Metabolomics in Central Sensitivity Syndromes
Abstract
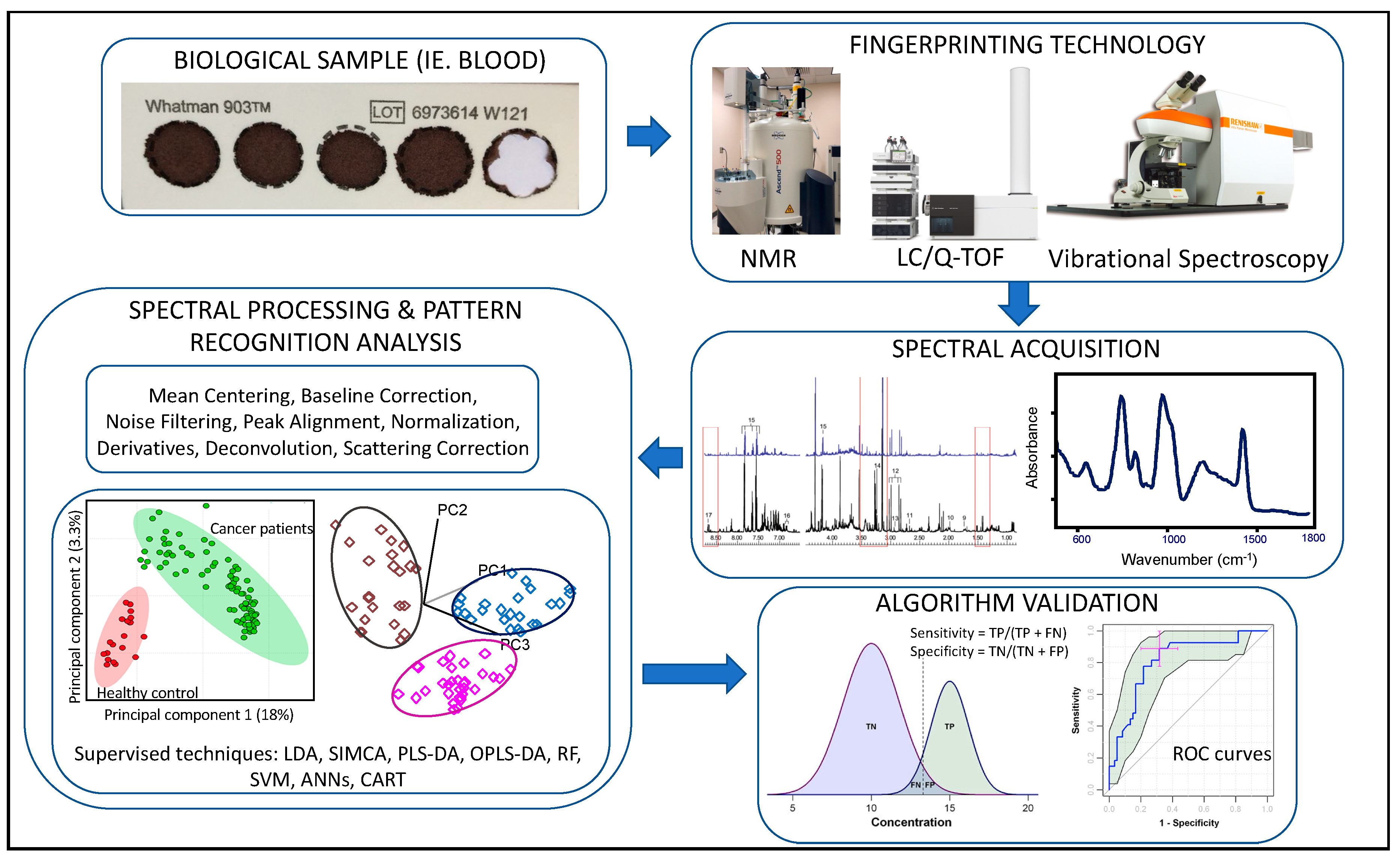
1. Potential of Metabolomics to Identify Biomarkers for Central Sensitivity Syndromes (CSS)
2. Current State of Metabolomics Research in CSS
2.1. Chronic Fatigue Syndrome (CFS)
2.2. Complex Regional Pain Syndrome (CRPS)
2.3. Endometriosis
2.4. Fibromyalgia (FM)
2.5. Headache
2.5.1. Tension-Type Headache (TTH)
2.5.2. Cluster Headache (CH)
2.6. Idiopathic Low Back Pain
2.7. Painful Bladder Syndrome (PBS)/Chronic Prostatitis (CP)/Interstitial Cystitis (IC)
2.8. Irritable Bowel Syndrome (IBS)
2.9. Migraine
2.10. Multiple Chemical Sensitivity (MCS) Syndrome
2.11. Myofascial Pain Syndrome (MPS)
2.12. Polycystic Ovary Syndrome (PCOS)
2.13. Primary Dysmenorrhea
2.14. Restless Leg Syndrome – Periodic Limb Movement in Sleep
2.15. Temporomandibular Disorders (TMD)
2.16. Vulvodynia
2.17. Post-Traumatic Stress Disorder (PTSD)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Acylcarnitines | AC |
| Amino Acids | AA |
| Anti-Dense Fine Speckled 70 | Anti-DFS70 |
| Area Under the Curve | AUC |
| Artificial Neural Networks | ANNs |
| Basal Ganglia | BG |
| Bladder Pain Syndrome | BPS |
| Capillary Electrophoresis | CE |
| Central Sensitization Syndrome | CSS |
| Cerebrospinal Fluid | CSF |
| Chronic Daily Headache | CDH |
| Chronic Fatigue Syndrome | CFS |
| Chronic Low Back Pain | CLBP |
| Chronic Prostatitis | CP |
| Cluster Headache | CH |
| Complex Regional Pain Syndrome | CRPS |
| Constipation Based Irritable Bowel Syndrome | IBS-C |
| Diarrhea Based Irritable Bowel Syndrome | IBS-D |
| Electrospray Ionization | ESI |
| Etiocholan-3α-ol-17-one Sulfate | Etio-S |
| Fibromyalgia | FM |
| Follicular Fluid | FF |
| Fourier Transform-Infrared Spectroscopy | FT-IR |
| Free Fatty Acids | FFA |
| Gas Chromatography | GC |
| High Resolution Mass Spectrometry | HRMS |
| High-Performance Liquid Chromatography | HPLC |
| Insulin resistance | IR |
| Interstitial Cystitis | IC |
| Irritable Bowel Syndrome | IBS |
| Irritable Bowel Syndrome Mice treated with C. butyricum | IBS+ |
| Liquid Chromatography-Mass Spectrometry | LC-MS |
| Mass spectrometry | MS |
| Matrix-Assisted Laser Desorption/Ionization | MALDI |
| Mid-Infrared Microspectroscopy | IMS |
| Mitochondrial DNA | mtDNA |
| Multiple Chemical Sensitivity | MCS |
| Multivariate Analysis | MVA |
| Myofascial Pain Syndrome | MPS |
| Nonbacterial Prostatitis | NBP |
| Nuclear Magnetic Resonance Spectroscopy | NMR |
| Osteoarthritis | OA |
| Painful Bladder Syndrome | PBS |
| Peripheral Blood Mononuclear Cells | PBMCs |
| Peritoneal Fluid | PF |
| Phenylacetylglutamine | PAGN |
| Polycystic Ovarian Syndrome | PCOS |
| Post-Traumatic Stress Disorder | PTSD |
| Principal Component Analysis | PCA |
| Proton Nuclear Magnetic Resonance Spectroscopy | 1H-NMR |
| Quadrupole | Q |
| Rheumatoid Arthritis | RA |
| Sequential Window Acquisition of all Theoretical fragment-ion spectra | SWATHTM |
| Soft Independent Modeling of Class Analogy | SIMCA |
| Support Vector Machines | SVM |
| Systemic Lupus Erythematosus | SLE |
| Tandem Mass Spectrometry | MS/MS |
| Temporomandibular Disorders | TMD |
| Tension-Type Headache | TTH |
| Thin Layer Chromatography | TLC |
| Time OF Flight/Mass Spectrometry | TOF/MS |
| Ultra High Performance Liquid Chromatography | UHPLC |
| Ultra-Performance Liquid Chromatography | UPLC |
| Volatile Organic Metabolites | VOMs |
References
- Pang, H.; Jia, W.; Hu, Z. Emerging applications of metabolomics in clinical pharmacology. Clin. Pharmacol. Ther. 2019, 106, 544–566. [Google Scholar] [CrossRef] [PubMed]
- Garrod, A.E. The incidence of alkaptonuria: A study in chemical individuality. Lancet 1902, 2, 1616–1620. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Wiley-Blackwell: Hoboken, NJ, USA, 1984; pp. 416–456. [Google Scholar]
- Hunt, D.F.; Yates, J.R., 3rd; Shabanowitz, J.; Winston, S.; Hauer, C.R. Protein sequencing by tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1986, 83, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.I.; Goodacre, R. Metabolic fingerprinting in disease diagnosis: Biomedical applications of infrared and Raman spectroscopy. Analyst 2006, 131, 875–885. [Google Scholar] [CrossRef]
- Wu, H.; Feng, F. Untargeted metabolomic analysis using LC-TOF/MS and LC-MS/MS for revealing metabolic alterations linked to alcohol-induced hepatic steatosis in rat serum and plasma. RSC Adv. 2016, 6, 28279–28288. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, W.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Yunus, M.B. Central sensitivity syndromes: A new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin. Arthritis Rheum. 2008, 37, 339–352. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Clauw, D.J.; Hassett, A.L. The role of centralised pain in osteoarthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 79–84. [Google Scholar]
- Melzack, A.R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Burgess, P.R.; Perl, E.R. Myelinated afferent fibres responding specifically to noxious stimulation ofthe skin. J. Physiol. 1967, 190, 541–562. [Google Scholar] [CrossRef]
- Perl, E.R. Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J. Physiol. 1968, 197, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Bessou, P.; Perl, E.R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol. 1969, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.M.; Wall, P.D. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965, 206, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Randic, M.; Jiang, M.C.; Cerne, R. Long-term potentiation and long-term depression of primaryafferent neurotransmission in the rat spinal cord. J. Neurosci. 1993, 13, 5228–5241. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.W.; Woolf, C.J.; Sivilotti, L.G. Small-caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal ratspinal cord in vitro. J. Neurophysiol. 1993, 69, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; King, A.E. Physiology and morphology of multireceptive neurons with C-afferent fiberinputs in the deep dorsal horn of the rat lumbar spinal cord. J. Neurophysiol. 1987, 58, 460–479. [Google Scholar] [CrossRef]
