A Metabolomic Approach for Predicting Diurnal Changes in Cortisol
Abstract
1. Introduction
2. Results
2.1. Multiple Regression and Diagnostics
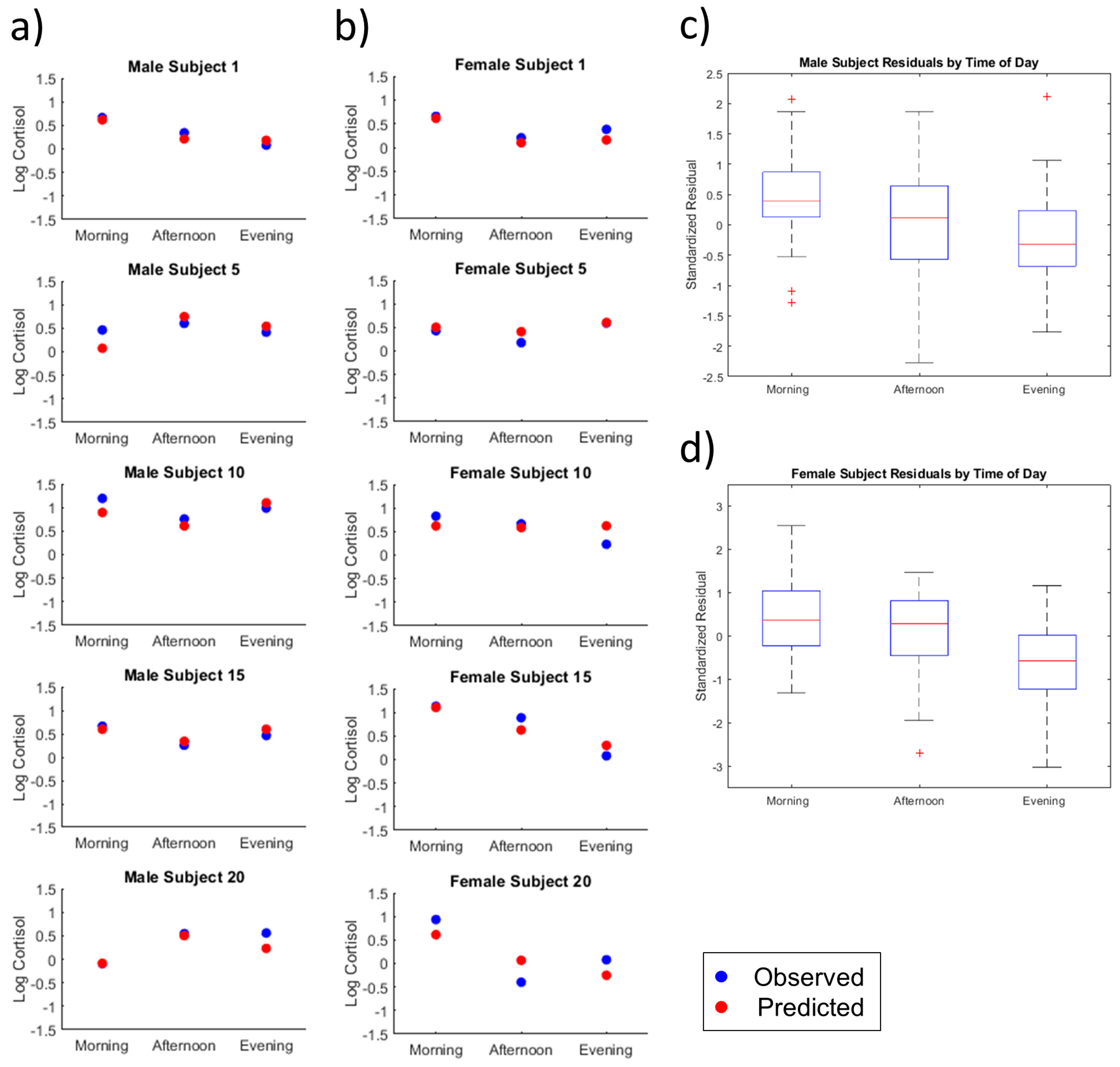
2.2. Individual Subject Analysis
2.2.1. Time of Day
2.2.2. Male Versus Female
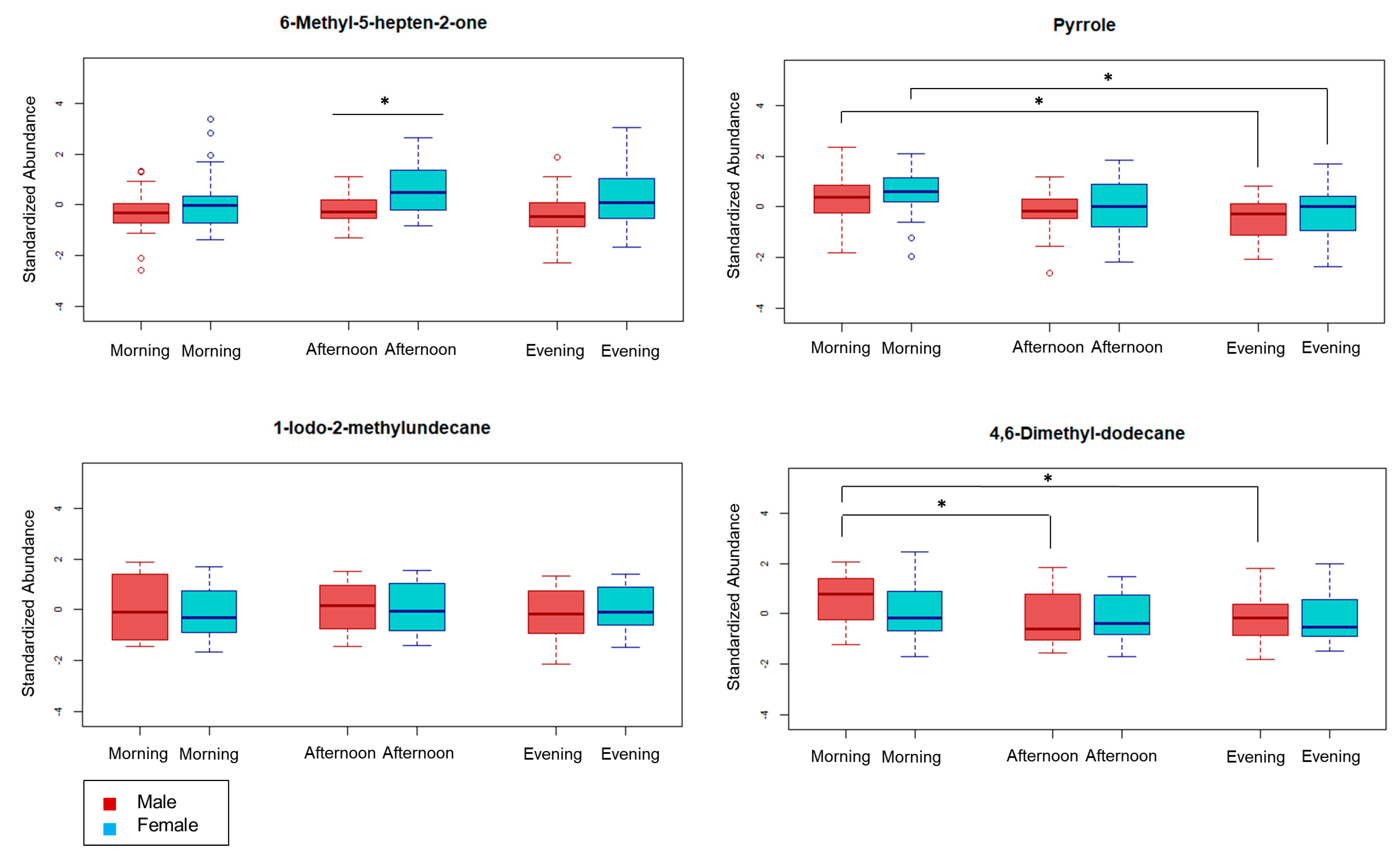
2.3. Review of Selected Metabolites
3. Discussion
4. Materials and Methods
4.1. Subject Recruitment
4.2. Sample Collection
4.3. Volatile Extraction (HS-SPME)
4.4. GC×GC-TOFMS Analysis
4.5. Data Processing
4.6. Statisical Analysis
4.6.1. Post-Processing
4.6.2. Variable Selection
- (i)
- morning samples received a 1 and all other samples received a 0
- (ii)
- afternoon samples received a 1 and all other samples received a 0.
