.H.; De Medina, F.S.; Martínez-Augustin, O. miR-146a regulates the crosstalk between intestinal epithelial cells, microbial components and inflammatory stimuli. Sci. Rep. 2018, 8, 17350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD + metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. et Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Aparicio-Ugarriza, R.; Olza, J.; Aranceta-Bartrina, J.; Gil, Á.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; González-Gross, M. Dietary Intake and Food Sources of Niacin, Riboflavin, Thiamin and Vitamin B6 in a Representative Sample of the Spanish Population. The ANIBES Study. Nutrients 2018, 10, 846. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Digby, J.E.; Martinez, F.; Jefferson, A.; Ruparelia, N.; Chai, J.; Wamil, M.; Greaves, D.R.; Choudhury, R. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arter. Thromb. Vasc. Boil. 2012, 32, 669–676. [Google Scholar] [CrossRef]
- La Paz, S.M.-D.; Naranjo, M.C.; Lopez, S.; Abia, R.; Muriana, F.J.G.; Bermudez, B. Niacin and its metabolites as master regulators of macrophage activation. J. Nutr. Biochem. 2017, 39, 40–47. [Google Scholar] [CrossRef]
- Petin, K.; Weiss, R.; Müller, G.A.; Garten, A.; Grahnert, A.; Sack, U.; Hauschildt, S. NAD metabolites interfere with proliferation and functional properties of THP-1 cells. Innate Immun. 2019, 25, 280–293. [Google Scholar] [CrossRef]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Genet. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Fouad, A.A.; AlBuali, W.H.; Jresat, I. Protective Effect of Naringenin against Lipopolysaccharide-Induced Acute Lung Injury in Rats. Pharmacology 2016, 97, 224–232. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Fan, C.-W.; Maa, M.-C.; Leu, T.-H. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. BioMedicine 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Casini-Raggi, V.; Kam, L.; Chong, Y.J.; Fiocchi, C.; Pizarro, T.T.; Cominelli, F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J. Immunol. 1995, 154, 154. [Google Scholar]
- Ludwiczek, O.; Vannier, E.; Borggraefe, I.; Käser, A.; Siegmund, B.; Dinarello, C.A.; Tilg, H. Imbalance between interleukin-1 agonists and antagonists: Relationship to severity of inflammatory bowel disease. Clin. Exp. Immunol. 2004, 138, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. et Biophys. Acta (BBA) Biomembr. 2017, 1860, 556–565. [Google Scholar] [CrossRef]
- Denis, M.C.; Furtos, A.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple Peel Polyphenols and Their Beneficial Actions on Oxidative Stress and Inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar] [CrossRef]
- Velikova, T.; Kyurkchiev, B.; Todorova, E.I.-; Spassova, Z.; Stanilova, S.; Altankova, I. Cytokines in Inflamed Mucosa of IBD Patients. New Insights Inflamm. Bowel Dis. 2016. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Triggianese, P.; Conigliaro, P.; Candi, E.; Melino, G.; Perricone, R. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 2015, 6, e1887. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Y.; Zhao, J.; Guo, S.; Zhai, L.; Yao, S.; Sang, H.; Yang, N.; Song, G.; Gu, J.; et al. Niacin Inhibits Vascular Inflammation via Downregulating Nuclear Transcription Factor-κB Signaling Pathway. Mediat. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Kong, L.; Chen, Z. Nicotinic acid regulates glucose and lipid metabolism through lipid independent pathways. Curr. Pharm. Biotechnol. 2015, 16, 16. [Google Scholar] [CrossRef]
- Hauri, H.P.; E Sterchi, E.; Bienz, D.; Fransen, J.; Marxer, A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J. Cell Boil. 1985, 101, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Liggi, S.; Hinz, C.; Hall, Z.; Santoru, M.L.; Poddighe, S.; Fjeldsted, J.; Atzori, L.; Griffin, J.L. KniMet: A pipeline for the processing of chromatography–mass spectrometry metabolomics data. Metabolomics 2018, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted Profiling: Quantitative Analysis of1H NMR Metabolomics Data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef] [PubMed]
| Intracellular Metabolites | |||
| Proinflammatory Stimuli | Proinflammatory Stimuli + NA | ||
| 2-Hydroxybutyrate | ↓ | - | |
| 3-Hydroxybutyrate | ↓ | 3-Hydroxybutyrate | ↓ |
| Acetate | ↑ | - | |
| Alanine | ↓ | Alanine | ↓ |
| - | ATP | ↑ | |
| Citrate | ↑ | - | |
| Creatine | ↑ | - | |
| Creatine Phosphate | ↑ | - | |
| Fructose | ↓ | - | |
| Fumarate | ↓ | - | |
| Glutamic acid | ↑ | Glutamic acid | ↓ |
| Glutamine | ↓ | Glutamine | ↑ |
| - | Glycerolphosphate | ↑ | |
| Glycerophosphocholine | ↓ | Glycerophosphocholine | ↑ |
| - | Glycine | ↑ | |
| Isoleucine | ↓ | Isoleucine | ↑ |
| Lactic acid | ↓ | - | |
| Malate | ↑ | - | |
| Ornithine | ↓ | Ornithine | ↑ |
| - | Proline | ↑ | |
| Pyruvate | ↓ | - | |
| Serine | ↓ | - | |
| Threonine | ↓ | - | |
| Extracellular Metabolites | |||
| Proinflammatory Stimuli | Proinflammatory Stimuli + NA | ||
| Alanine | ↑ | Alanine | ↓ |
| Cholesterol | ↑ | - | |
| Glycine | ↓ | Glycine | ↓ |
| Glucose | ↓ | - | |
| Glutamine | ↑ | Glutamine | ↓ |
| Lactic acid | ↑ | - | |
| Ornithine | ↓ | Ornithine | ↑ |
| Serine | ↑ | Serine | ↓ |
| - | Tyrosine | ↑ | |
| Uric acid | ↑ | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoru, M.L.; Piras, C.; Murgia, F.; Spada, M.; Tronci, L.; Leoni, V.P.; Serreli, G.; Deiana, M.; Atzori, L. Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1β and LPS. Metabolites 2020, 10, 204. https://doi.org/10.3390/metabo10050204
Santoru ML, Piras C, Murgia F, Spada M, Tronci L, Leoni VP, Serreli G, Deiana M, Atzori L. Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1β and LPS. Metabolites. 2020; 10(5):204. https://doi.org/10.3390/metabo10050204
Chicago/Turabian StyleSantoru, Maria Laura, Cristina Piras, Federica Murgia, Martina Spada, Laura Tronci, Vera Piera Leoni, Gabriele Serreli, Monica Deiana, and Luigi Atzori. 2020. "Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1β and LPS" Metabolites 10, no. 5: 204. https://doi.org/10.3390/metabo10050204
APA StyleSantoru, M. L., Piras, C., Murgia, F., Spada, M., Tronci, L., Leoni, V. P., Serreli, G., Deiana, M., & Atzori, L. (2020). Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1β and LPS. Metabolites, 10(5), 204. https://doi.org/10.3390/metabo10050204