- Woolf, C.J.; King, A.E. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. J. Neurophysiol. 1989, 62, 907–916. [Google Scholar] [CrossRef]
- Chacur, M.; Lambertz, D.; Hoheisel, U.; Mense, S. Role of spinal microglia in myositis-induced centralsensitisation: An immunohistochemical and behavioural study in rats. Eur. J. Pain 2009, 13, 915–923. [Google Scholar] [CrossRef]
- Chang, Y.W.; Waxman, S.G. Minocycline attenuates mechanical allodynia and central sensitization following peripheral second-degree burn injury. J. Pain 2010, 11, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Li, Z.; Dostrovsky, J.O.; Sessle, B.J. Central sensitization in medullary dorsal horninvolves gap junctions and hemichannels. Neuroreport 2010, 21, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Zammataro, M.; Morales, M.E.; Busceti, C.L.; Drago, F.; Gereau, R.W.T.; Copani, A.; Nicoletti, F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment ofinflammatory pain. Mol. Pharmacol. 2009, 75, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.Z.; Park, J.Y.; Lind, A.L.; Ma, Q.; Ji, R.R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization andneuropathic pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef] [PubMed]
- Hathway, G.J.; Vega-Avelaira, D.; Moss, A.; Ingram, R.; Fitzgerald, M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitizationin adults but not in neonates. Pain 2009, 144, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Arconada, I.; Lopez-Garcia, J.A. Changes in membrane excitability and potassium currentsin sensitized dorsal horn neurons of mice pups. J. Neurosci. 2010, 30, 5376–5383. [Google Scholar] [CrossRef]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory painhypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef]
- Seybold, V.S. The role of peptides in central sensitization. Handb. Exp. Pharmacol. 2009, 194, 451–491. [Google Scholar]
- Torres Tortosa, M.; Roman Rico, D. Risk of transmission of human immunodeficiency virus type 1 after accidents with needles from drug addicts, occurred in the community. Rev. Clin. Esp. 1991, 189, 95–96. [Google Scholar]
- Clauw, D.J.; Chrousos, G.P. Chronic pain and fatigue syndromes: Overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulat 1997, 4, 134–153. [Google Scholar] [CrossRef]
- Wasserman, R.A.; Brummett, C.M.; Goesling, J.; Tsodikov, A.; Hassett, A.L. Characteristics of chronic pain patients who take opioids and persistently report high pain intensity. Reg. Anesth Pain M 2014, 39, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Gamsa, P.A.; Ware, M.A.; Shir, Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am. J. Med. 2011, 124, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Collado, R.; Quiles, J.L.; Ramírez-Tortosa, M.; Sol, J.; Ruiz-Sanjuan, M.; Fernandez, M.; Ramírez-Tortosa, C.; Granados-Principal, S. A plasma metabolomic signature discloses human breast cancer. Oncotarget 2017, 8, 19522–19533. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, K.V.; Aykas, D.P.; Sigurdson, G.T.; Plans, M.; Madiai, F.; Yu, L.; Buffington, C.A.T.; Giusti, M.M.; Rodriguez-Saona, L. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J. Biol. Chem. 2019, 294, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.H.; Wevers, R.A.; van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Schmukler, J.; Jamal, S.; Castrejon, I.; Gibson, K.A.; Srinivasan, S.; Häuser, W.; Pincus, T. Diagnosis of fibromyalgia: Disagreement between fibromyalgia criteria and clinician-based fibromyalgia diagnosis in a university clinic. Arthritis Care Res. 2019, 71, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.B.; van der Meer, J.W.; Bleijenberg, G. Chronic fatigue syndrome. Lancet 2006, 367, 346–355. [Google Scholar] [CrossRef]
- Aronowitz, R.A. From myalgic encephalitis to yuppie flu: A history of chronic fatigue syndromes. In Framing Disease: Studies in Cultural History; Rosenberg, C.E., Golden, J., Eds.; Rutgers University Press: New Brunswick, NJ, USA, 1992; pp. 155–181. [Google Scholar]
- Kim, E. A brief history of chronic fatigue syndrome. JAMA 1994, 272, 1070–1071. [Google Scholar] [CrossRef]
- Institute of Medicine, I. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Lwai, K.; Fukuda, S.; et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci. Rep. 2016, 6, 34990. [Google Scholar] [CrossRef]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Prospective biomarkers from plasma metabolomics of myalgic encephalomyelitis/chronic fatigue syndrome implicate redox imbalance in disease symptomatology. Metabolites 2018, 8, 90. [Google Scholar] [CrossRef]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol. Biosyst. 2017, 13, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Correction for Naviaux et al., Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E3749. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, E.M.; Van Der Westhuizen, F.H.; Erasmus, E.; van Dyk, E.; Knowles, C.V.; Al-Ali, S.; Ng, W.F. Clinically proven mtDNA mutations are not common in those with chronic fatigue syndrome. BMC Med. Genet. 2017, 18, 29. [Google Scholar] [CrossRef]
- Venter, M.; Tomas, C.; Pienaar, I.S.; Strassheim, V.; Erasmus, E.; Ng, W.F.; Howell, N.; Newton, J.L.; Van der Westhuizen, F.H.; Elson, J.L. MtDNA population variation in myalgic encephalomyelitis/chronic fatigue syndrome in two populations: A study of mildly deleterious variants. Sci. Rep. 2019, 9, 2914. [Google Scholar] [CrossRef]
- Lyall, M.; Peakman, M.; Wessely, S. A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. J. Psychosom. Res. 2003, 55, 79–90. [Google Scholar] [CrossRef]