4.6.3. Multiple Regression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Takei, Y.; Ando, H.; Tsutsui, K. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0128010280. [Google Scholar]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef]
- Moreira, A.C.; Antonini, S.R.; De Castro, M. Mechanisms in Endocrinology: A sense of time of the glucocorticoid circadian clock: From the ontogeny to the diagnosis of Cushing’s syndrome. Eur. J. Endocrinol. 2018, 179, R1–R18. [Google Scholar] [CrossRef] [PubMed]
- E Stokes, P. The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur. Neuropsychopharmacol. 1995, 5, 77–82. [Google Scholar] [CrossRef]
- Yehuda, R.; Teicher, M.H.; Trestman, R.; Levengood, R.A.; Siever, L.J. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Boil. Psychiatry 1996, 40, 79–88. [Google Scholar] [CrossRef]
- McQuade, R.; Young, A.H. Future therapeutic targets in mood disorders: The glucocorticoid receptor. Br. J. Psychiatry 2000, 177, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Boil. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Vrshek-Schallhorn, S.; Doane, L.D.; Mineka, S.; Zinbarg, R.E.; Craske, M.G.; Adam, E.K. The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychol. Med. 2012, 43, 483–493. [Google Scholar] [CrossRef]
- Dedovic, K.; Ngiam, J. The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 1181–1189. [Google Scholar] [CrossRef]
- Jia, M.; Chew, W.M.; Feinstein, Y.; Skeath, P.; Sternberg, E.M. Quantification of Cortisol in Human Eccrine Sweat by Liquid Chromatography - Tandem Mass Spectrometry. Analyst 2016, 141, 2053–2060. [Google Scholar] [CrossRef]
- Wood, L.; Ducroq, D.H.; Fraser, H.L.; Gillingwater, S.; Evans, C.; Pickett, A.J.; Rees, D.W.; John, R.; Turkes, A. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann. Clin. Biochem. Int. J. Lab. Med. 2008, 45, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.K.G.; Singh, R.J. LC-MS/MS in the Clinical Laboratory – Where to From Here? Clin. Biochem. Rev. 2011, 32, 5–31. [Google Scholar] [PubMed]
- Taylor, R.L.; Machacek, D.; Singh, R.J. Validation of a High-Throughput Liquid Chromatography–Tandem Mass Spectrometry Method for Urinary Cortisol and Cortisone. Clin. Chem. 2002, 48, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Shibasaki, H.; Nakayama, H.; Furuta, T.; Kasuya, Y.; Tsuchiya, M.; Soejima, A.; Yamada, A.; Nagasawa, T. Simultaneous determination of prednisolone, prednisone, cortisol, and cortisone in plasma by GC–MS: Estimating unbound prednisolone concentration in patients with nephrotic syndrome during oral prednisolone therapy. J. Chromatogr. B 2008, 870, 164–169. [Google Scholar] [CrossRef]
- Watson, S.; Mackin, P. HPA axis function in mood disorders. Psychiatry 2006, 5, 166–170. [Google Scholar] [CrossRef]
- Jansen, L.M.; Wied, C.C.G.-D.; Gademan, P.J.; De Jonge, R.C.; Van Der Linden, J.A.; Kahn, R.S. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr. Res. 1998, 33, 87–94. [Google Scholar] [CrossRef]
- Ehlert, U.; Gaab, J.; Heinrichs, M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Boil. Psychol. 2001, 57, 141–152. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef]
- Martins-De-Souza, D. Proteomics, metabolomics, and protein interactomics in the characterization of the molecular features of major depressive disorder. Dialog- Clin. Neurosci. 2014, 16, 63–73. [Google Scholar]
- Sheather, S. A Modern Approach to Regression with R; Springer Science and Business Media LLC: Berlin, Germany, 2009. [Google Scholar]
- Dallmann, R.; Viola, A.U.; Tarokh, L.; Cajochen, C.; Brown, S.A. The human circadian metabolome. Proc. Natl. Acad. Sci. USA 2012, 109, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.E.; Revell, V.; Anuska, M.; Mäntele, S.; Otway, D.T.; Johnston, J.D.; Thumser, A.E.; Skene, D.J.; Raynaud, F.I. Identification of Human Plasma Metabolites Exhibiting Time-of-Day Variation Using an Untargeted Liquid Chromatography–Mass Spectrometry Metabolomic Approach. Chrono- Int. 2012, 29, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Sinues, P.; Tarokh, L.; Li, X.; Kohler, M.; Brown, S.A.; Zenobi, R.; Dallmann, R. Circadian Variation of the Human Metabolome Captured by Real-Time Breath Analysis. PLOS ONE 2014, 9, e114422. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Davies, S.K.; Revell, V.L.; Keun, H.; Skene, D.J. Diurnal rhythms in the human urine metabolome during sleep and total sleep deprivation. Sci. Rep. 2015, 5, 14843. [Google Scholar] [CrossRef]
- An, M.; Gao, Y. Urinary Biomarkers of Brain Diseases. Genom. Proteom. Bioinform. 2016, 13, 345–354. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, J.; Huang, T.; Wang, M.-J.; Wang, Y.; Dong, M.-X.; Huang, Y.-J.; Zhou, L.-K.; Xie, P. A Novel Urinary Metabolite Signature for Diagnosing Major Depressive Disorder. J. Proteome Res. 2013, 12, 5904–5911. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Fryer, A.A.; Hanna, F.; A A Ferns, G. Can volatile compounds in exhaled breath be used to monitor control in diabetes mellitus? J. Breath Res. 2011, 5, 22001. [Google Scholar] [CrossRef]
- Mikirova, N. Cross-sectional analysis of pyrroles in psychiatric disorders: Association with nutritional and immunological markers. J. Orthomol. Med. 2015, 30, 25–31. [Google Scholar]
- Jackson, J.A.; Riordan, H.D.; Neathery, S.; Riordan, N. Urinary pyrrole in health and disease. J. Orthomol. Med. 1997, 12, 96–98. [Google Scholar]
- McGinnis, W.R.; Audhya, T.; Walsh, W.J.; A Jackson, J.; McLaren-Howard, J.; Lewis, A.; Lauda, P.H.; Bibus, D.M.; Jurnak, F.; Lietha, R.; et al. Discerning the Mauve Factor, Part 1. Altern. Ther. Heal. Med. 2008, 14, 40. [Google Scholar]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018, D633, D639. [Google Scholar]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 34001. [Google Scholar] [CrossRef] [PubMed]
- Costello, B.D.L.; Amann, A.; Alkateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 14001. [Google Scholar] [CrossRef]
- Hall, M.; Hauer, B.; Stuermer, R.; Kroutil, W.; Faber, K. Asymmetric whole-cell bioreduction of an α,β-unsaturated aldehyde (citral): Competing prim-alcohol dehydrogenase and C–C lyase activities. Tetrahedron Asymmetry 2006, 17, 3058–3062. [Google Scholar] [CrossRef]
- Talukdar, S.; Bayan, U.; Saikia, K.K. In silico identification of vaccine candidates against Klebsiella oxytoca. Comput. Boil. Chem. 2017, 69, 48–54. [Google Scholar] [CrossRef]
- Marmulla, R.; Harder, J. Microbial monoterpene transformations—a review. Front. Microbiol. 2014, 5, 346. [Google Scholar] [CrossRef]