- Chi, A.P.; Wang, Z.N.; Shi, B.; Yang, X.F.; Min, R.X.; Song, J. Comparison of differential metabolites in urine of the middle school students with chronic fatigue syndrome before and after exercise. Chin. J. Appl. Physiol. 2018, 34, 340–344. [Google Scholar] [CrossRef]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Correction: Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 2018, 13, e0192817. [Google Scholar] [CrossRef]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 2017, 12, e0186802. [Google Scholar] [CrossRef]
- Li, K.; Naviaux, J.C.; Bright, A.T.; Wang, L.; Naviaux, R.K. A robust, single-injection method for targeted, broad-spectrum plasma metabolomics. Metabolomics 2017, 13, 122. [Google Scholar] [CrossRef]
- Natelson, B.H.; Vu, D.; Coplan, J.D.; Mao, X.; Blate, M.; Kang, G.; Soto, E.; Kapusuz, T.; Shungu, D.C. Elevations of ventricular lactate levels occur in both chronic fatigue syndrome and fibromyalgia. Fatigue 2017, 5, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaro, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.N.; Bruehl, S.; Stanton-Hicks, M.; Wilson, P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Stanton-Hicks, M.; Jänig, W.; Hassenbusch, S.; Haddox, J.D.; Boas, R.; Wilson, P. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain 1995, 63, 127–133. [Google Scholar] [CrossRef]
- Harden, R.N.; Oaklander, A.L.; Burton, A.W.; Perez, R.S.G.M.; Richardson, K.; Swan, M.; Barthel, J.; Costa, B.; Graciosa, J.R.; Bruehl, S.; et al. Complex regional pain syndrome: Practical diagnostic and treatment guidelines. Pain Med. 2013, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 2010, 113, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; van der Plas, A.A.; van Dasselaar, N.T.; Deelder, A.M.; van Hilten, J.J.; Mayboroda, O.A. 1H-NMR metabolic profiling of cerebrospinal fluid in patients with complex regional pain syndrome-related dystonia. Pain 2014, 155, 190–196. [Google Scholar] [CrossRef]
- Ramautar, R.; van der Plas, A.A.; Nevedomskaya, E.; Derks, R.J.; Somsen, G.W.; de Jong, G.J.; van Hilten, J.J.; Deelder, A.M.; Mayboroda, O.A. Explorative analysis of urine by capillary electrophoresis-mass spectrometry in chronic patients with complex regional pain syndrome. J. Proteome Res. 2009, 8, 5559–5567. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Jansen, R.P.; Russell, P. Nonpigmented endometriosis: Clinical, laparoscopic, and pathologic definition. Am. J. Obstet. Gynecol. 1986, 155, 1154–1159. [Google Scholar] [CrossRef]
- Clement, P.B. The pathology of endometriosis: A survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Cuviello, F.; Ostuni, A. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: A pilot study. Metabolomics 2019, 15, 19. [Google Scholar] [CrossRef]
- Sun, Z.; Song, J.; Zhang, X.; Wang, A.; Guo, Y.; Yang, Y.; Wang, X.; Xu, K.; Deng, J. Novel SWATHTM technology for follicular fluid metabolomics in patients with endometriosis. Pharmazie 2018, 73, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef]
- Marianna, S.; Alessia, P.; Susan, C.; Francesca, C.; Angela, S.; Francesca, C.; Antonella, N.; Patrizia, I.; Nicola, C.; Emilio, C. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 2017, 13, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, F.B.; Cataldi, T.R.; Perkel, K.J.; do Vale Teixeira da Costa, L.; Rochetti, R.C.; Stevanato, J.; Eberlin, M.N.; Zylbersztejn, D.S.; Cedenho, A.P.; Turco, E.G. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J. Assist. Reprod. Genet. 2015, 32, 1817–1825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Guan, L.; Zhang, H.; Gao, Y.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Liang, X.; Huang, M.; et al. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod. Biol. Endocrinol. 2018, 16, 42. [Google Scholar] [CrossRef]
- Li, J.; Guan, L.; Zhang, H.; Gao, Y.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Liang, X.; Huang, M.; et al. Correction to: Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod. Biol. Endocrinol. 2019, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Singh, B.; Joshi, M.; Das, D.; Subramani, E.; Maan, M.; Jana, S.K.; Sharma, U.; Das, S.; Dasgupta, S.; et al. Metabolomics reveals perturbations in endometrium and serum of minimal and mild endometriosis. Sci. Rep. 2018, 8, 6466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Guan, L.; Zhang, H.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Ma, Z.; Liang, X.; et al. Discovery of Phosphatidic Acid, Phosphatidylcholine, and Phosphatidylserine as Biomarkers for Early Diagnosis of Endometriosis. Front. Physiol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Peterse, D.P.; Fassbender, A.; Hendriks, M.M.; van den Broek, N.J.; Berger, R.; Vanhie, A.; Vodolazkaia, A.; Van Langendonckt, A.; Donnez, J.; et al. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil. Steril. 2017, 107, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Muñoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Payá, V.; Pellicer, A.; Pineda-Lucena, A. Pathophysiologic processes have an impact on the plasma metabolomic signature of endometriosis patients. Fertil. Steril. 2016, 106, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Vouk, K.; Hevir, N.; Ribić-Pucelj, M.; Haarpaintner, G.; Scherb, H.; Osredkar, J.; Möller, G.; Prehn, C.; Rižner, T.L.; Adamski, J. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum. Reprod. 2012, 27, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.; Arjmand, M.; Akbari, Z.; Mellati, A.O.; Saheb-Kashaf, H.; Zamani, Z. (1) H NMR- based metabolomics approaches as non- invasive tools for diagnosis of endometriosis. Int. J. Reprod. Biomed. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Jana, S.K.; Dutta, M.; Joshi, M.; Srivastava, S.; Chakravarty, B.; Chaudhury, K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. Biomed. Res. Int. 2013, 2013, 329058. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Joshi, M.; Srivastava, S.; Lodh, I.; Chakravarty, B.; Chaudhury, K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol. Biosyst. 2012, 8, 3281–3287. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Montani, D.A.; Setti, A.S.; Lo Turco, E.G.; Oliveira-Silva, D.; Borges, E., Jr. Metabolomic profile as a noninvasive adjunct tool for the diagnosis of Grades III and IV endometriosis-related infertility. Mol. Reprod. Dev. 2019, 86, 1044–1052. [Google Scholar] [CrossRef]