- Achiraman, S.; Archunan, G.; Ponmanickam, P.; Rameshkumar, K.; Kannan, S.; John, G. 1–Iodo-2 methylundecane [1I2MU]: An estrogen-dependent urinary sex pheromone of female mice. Theriogenology 2010, 74, 345–353. [Google Scholar] [CrossRef]
- Karmakar, S.; Jin, Y.; Nagaich, A.K. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J. Boil. Chem. 2013, 288, 24020–24034. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Rasoel, S.S.; Toth, J.; Holvoet, L.; Farré, R.; et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2013, 63, 1293–1299. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.G.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Baptista, I.; Santos, M.; Rudnitskaya, A.; Saraiva, J.A.; Almeida, A.; Rocha, S.M. A comprehensive look into the volatile exometabolome of enteroxic and non-enterotoxic Staphylococcus aureus strains. Int. J. Biochem. Cell Boil. 2019, 108, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Krapf, K.; Ager, C.; Wiesenhofer, H.; Agapiou, A.; Statheropoulos, M.; Fuchs, D.; Ellmerer, E.; Buszewski, B.; Amann, A. Temporal profiling of human urine VOCs and its potential role under the ruins of collapsed buildings. Toxicol. Mech. Methods 2012, 22, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, W.; Dai, W.; Tong, T.; Guo, P.; He, S.; Chang, Z.; Gao, X. A simple model for separation prediction of comprehensive two-dimensional gas chromatography and its applications in petroleum analysis. Anal. Methods 2014, 6, 2608. [Google Scholar] [CrossRef]
- Griffith, G.; Owen, G.; Kirkman, S.; Shields, R. MEASUREMENT OF RATE OF GASTRIC EMPTYING USING CHROMIUM-51. Lancet 1966, 287, 1244–1245. [Google Scholar] [CrossRef]
- Risticevic, S.; Pawliszyn, J. Solid-Phase Microextraction in Targeted and Nontargeted Analysis: Displacement and Desorption Effects. Anal. Chem. 2013, 85, 8987–8995. [Google Scholar] [CrossRef]
- Marine, S.S.; Clemons, J. Determination of limonene oxidation products using SPME and GC-MS. J. Chromatogr. Sci. 2003, 41, 31–35. [Google Scholar] [CrossRef]
- Eshima, J.; Ong, S.; Davis, T.J.; Miranda, C.; Krishnamurthy, D.; Nachtsheim, A.; Stufken, J.; Plaisier, C.; Fricks, J.; Bean, H.D.; et al. Monitoring changes in the healthy female metabolome across the menstrual cycle using GC × GC-TOFMS. J. Chromatogr. B 2019, 1121, 48–57. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.A.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Bean, H.D.; Rees, C.A.; Hill, J.E. Comparative analysis of the volatile metabolomes of Pseudomonas aeruginosa clinical isolates. J. Breath Res. 2016, 10, 047102. [Google Scholar] [CrossRef]
- Li, F.; Feng, X.; Zhang, D.; Li, C.; Xu, X.; Zhou, G.; Liu, Y. Physical properties, compositions and volatile profiles of Chinese dry-cured hams from different regions. J. Food Meas. Charact. 2019, 14, 492–504. [Google Scholar] [CrossRef]
- Raffo, A.; Carcea, M.; Castagna, C.; Magrì, A. Improvement of a headspace solid phase microextraction-gas chromatography/mass spectrometry method for the analysis of wheat bread volatile compounds. J. Chromatogr. A 2015, 1406, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Bean, H.D.; Dimandja, J.-M.D.; Hill, J.E. Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. B 2012, 901, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Laboratories. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8546 (accessed on 1 May 2020).