- Vouk, K.; Ribič-Pucelj, M.; Adamski, J.; Rižner, T.L. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. J. Steroid. Biochem. Mol. Biol. 2016, 159, 60–69. [Google Scholar] [CrossRef]
- Vicente-Muñoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Payá, V.; Pellicer, A.; Pineda-Lucena, A. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endometriosis. Fertil. Steril. 2015, 104, 1202–1209. [Google Scholar] [CrossRef]
- Yunus, M.B. Editorial review: An update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr. Rheumatol. Rev. 2015, 11, 70–85. [Google Scholar] [CrossRef]
- Arnold, L.M.; Clauw, D.J.; McCarberg, B.H. FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin. Proc. 2011, 86, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S.; Harris, R.; Clauw, D. Fibromyalgia: An afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 2011, 14, E217–E245. [Google Scholar] [PubMed]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Maixner, W.; Fillingim, R.B.; Williams, D.A.; Smith, S.B.; Slade, G.D. Overlapping chronic pain conditions: Implications for diagnosis and classification. J. Pain 2016, 17, T93–T107. [Google Scholar] [CrossRef]
- Buskila, D.; Sarzi-Puttini, P.; Ablin, J.N. The genetics of fibromyalgia syndrome. Pharmacogenomics 2007, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dadabhoy, D.; Clauw, D.J. Musculoskeletal signs and symptoms: The fibromyalgia syndrome. In Primer on the Rheumatic Diseases; Klippel, J.H., Ed.; Springer: New York, NY, USA, 2008; pp. 87–93. [Google Scholar]
- Clauw, D.J. The pathogenesis of chronic pain and fatigue syndromes, with special reference to fibromyalgia. Med. Hypotheses 1995, 44, 369–378. [Google Scholar] [CrossRef]
- Ablin, J.N.; Wolfe, F. A comparative evaluation of the 2011 and 2016 criteria for fibromyalgia. J. Rheumatol. 2017, 44, 1271–1276. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Dumas, M.-E.; Davidovic, L. Metabolic phenotyping and systems biology approaches to understanding neurological disorders. F1000Prime Rep. 2013, 5, 18. [Google Scholar] [CrossRef]
- Vincent, A.; Hoskin, T.L.; Whipple, M.O.; Clauw, D.J.; Barton, D.L.; Benzo, R.P.; Williams, D.A. OMERACT-based fibromyalgia symptom subgroups: An exploratory cluster analysis. Arthritis Res. Ther. 2014, 16, 463. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Soriano-Maldonado, A.; Álvarez Gallardo, I.C.; Estévez-López, F.; Carbonell-Baeza, A.; Delgado-Fernández, M. Subgroups of fibromyalgia patients using the 1990 American College of Rheumatology criteria and the modified 2010 preliminarydiagnostic criteria: The al-Ándalus project. Clin. Exp. Rheumatol. 2015, 34, S26–S33. [Google Scholar] [PubMed]
- Richards, S.C.M.; Bell, J.; Cheung, Y.-L.; Cleare, A.; Scott, D.L. Abstract 382: Muscle metabolites detected in urine in fibromyalgia and chronic fatigue syndrome may suggest ongoing muscle damage. Rheumatology 2001, 40, 135. [Google Scholar]
- Malatji, B.G.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Meyer, H.; van Reenen, M.; Reinecke, C.J. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Liori, B.; Kumar, A.; Santoru, M.L.; Asthana, S.; Pieroni, E.; Fais, A.; Era, B.; Cacace, E.; Ruggiero, V.; et al. Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS ONE 2014, 9, e107626. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, D.H.; Park, G.; Park, S.; Kim, H.S. Clinical significance of anti-dense fine speckled 70 antibody in patients with fibromyalgia. Korean J. Intern. Med. 2019, 34, 426–433. [Google Scholar] [CrossRef]
- Hackshaw, K.V.; Rodriguez-Saona, L.; Plans, M.; Bell, L.N.; Buffington, C.A. A bloodspot-based diagnostic test for fibromyalgia syndrome and related disorders. Analyst 2013, 138, 4453–4462. [Google Scholar] [CrossRef]
- Jensen, R.H. Tension-type headache—the normal and most prevalent headache. Headache 2018, 58, 339. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Birbeck, G.L.; Katsarava, Z.; Jensen, R.H.; Stovner, L.J.; Steiner, T.J. The Global Burden of Disease survey 2010, lifting the Burden and thinking outside-the-box on headache disorders. J. Headache Pain 2013, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Son, C.N.; Kim, S.H.; Chang, H.W.; Kim, J.M. A neurometabolite study of chronic daily headache in patients with systemic lupus erythematosus using magnetic resonance spectroscopy: Comparison with fibromyalgia patients and healthy controls. Korean J. Intern. Med. 2016, 31, 1171–1177. [Google Scholar] [CrossRef][Green Version]
- Nesbitt, A.D.; Goadsby, P.J. Cluster headache. Br. Med. J. 2012, 344, e2407. [Google Scholar] [CrossRef] [PubMed]
- Fischera, M.; Marziniak, M.; Gralow, I.; Evers, S. The incidence and prevalence of cluster headache: A meta-analysis of population-based studies. Cephalalgia 2008, 28, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, B.K.; Chung, P.W. Impact of cluster headache on employment status and job burden: A prospective cross-sectional multicenter study. J. Headache Pain 2018, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Gucciardi, A.; Perini, F.; Leon, A. The role of neurotransmitters and neuromodulators in the pathogenesis of cluster headache: A review. Neurol. Sci. 2019, 40 (Suppl. 1), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Tsui-Wu, Y.J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987, 12, 264–268. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Carroll, L.J.; Côté, P. The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine 1998, 23, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Chou, R. Low back pain. Ann. Intern. Med. 2014, 160, ITC6-1. [Google Scholar] [CrossRef]
- Rui, P.; Okeyode, T. National Ambulatory Medical Care Survey: 2016 National Summary Tables. Available online: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_ web_tables.pdf (accessed on 25 January 2020).
- Trbojević-Akmačić, I.; Vučković, F.; Vilaj, M.; Skelin, A.; Karssen, L.C.; Krištić, J.; Jurić, J.; Momčilović, A.; Šimunović, J.; Mangino, M.; et al. Plasma N-glycome composition associates with chronic low back pain. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2124–2133. [Google Scholar] [CrossRef]
- Hanno, P.M. Painful bladder syndrome/interstitial cystitis and related disorders. In Campbell-Walsh Urology; Wein, A.J., Kavoussi, L.R., Novick, A.C., Partin, A.W., Peters, C.A., Eds.; Elsevier: Philadelphia, PA, USA, 2007; pp. 330–370. [Google Scholar]
- Parsons, C.L.; Tatsis, V. Prevalence of interstitial cystitis in young women. Urology 2004, 64, 866–870. [Google Scholar] [CrossRef]
- Berry, S.H.; Elliott, M.N.; Suttorp, M. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 2011, 186, 540–544. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Markossian, T.; Calhoun, E.A. Comparison of economic impact of chronic prostatitis ⁄ chronic pelvic pain syndrome and interstitial cystitis/painful bladder syndrome. Urology 2009, 73, 743–746. [Google Scholar] [CrossRef]
- Wilkinson, D.R.; Erickson, A.D. Urinary and serologic markers for interstitial cystitis: An update. Curr. Urol. Rep. 2006, 7, 414–422. [Google Scholar] [CrossRef]
- Keay, S.K.; Zhang, C.O.; Shoenfelt, J.; Erickson, D.R.; Whitmore, K.; Warren, J.W.; Marvel, R.; Chai, T. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology 2001, 57, 9–14. [Google Scholar] [CrossRef]
- You, S.; Yang, W.; Anger, J.T.; Freeman, M.R.; Kim, J. Omics approaches to understanding interstitial cystitis/painful bladder syndrome/bladder pain syndrome. Int. Neurourol. J. 2012, 16, 159–168. [Google Scholar] [CrossRef]
- Fiehn, O.; Kim, J. Metabolomics insights into pathophysiological mechanisms of interstitial cystitis. Int. Neurourol. J. 2014, 18, 106–114. [Google Scholar] [CrossRef]
- Fukui, Y.; Kato, M.; Inoue, Y.; Matsubara, A.; Itoh, K. A metabonomic approach identifies human urinary phenylacetylglutamine as a novel marker of interstitial cystitis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3806–3812. [Google Scholar] [CrossRef]
- Wen, H.; Lee, T.; You, S.; Park, S.H.; Song, H.; Eilber, K.S.; Anger, J.T.; Freeman, M.R.; Park, S.; Kim, J. Urinary metabolite profiling combined with computational analysis predicts interstitial cystitis-associated candidate biomarkers. J. Proteome Res. 2015, 14, 541–548. [Google Scholar] [CrossRef]
- Parker, K.S.; Crowley, J.R.; Stephens-Shields, A.J.; van Bokhoven, A.; Lucia, M.S.; Lai, H.H.; Andriole, G.L.; Hooton, T.M.; Mullins, C.; Henderson, J.P. Urinary metabolomics identifies a molecular correlate of interstitial cystitis/bladder pain syndrome in a multidisciplinary approach to the study of chronic pelvic pain (MAPP) research network cohort. EBioMedicine 2016, 7, 167–174. [Google Scholar] [CrossRef]
- Kind, T.; Cho, E.; Park, T.D.; Deng, N.; Liu, Z.; Lee, T.; Fiehn, O.; Kim, J. Interstitial cystitis-associated urinary metabolites identified by mass-spectrometry based metabolomics analysis. Sci. Rep. 2016, 6, 39227. [Google Scholar] [CrossRef]
- Shahid, M.; Lee, M.Y.; Yeon, A.; Cho, E.; Sairam, V.; Valdiviez, L.; You, S.; Kim, J. Menthol, a unique urinary volatile compound, is associated with chronic inflammation in interstitial cystitis. Sci. Rep. 2018, 8, 10859. [Google Scholar] [CrossRef]
- Rubio-Diaz, D.E.; Pozza, M.E.; Dimitrakov, J.; Gilleran, J.P.; Giusti, M.M.; Stella, J.L.; Rodriguez-Saona, L.E.; Buffington, C.A. A candidate serum biomarker for bladder pain syndrome/interstitial cystitis. Analyst 2009, 134, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Van, Q.N.; Klose, J.R.; Lucas, D.A.; Prieto, D.A.; Luke, B.; Collins, J.; Burt, S.K.; Chmurny, G.N.; Issaq, H.J.; Conrads, T.P.; et al. The use of urine proteomic and metabonomic patterns for the diagnosis of interstitial cystitis and bacterial cystitis. Dis. Markers 2004, 19, 169–183. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, H.; Sun, H.; Zhang, Y.; An, N.; Yan, G.; Meng, X.; Wang, X. Metabolomics strategy reveals therapeutical assessment of limonin on nonbacterial prostatitis. Food Funct. 2015, 6, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Zinsmeister, A.R.; Van Dyke, C.; Melton, L.J. 3rd. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology 1991, 101, 927–934. [Google Scholar] [CrossRef]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, S0016-5085, 00222–00225. [Google Scholar] [CrossRef]
- Drossman, D.A.; Li, Z.; Andruzzi, E.; Temple, R.D.; Talley, N.J.; Thompson, W.G.; Whitehead, W.E.; Janssens, J.; Funch-Jensen, P.; Corazziari, E.; et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig. Dis. Sci. 1993, 38, 1569–1580. [Google Scholar] [CrossRef]
- Jones, R.; Lydeard, S. Irritable bowel syndrome in the general population. Br. Med. J. 1992, 304, 87–90. [Google Scholar] [CrossRef]
- Heaton, K.W.; O’Donnell, L.J.; Braddon, F.E.; Mountford, R.A.; Hughes, A.O.; Cripps, P.J. Symptoms of irritable bowel syndrome in a British urban community: Consulters and nonconsulters. Gastroenterology 1992, 102, 1962–1967. [Google Scholar] [CrossRef]
- Everhart, J.E.; Renault, P.F. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology 1991, 100, 998–10005. [Google Scholar] [CrossRef]
- Ford, A.C.; Forman, D.; Bailey, A.G.; Axon, A.T.R.; Moayyedi, P. Irritable bowel syndrome: A 10-yr natural history of symptoms and factors that influence consultation behavior. Am. J. Gastroenterol. 2008, 103, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Sayuk, G.S.; Wolf, R.; Chang, L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am. J. Gastroenterol. 2017, 112, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.M. Diagnostic evaluation of the irritable bowel syndrome. Gastroenterol. Clin. N. Am. 1991, 20, 269–278. [Google Scholar]