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Statistical Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer Science and Business Media LLC: Berlin, Germany, 2002. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Statistical Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Haug, K.; Salek, R.M.; Conesa, P.; Hastings, J.; De Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P.; et al. MetaboLights—An open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2012, 41, D781–D786. [Google Scholar] [CrossRef]


| Multiple Regression Model–Metabolite Term Breakdown | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model Variable | Compound Name | HMDB ID | Chemical Classification | Regression Coefficient | 95% Confidence Interval | Benjamini-Hochberg Adjusted p-Value | 1tR(s) | 2tR(s) | RI | ID |
| β0 | Intercept | NA | NA | 0.496 | (0.446, 0.548) | < 2 × 10−16 | NA | NA | NA | NA |
| x1 | 6-Methyl-5-hepten-2-one | HMDB0035915 | Ketone | −0.116 | (−0.174, −0.058) | 2.93 × 10−6 | 1364 | 0.76 | 1031 | 1 |
| x2 | Ketone 1 | ‒ | Ketone | 0.170 | (0.093, 0.247) | 4.18 × 10−8 | 2056 | 0.75 | 1403 | 4 |
| x3 | Unknown 1 | ‒ | ‒ | 0.035 | (−0.048, 0.117) | 2.16 × 10−8 | 1102 | 0.94 | 911 | 4 |
| x4 | Hydrocarbon 1 | ‒ | Hydrocarbon | −0.069 | (−0.127, 0.012) | 2.70 × 10−3 | 1926 | 0.57 | 1326 | 4 |
| x5 | Unknown 2 | ‒ | ‒ | −0.112 | (−0.169, −0.055) | 4.57 × 10−3 | 1510 | 1.03 | 1102 | 4 |
| x6 | Unknown 3 | ‒ | ‒ | 0.207 | (0.105, 0.308) | 4.79 × 10−3 | 2270 | 1.00 | 1538 | 4 |
| x7 | Unknown 4 | ‒ | ‒ | −0.040 | (−0.095, 0.014) | 4.08 × 10−2 | 512 | 1.47 | 644 * | 4 |
| x8 | Unknown 5 | ‒ | ‒ | −0.115 | (−0.173, −0.057) | 6.37 × 10−2 | 918 | 0.64 | 830 | 4 |
| x9 | Unknown 6 | ‒ | ‒ | −0.035 | (−0.112, 0.042) | 6.80 × 10−2 | 2038 | 0.52 | 1392 | 4 |
| x10 | Unknown 7 | ‒ | ‒ | 0.004 | (−0.095, 0.104) | 1.57 × 10−2 | 2156 | 0.54 | 1456 | 4 |
| x11 | Pyrrole | HMDB0035924 | Heteroaromatic | 0.070 | (0.001, 0.139) | 1.39 × 10−1 | 926 | 1.87 | 833 | 1 |
| x12 | 1-Iodo-2-methylundecane ** | HMDB0062727 | Halogenated Hydrocarbon | −0.063 | (−0.124, −0.003) | 5.47 × 10−2 | 2322 | 0.59 | 1571 | 3 |
| x13 | Unknown 8 | ‒ | ‒ | 0.070 | (0.003, 0.137) | 8.97 × 10−2 | 2304 | 1.07 | 1560 | 4 |
| x14 | 4,6-Dimethyl-dodecane *** | HMDB0062598 | Hydrocarbon | 0.081 | (0.001, 0.161) | 8.97 × 10−2 | 1784 | 0.56 | 1246 | 3 |
| Multiple Regression Model–Interaction Term Breakdown | |||||
|---|---|---|---|---|---|
| Interaction Terms | Compound Name | Dummy Term | Regression Coefficient | 95% Confidence Interval | Benjamini-Hochberg Adjusted p-Value |
| x2 × Male | Ketone 1 | Male | −0.121 | (−0.228, −0.013) | 1.81 × 10−2 |
| x9 × Male | Unknown 6 | Male | −0.100 | (−0.203, 0.005) | 3.18 × 10−2 |
| x10 × Male | Unknown 7 | Male | 0.121 | (0.004, 0.237) | 7.41 × 10−3 |
| x3 × Morning | Unknown 1 | Morning | 0.210 | (0.076, 0.345) | 6.82 × 10−2 |
| x6 × Morning | Unknown 3 | Morning | −0.295 | (−0.434, −0.155) | 1.25 × 10−1 |
| x14 × Morning | 4,6-Dimethyl-dodecane *** | Morning | −0.075 | (−0.182, 0.031) | 1.91 × 10−1 |
| x6 × Afternoon | Unknown 3 | Afternoon | −0.092 | (−0.218, 0.035) | 1.80 × 10−1 |