- Sandler, R.S.; Everhart, J.E.; Donowitz, M.; Adams, E.; Cronin, K.; Goodman, C.; Gemmen, E.; Shah, S.; Avdic, A.; RobertRubin, R. The burden of selected digestive diseases in the United States. Gastroenterology 2002, 122, 1500–1511. [Google Scholar] [CrossRef]
- Ahmed, I.; Niaz, Z.; Ewbank, F.; Akarca, D.; Felwick, R.; Furnari, M. Sniffing out causes of gastrointestinal disorders: A review of volatile metabolomic biomarkers. Biomark. Med. 2018, 12, 1139–1148. [Google Scholar] [CrossRef]
- Baranska, A.; Mujagic, Z.; Smolinska, A.; Dallinga, J.W.; Jonkers, D.M.; Tigchelaar, E.F.; Dekens, J.; Zhernakova, A.; Ludwig, T.; Masclee, A.A.; et al. Volatile organic compounds in breath as markers for irritable bowel syndrome: A metabolomic approach. Aliment Pharmacol. Ther. 2016, 44, 45–56. [Google Scholar] [CrossRef]
- Yu, L.M.; Zhao, K.J.; Wang, S.S.; Wang, X.; Lu, B. Gas chromatography/mass spectrometry based metabolomic study in a murine model of irritable bowel syndrome. World J. Gastroenterol. 2018, 24, 894–904. [Google Scholar] [CrossRef]
- Noorbakhsh, H.; Yavarmanesh, M.; Mortazavi, S.A.; Adibi, P.; Moazzami, A.A. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. Eur. J. Nutr. 2018, 58, 3109–3119. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Han, C.J.; Jarrett, M.E.; Gu, H.; Djukovic, D.; Shulman, R.J.; Raftery, D.; Henderson, W.A.; Cain, K.C. Serum tryptophan metabolite levels during sleep in patients with and without irritable bowel syndrome (IBS). Biol. Res. Nurs. 2016, 18, 193–198. [Google Scholar] [CrossRef]
- Charles, A. The evolution of a migraine attack—a review of recent evidence. Headache 2013, 53, 413–419. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Smith, R.A.; Griffiths, L.R. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J. Headache Pain 2017, 18, 20. [Google Scholar] [CrossRef]
- Lionetto, L.; Gentile, G.; Bellei, E.; Capi, M.; Sabato, D.; Marsibilio, F.; Simmaco, M.; Pini, L.A.; Martelletti, P. The omics in migraine. J. Headache Pain. 2013, 14, 55. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. Low levels of serum serotonin and amino acids identified in migraine patients. Biochem. Biophys. Res. Commun. 2018, 496, 267–273. [Google Scholar] [CrossRef]
- Onderwater, G.L.J.; Ligthart, L.; Bot, M.; Demirkan, A.; Fu, J.; van der Kallen, C.J.H.; Vijfhuizen, L.S.; Pool, R.; Liu, J.; Vanmolkot, F.H.M.; et al. BBMRI Metabolomics Consortium. Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology 2019, 92, e1899–e1911. [Google Scholar] [CrossRef]
- Zielman, R.; Postma, R.; Verhoeven, A.; Bakels, F.; van Oosterhout, W.P.; Meissner, A.; van den Maagdenberg, A.M.; Terwindt, G.M.; Mayboroda, O.A.; Ferrari, M.D. Metabolomic changes in CSF of migraine patients measured with 1H-NMR spectroscopy. Mol. Biosyst. 2016, 12, 3674–3682. [Google Scholar] [CrossRef]
- Esteve, C.; Tolner, E.A.; Shyti, R.; van den Maagdenberg, A.M.; McDonnell, L.A. Mass spectrometry imaging of amino neurotransmitters: A comparison of derivatization methods and application in mouse brain tissue. Metabolomics 2016, 12, 30. [Google Scholar] [CrossRef]
- College of Occupational and Environmental Medicine. ACOEM position statement Multiple chemical sensitivities: Idiopathic environmental intolerance. J. Occup. Environ. Med. 1999, 41, 940–942. [Google Scholar] [CrossRef]
- Levin, A.S.; Byers, V.S. Environmental illness: A disorder of immune regulation. Occup. Med. 1987, 2, 669–681. [Google Scholar]
- Meggs, W.J.; Dunn, K.A.; Bloch, R.M.; Dunn, K.A.; Goodman, P.E.; Davidoff, A.L. Prevalence and nature of allergy and chemical sensitivity in a general population. Arch. Environ. Health 1996, 51, 275–282. [Google Scholar] [CrossRef]
- Miller, C.S. White paper: Chemical sensitivity: History and phenomenology. Toxicol. Ind. Health 1994, 10, 253–276. [Google Scholar]
- American Academy of Allergy Asthma and immunology (AAAAI) Board of Directors Idiopathic environmental intolerances. J. Allergy Clin. Immunol. 1999, 103, 36–40. [CrossRef]
- Das-Munshi, J.; Rubin, G.J.; Wessely, S. Multiple chemical sensitivities: Review. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 274–280. [Google Scholar] [CrossRef]
- Bornschein, S.; Hausteiner, C.; Zilker, T.; Förstl, H. Psychiatric and somatic disorders and multiple chemical sensitivity (MCS) in 264 ‘environmental patients’. Psychol. Med. 2002, 32, 1387–1394. [Google Scholar] [CrossRef]
- Katoh, T.; Fujiwara, Y.; Nakashita, C.; Lu, X.; Hisada, A.; Miyazaki, W.; Azuma, K.; Tanigawa, M.; Uchiyama, I.; Kunugita, N. Application of metabolomics to multiple chemical sensitivity research. Nihon Eiseigaku Zasshi 2016, 71, 94–99. [Google Scholar] [CrossRef]
- Yap, E.C. Myofascial pain—an overview. Ann. Acad. Med. Singap. 2007, 36, 43–48. [Google Scholar] [PubMed]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction in the Trigger Point Manual: Upper Half of Body, Lippincott; Williams & Wilkins: Baltimore, MD, USA, 1988. [Google Scholar]
- Gowers, W.R. A lecture on lumbago: Its lessons and analogues: Delivered at the national hospital for the paralysed and epileptic. Br. Med. J. 1904, 1, 117–121. [Google Scholar] [CrossRef]
- Skootsky, S.A.; Jaeger, B.; Oye, R.K. Prevalence of myofascial pain in general internal medicine practice. West. J. Med. 1989, 151, 157–160. [Google Scholar]
- Thibaut, A.; Zeng, D.; Caumo, W.; Liu, J.; Fregni, F. Corticospinal excitability as a biomarker of myofascial pain syndrome. Pain Rep. 2017, 2, e594. [Google Scholar] [CrossRef]
- Meier, R.K. Polycystic ovary syndrome. Nurs. Clin. N. Am. 2018, 53, 407–420. [Google Scholar] [CrossRef]
- Azziz, R.; Woods, K.S.; Reyna, R. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef]
- Rosenfield, R.L. What every physician should know about polycystic ovary syndrome. Dermatol. Ther. 2008, 21, 354–361. [Google Scholar] [CrossRef]