| Male Subject | Age (years) | Ethnicity | Height (cm) | Weight (kg) | Stress Score (0–10) | Female Subject | Age (years) | Ethnicity | Height (cm) | Weight (kg) | Stress Score (0–10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | Caucasian | 180 | 64 | 3 | 1 | 26 | Latino | 163 | 56 | 0 |
| 2 | 18 | Caucasian | 168 | 59 | 3 | 2 | 18 | Asian | 165 | 51 | 4 |
| 3 | 28 | Asian | 178 | 62 | 5 | 3 * | 20 | Latino | 157 | 43 | 4 |
| 4 | 19 | African American | 185 | 95 | 3 | 4 | 23 | Caucasian | 163 | 59 | 2 |
| 5 | 26 | Asian | 178 | 50 | 4 | 5 | 18 | Asian | 163 | 45 | 6 |
| 6 | 18 | Caucasian | 183 | 79 | 0 | 6 | 19 | Caucasian | 178 | 64 | 5 |
| 7 | 21 | Asian | 180 | 75 | 0 | 7 * | 21 | Caucasian | 163 | 54 | 4 |
| 8 | 22 | Caucasian | 179 | 74 | 0 | 8 * | 32 | African American | 160 | 75 | 4 |
| 9 | 20 | Caucasian | 170 | 70 | 2 | 9 | 18 | Latino | 165 | 54 | 3 |
| 10 | 20 | Asian | 152 | 50 | 4 | 10* | 25 | Latino | 170 | 63 | 3 |
| 11 | 20 | Asian | 173 | 52 | 1 | 11 | 28 | Caucasian | 170 | 67 | 3 |
| 12 | 19 | Latino | 170 | 82 | 0 | 12 | 44 | Asian | 165 | 58 | 2 |
| 13 | 20 | Latino | 183 | 73 | 6 | 13 | 30 | Latino | 163 | 51 | 2 |
| 14 | 21 | Caucasian | 180 | 84 | 2 | 14 | 18 | Caucasian/ Latino | 170 | 82 | 3 |
| 15 | 18 | Latino | 160 | 61 | 2 | 15 * | 20 | Caucasian | 170 | 66 | 5 |
| 16 | 22 | Asian | 179 | 70 | 0 | 16 * | 19 | Latino | 173 | 75 | 6 |
| 17 | 18 | Caucasian | 180 | 68 | 3 | 17 * | 25 | African American | 168 | 70 | 4 |
| 18 | 20 | Caucasian | 183 | 86 | 2 | 18 | 23 | African American | 165 | 53 | 2 |
| 19 | 18 | Caucasian | 183 | 68 | 6 | 19 | 22 | Caucasian/ Asian | 157 | 61 | 6 |
| 20 | 21 | Caucasian | 178 | 68 | 4 | 20 | 54 | Caucasian | 157 | 51 | 0 |
| 21 | 23 | Caucasian | 193 | 77 | 4 | 21 | 20 | African American | 165 | 62 | 0 |
| 22 | 18 | Asian | 173 | 73 | 4 | 22 | 21 | Caucasian | 170 | 73 | 6 |
| 23 | 26 | Indian | 170 | 70 | 6 | 23 | 18 | Latino | 157 | 48 | 4 |
| 24 | 20 | Asian | 178 | 77 | 6 | 24 * | 18 | Caucasian/ Asian | 165 | 59 | 6 |
| 25 | 19 | Latino | 178 | 77 | 4 | 25 * | 21 | Caucasian | 168 | 64 | 6 |
| 26 | 22 | Asian | 183 | 84 | 3 | 26 * | 19 | Caucasian | 157 | 68 | 3 |
| 27 | 22 | Caucasian | 183 | 77 | 0 | 27 * | 23 | African American | 157 | 52 | 2 |
| 28 | 20 | Asian | 175 | 79 | 0 | 28 | 23 | African | 158 | 55 | 5 |
| 29 | 18 | Asian | 170 | 61 | 4 | 29 * | 18 | Caucasian | 160 | 64 | 5 |
| 30 | 18 | Asian | 185 | 77 | 5 | 30 * | 21 | Latino | 160 | 52 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshima, J.; Davis, T.J.; Bean, H.D.; Fricks, J.; Smith, B.S. A Metabolomic Approach for Predicting Diurnal Changes in Cortisol. Metabolites 2020, 10, 194. https://doi.org/10.3390/metabo10050194
Eshima J, Davis TJ, Bean HD, Fricks J, Smith BS. A Metabolomic Approach for Predicting Diurnal Changes in Cortisol. Metabolites. 2020; 10(5):194. https://doi.org/10.3390/metabo10050194
Chicago/Turabian StyleEshima, Jarrett, Trenton J. Davis, Heather D. Bean, John Fricks, and Barbara S. Smith. 2020. "A Metabolomic Approach for Predicting Diurnal Changes in Cortisol" Metabolites 10, no. 5: 194. https://doi.org/10.3390/metabo10050194
APA StyleEshima, J., Davis, T. J., Bean, H. D., Fricks, J., & Smith, B. S. (2020). A Metabolomic Approach for Predicting Diurnal Changes in Cortisol. Metabolites, 10(5), 194. https://doi.org/10.3390/metabo10050194