- Omabe, M.; Elom, S.; Omabe, K.N. Emerging metabolomics biomarkers of polycystic ovarian syndrome; targeting the master metabolic disrupters for diagnosis and treatment. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 221–229. [Google Scholar] [CrossRef]
- Daghestani, M.H.; Daghestani, M.; Daghistani, M.; El-Mazny, A.; Bjørklund, G.; Chirumbolo, S.; Al Saggaf, S.H.; Warsy, A. A study of ghrelin and leptin levels and their relationship to metabolic profiles in obese and lean Saudi women with polycystic ovary syndrome (PCOS). Lipids Health Dis. 2018, 17, 195. [Google Scholar] [CrossRef]
- Couto Alves, A.; Valcarcel, B.; Mäkinen, V.P.; Morin-Papunen, L.; Sebert, S.; Kangas, A.J.; Soininen, P.; Das, S.; De Iorio, M.; Coin, L.; et al. Metabolic profiling of polycystic ovary syndrome reveals interactions with abdominal obesity. Int. J. Obes. 2017, 41, 1331–1340. [Google Scholar] [CrossRef]
- RoyChoudhury, S.; Mishra, B.P.; Khan, T.; Chattopadhayay, R.; Lodh, I.; Datta Ray, C.; Bose, G.; Sarkar, H.S.; Srivastava, S.; Joshi, M.V.; et al. Serum metabolomics of Indian women with polycystic ovary syndrome using 1H NMR coupled with a pattern recognition approach. Mol. Biosyst. 2016, 12, 3407–3416. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M.; Rachoń, D.; Stefaniak, A.; Wawrzyniak, R.; Konieczna, A.; Kowalewska, A.; Markuszewski, M.J. Identification of the metabolic fingerprints in women with polycystic ovary syndrome using the multiplatform metabolomics technique. J. Steroid Biochem. Mol. Biol. 2019, 186, 176–184. [Google Scholar] [CrossRef]
- Jia, C.; Xu, H.; Xu, Y.; Xu, Y.; Shi, Q. Serum metabolomics analysis of patients with polycystic ovary syndrome by mass spectrometry. Mol. Reprod. Dev. 2019, 86, 292–297. [Google Scholar] [CrossRef]
- Troisi, J.; Cinque, C.; Giugliano, L.; Symes, S.; Richards, S.; Adair, D.; Cavallo, P.; Sarno, L.; Scala, G.; Caiazza, M.; et al. Metabolomic change due to combined treatment with myo-inositol, D-chiro-inositol and glucomannan in polycystic ovarian syndrome patients: A pilot study. J. Ovarian Res. 2019, 12, 25. [Google Scholar] [CrossRef]
- Halama, A.; Aye, M.M.; Dargham, S.R.; Kulinski, M.; Suhre, K.; Atkin, S.L. Metabolomics of dynamic changes in insulin resistance before and after exercise in PCOS. Front. Endocrinol. 2019, 10, 116. [Google Scholar] [CrossRef]
- Baranova, A.; Tran, T.P.; Birerdinc, A.; Younossi, Z.M. Systematic review: Association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. 2011, 33, 801–814. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Liljenquist, D.R.; Kasza, K.; Azziz, R.; Legro, R.S.; Ghazzi, M.N. PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 48–53. [Google Scholar] [CrossRef]
- Cree-Green, M.; Newcomer, B.R.; Coe, G.; Newnes, L.; Baumgartner, A.; Brown, M.S.; Pyle, L.; Reusch, J.E.; Nadeau, K.J. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E726–E733. [Google Scholar] [CrossRef]
- Cree-Green, M.; Carreau, A.M.; Rahat, H.; Garcia-Reyes, Y.; Bergman, B.C.; Pyle, L.; Nadeau, K.J. Amino acid and fatty acid metabolomic profile during fasting and hyperinsulinemia in girls with polycystic ovarian syndrome. Am. J. Physiol. Endocrinol. Metabol. 2019, 316, E707–E718. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, J.; Huang, Z.; Gong, J.; Wang, Y.; Xue, W.; Deng, Y.; Wang, Y.; Zheng, T.; Sun, A.; et al. UPLC/Q-TOF-MS based plasma metabolomics and clinical characteristics of polycystic ovarian syndrome. Mol. Med. Rep. 2019, 19, 280–292. [Google Scholar] [CrossRef]
- Zou, Y.; Zhu, F.F.; Fang, C.Y.; Xiong, X.Y.; Li, H.Y. Identification of potential biomarkers for urine metabolomics of polycystic ovary syndrome based on gas chromatography-mass spectrometry. Chin. Med. J. 2018, 131, 945–949. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Tan, S.; Wen, M.; Qian, Y.; Zeng, X.; Guo, Y.; Yu, C. Detection of urine metabolites in polycystic ovary syndrome by UPLC triple-TOF-MS. Clin. Chim. Acta 2015, 448, 39–47. [Google Scholar] [CrossRef]
- Campbell, M.A.; McGrath, P.J. Use of medication by adolescents for the management of menstrual discomfort. Arch. Pediatr. Adolesc. Med. 1997, 151, 905–913. [Google Scholar] [CrossRef]
- Wilson, C.A.; Keye, W.R., Jr. A survey of adolescent dysmenorrhea and premenstrual symptom frequency. A model program for prevention, detection, and treatment. J. Adolesc. Health Care 1989, 10, 317–322. [Google Scholar] [CrossRef]
- Klein, J.R.; Litt, I.F. Epidemiology of adolescent dysmenorrhea. Pediatrics 1981, 68, 661–664. [Google Scholar] [CrossRef]
- Johnson, J. Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. J. Adolesc. Health Care 1988, 9, 398–402. [Google Scholar] [CrossRef]
- Burnett, M.A.; Antao, V.; Black, A.; Feldman, K.; Grenville, A.; Lea, A.; Lefebvre, G.; Pinsonneaul, O.; Robert, M. Prevalence of primary dysmenorrhea in Canada. J. Obstet. Gynaecol. Can. 2005, 27, 765–770. [Google Scholar] [CrossRef]
- Andersch, B.; Milsom, I. An epidemiologic study of young women with dysmenorrhea. Am. J. Obstet. Gynecol. 1982, 144, 655–660. [Google Scholar] [CrossRef]
- Ortiz, M.I. Primary dysmenorrhea among Mexican university students: Prevalence, impact and treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Rangel-Flores, E.; Carrillo-Alarcón, L.C.; Veras-Godoy, H.A. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int. J. Gynaecol. Obstet. 2009, 107, 240–243. [Google Scholar] [CrossRef]
- Polat, A.; Celik, H.; Gurates, B.; Kaya, D.; Nalbant, M.; Kavak, E.; Hanay, F. Prevalence of primary dysmenorrhea in young adult female university students. Arch. Gynecol. Obstet. 2009, 279, 527–532. [Google Scholar] [CrossRef]
- Hillen, T.I.; Grbavac, S.L.; Johnston, P.J.; Straton, J.A.Y.; Keogh, J.M.F. Primary dysmenorrhea in young Western Australian women: Prevalence, impact, and knowledge of treatment. J. Adolesc. Health 1999, 25, 40–45. [Google Scholar] [CrossRef]
- Fang, L.; Gu, C.; Liu, X.; Xie, J.; Hou, Z.; Tian, M.; Yin, J.; Li, A.; Li, Y. Metabolomics study on primary dysmenorrhea patients during the luteal regression stage based on ultra performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Mol. Med. Rep. 2017, 15, 1043–1050. [Google Scholar] [CrossRef]
- Massey, T.H.; Robertson, N.P. Restless legs syndrome: Causes and consequences. J. Neurol. 2020, 267, 575–577. [Google Scholar] [CrossRef]
- Rizzo, G.; Tonon, C.; Manners, D.; Testa, C.; Lodi, R. Imaging brain functional and metabolic changes in restless legs syndrome. Curr. Neurol. Neurosci. Rep. 2013, 13, 372. [Google Scholar] [CrossRef]
- Trotti, L.M.; Becker, L.A. Iron for the treatment of restless legs syndrome. Cochrane Database Syst. Rev. 2019, 1, CD007834. [Google Scholar] [CrossRef]
- Maixner, W.; Diatchenko, L.; Dubner, R.; Fillingim, R.B.; Greenspan, J.D.; Knott, C.; Ohrbach, R.; Weir, B.; Slade, G.D. Orofacial pain prospective evaluation and risk assessment study—the OPPERA study. J. Pain 2011, 12, T4–T12. [Google Scholar] [CrossRef]
- Macfarlane, T.V.; Blinkhorn, A.S.; Davies, R.M.; Kincey, J.; Worthington, H.V. Oro-facial pain in the community: Prevalence and associated impact. Community Dent. Oral Epidemiol. 2002, 30, 52–60. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldstein, A.T.; Stockdale, C.K.; Bergeron, S.; Pukall, C.; Zolnoun, D.; Coady, D. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet. Gynecol. 2016, 127, 745–751. [Google Scholar] [CrossRef]
- Bonham, A. Vulvar vestibulodynia: Strategies to meet the challenge. Obstet. Gynecol. Surv. 2015, 70, 274–278. [Google Scholar] [CrossRef]
- Friedrich, E.G., Jr. Vulvar vestibulitis syndrome. J. Reprod. Med. 1987, 32, 110–114. [Google Scholar]
- Bergeron, S.; Binik, Y.M.; Khalifé, S.; Pagidas, K.; Glazer, H.I. Vulvar vestibulitis syndrome: Reliability of diagnosis and evaluation of current diagnostic criteria. Obstet. Gynecol. 2001, 98, 45–51. [Google Scholar] [CrossRef]
- Harlow, B.L.; Stewart, E.G. A population-based assessment of chronic unexplained vulvar pain: Have we underestimated the prevalence of vulvodynia? J. Am. Med. Womens Assoc. 2003, 58, 82–88. [Google Scholar]
- Govorov, I.; Sitkin, S.; Pervunina, T.; Moskvin, A.; Baranenko, D.; Komlichenko, E. Metabolomic biomarkers in gynecology: A treasure path or a false path? Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 271–272. [Google Scholar]
- Van der Kolk, B.A.; Pelcovitz, D.; Roth, S.; Mandel, F.S.; McFarlane, A.; Herman, J.L. Dissociation, somatization, and affect dysregulation: The complexity of adaptation of trauma. Am. J. Psychiatr. 1996, 153, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Grinage, B.D. Diagnosis and management of post-traumatic stress disorder. Am. Fam. Physician 2003, 68, 2401–2409. [Google Scholar]
- Gautam, A.; D’Arpa, P.; Donohue, D.E.; Muhie, S.; Chakraborty, N.; Luke, B.T.; Grapov, D.; Carroll, E.E.; Meyerhoff, J.L.; Hammamieh, R.; et al. Acute and chronic plasma metabolomic and liver transcriptomic stress effects in a mouse model with features of post-traumatic stress disorder. PLoS ONE 2015, 10, e0117092. [Google Scholar] [CrossRef]
- Konjevod, M.; Tudor, L.; Svob Strac, D.; Nedic Erjavec, G.; Barbas, C.; Zarkovic, N.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Lauc, G.; et al. Metabolomic and glycomic findings in posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2019, 88, 181–193. [Google Scholar] [CrossRef]
- Su, X.; Xia, C.; Wang, W.; Sun, H.; Tan, Q.; Zhang, S.; Li, L.; Kemp, G.J.; Yue, Q.; Gong, Q. Abnormal metabolite concentrations and amygdala volume in patients with recent-onset posttraumatic stress disorder. J. Affect. Disord. 2018, 241, 539–545. [Google Scholar] [CrossRef]
- Tükel, R.; Aydın, K.; Yüksel, Ç.; Ertekin, E.; Koyuncu, A. Proton magnetic resonance spectroscopy in social anxiety disorder. J. Neuropsychiatr. Clin. Neurosci. 2016, 28, 138–142. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Su, X.; Tan, Q.; Zhang, S.; Xia, C.; Li, L.; Kemp, G.J.; Tue, Q.; Gong, Q. Increased right amygdala metabolite concentrations in the absence of atrophy in children and adolescents with PTSD. Eur. Child Adolesc. Psychiatr. 2019, 28, 807–817. [Google Scholar] [CrossRef]
- Ham, B.J.; Chey, J.; Yoon, S.J.; Sung, Y.; Jeong, D.U.; Ju Kim, S.; Sim, M.E.; Choi, N.; Choi, I.G.; Renshaw, P.F.; et al. Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: A proton magnetic resonance spectroscopy study. Eur. J. Neurosci. 2007, 25, 324–329. [Google Scholar] [CrossRef]
- Meyerhoff, D.J.; Mon, A.; Metzler, T.; Neylan, T.C. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 2014, 37, 893–900. [Google Scholar] [CrossRef]
- Mellon, S.H.; Bersani, F.S.; Lindqvist, D.; Hammamieh, R.; Donohue, D.; Dean, K.; Jett, M.; Yehuda, R.; Flory, J.; Reus, V.I.; et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS ONE 2019, 14, e0213839. [Google Scholar] [CrossRef]
- Koulman, A.; Lane, G.A.; Harrison, S.J.; Volmer, D.A. From differentiating metabolites to biomarkers. Anal. Bioanal. Chem. 2009, 394, 663–670. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, J.S.; Rodriguez-Saona, L.; Hackshaw, K.V. Metabolomics in Central Sensitivity Syndromes. Metabolites 2020, 10, 164. https://doi.org/10.3390/metabo10040164
Miller JS, Rodriguez-Saona L, Hackshaw KV. Metabolomics in Central Sensitivity Syndromes. Metabolites. 2020; 10(4):164. https://doi.org/10.3390/metabo10040164
Chicago/Turabian StyleMiller, Joseph S., Luis Rodriguez-Saona, and Kevin V. Hackshaw. 2020. "Metabolomics in Central Sensitivity Syndromes" Metabolites 10, no. 4: 164. https://doi.org/10.3390/metabo10040164
APA StyleMiller, J. S., Rodriguez-Saona, L., & Hackshaw, K. V. (2020). Metabolomics in Central Sensitivity Syndromes. Metabolites, 10(4), 164. https://doi.org/10.3390/metabo10040164