Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS Analysis
Abstract
1. Introduction
2. Results
2.1. Metabolite Identification and Submetabolomic Changes in Brain Tissue
2.2. Metabolite Identification and Submetabolomic Changes in Heart Tissue
2.3. Metabolite Identification and Submetabolomic Changes in Kidney Tissue
2.4. Metabolite Identification and Submetabolomic Changes in Liver Tissue
2.5. Metabolite Identification and Submetabolomic Changes in Skeletal Muscle Tissue
3. Discussion
3.1. Important Pathways Altered in the Brain after Dex Treatment
3.2. Important Pathways Altered in the Heart after Dex Treatment
3.3. Important Pathways Altered in the Kidney after Dex Treatment
3.4. Important Pathways Altered in the Liver after Dex Treatment
3.5. Important Pathways Altered in the Skeletal Muscle after Dex Treatment
3.6. Glutathione Metabolism: Commonly Altered Pathway in the Brain, Kidney, Liver, and Skeletal Muscle
3.7. Dipeptide Profiling
3.8. Clinical Implications
4. Materials and Methods
4.1. Ethical Considerations
4.2. Experimental Design
4.3. Chemicals and Reagents
4.4. Metabolomics
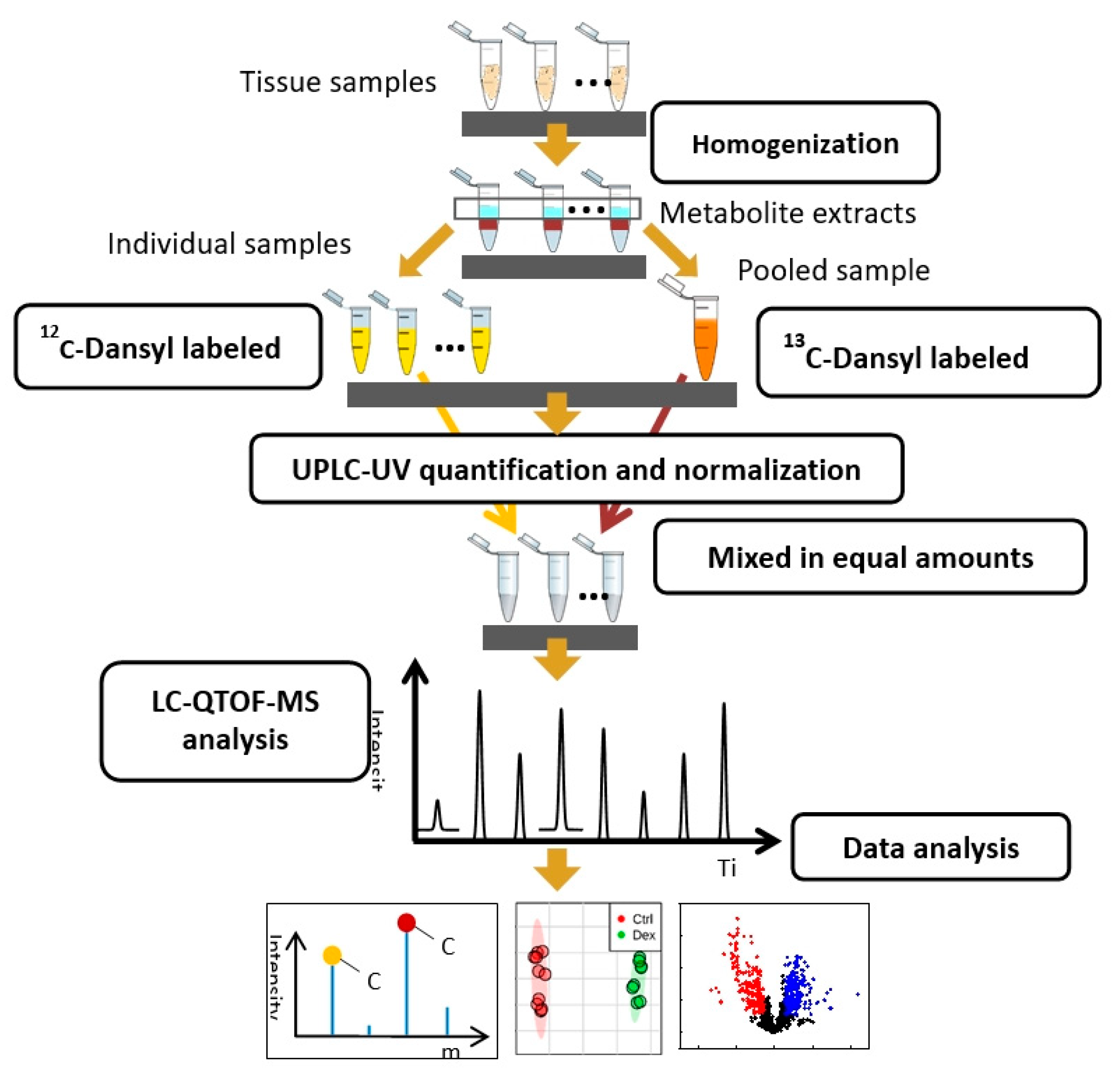
4.4.1. Sample Preparation for Metabolite Profiling
4.4.2. Metabolite Profiling Using LC-QTOF-MS
4.4.3. Data Analysis and Informatics
4.4.4. Metabolite Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waljee, A.K.; Rogers, M.A.M.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Begaud, B.; Zhang, B.; Cooper, C. Use of oral corticosteroids in the United Kingdom. QJM 2000, 93, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Mitre-Aguilar, I.B.; Cabrera-Quintero, A.J.; Zentella-Dehesa, A. Genomic and non-genomic effects of glucocorticoids: Implications for breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 1–10. [Google Scholar] [PubMed]
- Kroot, E.J.A.; Huisman, A.M.; Van Zeben, J.; Wouters, J.; Van Paassen, H.C. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: A pilot study. Ann. N. Y. Acad. Sci. 2006, 1069, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Okano, M. Mechanisms and clinical implications of glucocorticosteroids in the treatment of allergic rhinitis. Clin. Exp. Immunol. 2009, 158, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Strange, C. Inhaled corticosteroids for chronic obstructive pulmonary disease: What is their role in therapy? Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2587–2601. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef]
- Malkawi, A.K.; Masood, A.; Shinwari, Z.; Jacob, M.; Benabdelkamel, H.; Matic, G.; Almuhanna, F.; Dasouki, M.; Alaiya, A.A.; Rahman, A.M.A. Proteomic Analysis of Morphologically Changed Tissues after Prolonged Dexamethasone Treatment. Int. J. Mol. Sci. 2019, 20, 3122. [Google Scholar] [CrossRef]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Sun, H.; Wang, P.; Han, Y.; Wang, X.J. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Barrington, W.T.; Dearth, S.; May, A.; Threadgill, D.W.; Campagna, S.R.; Voy, B.H. Tissue Level Diet and Sex-by-Diet Interactions Reveal Unique Metabolite and Clustering Profiles Using Untargeted Liquid Chromatography–Mass Spectrometry on Adipose, Skeletal Muscle, and Liver Tissue in C57BL6/J Mice. J. Proteome Res. 2018, 17, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Sapkota, S.; Camicioli, R.; Dixon, R.A.; Li, L. Profiling novel metabolic biomarkers for Parkinson’s disease using in-depth metabolomic analysis. Mov. Disord. 2017, 32, 1720–1728. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Westaway, D.; Li, L. Development of chemical isotope labeling LC-MS for tissue metabolomics and its application for brain and liver metabolome profiling in Alzheimer’s disease mouse model. Anal. Chim Acta 2019, 1050, 95–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Wang, Z.X.; Bi, Y.N.; Yuan, X.M.; Song, L.; Jiang, M.M.; Sun, L.K.; Zhou, K. A Study of NMR-Based Hepatic and Serum Metabolomics in a Liver Injury Sprague-Dawley Rat Model Induced by Psoralen. Chem. Res. Toxicol. 2018, 31, 852–860. [Google Scholar] [CrossRef]
- Xia, T.S.; Dong, X.; Jiang, Y.P.; Lin, L.Y.; Dong, Z.M.; Shen, Y.; Xin, H.L.; Zhang, Q.Y.; Qin, L.P. Metabolomics Profiling Reveals Rehmanniae Radix Preparata Extract Protects against Glucocorticoid-Induced Osteoporosis Mainly via Intervening Steroid Hormone Biosynthesis. Molecules 2019, 24, 253. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.Y.; Zhao, L.S.; Li, F.M.; Xiong, Z.L. Kidney tissue targeted metabolic profiling of glucocorticoid-induced osteoporosis and the proposed therapeutic effects of Rhizoma Drynariae studied using UHPLC/MS/MS. Biomed. Chromatogr. 2014, 28, 878–884. [Google Scholar] [CrossRef]
- Ho, W.E.; Xu, Y.J.; Xu, F.G.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.F.; Ong, C.N. Metabolomics Reveals Altered Metabolic Pathways in Experimental Asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef]
- Jacob, M.; Malkawi, A.; Albast, N.; Al Bougha, S.; Lopata, A.; Dasouki, M.; Abdel Rahman, A.M. A targeted metabolomics approach for clinical diagnosis of inborn errors of metabolism. Anal. Chim Acta 2018, 1025, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.K.; Alzoubi, K.H.; Jacob, M.; Matic, G.; Ali, A.; Al Faraj, A.; Almuhanna, F.; Dasouki, M.; Rahman, A.M.A. Metabolomics Based Profiling of Dexamethasone Side Effects in Rats. Front. Pharmacol. 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, H.; Han, W.; Chan, W.; Li, L. Metabolomic Coverage of Chemical-Group-Submetabolome Analysis: Group Classification and Four-Channel Chemical Isotope Labeling LC-MS. Anal. Chem. 2019, 91, 12108–12115. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Gilson, H.; Kalista, S.; Thissen, J.P. Mechanisms of Muscle Atrophy Induced by Glucocorticoids. Horm. Res. 2009, 72, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gross, V.; Andus, T.; Tranthi, T.A.; Bauer, J.; Decker, K.; Heinrich, P.C. Induction of Acute Phase Proteins by Dexamethasone in Rat Hepatocyte Primary Cultures. Exp. Cell Res. 1984, 151, 46–54. [Google Scholar] [CrossRef]
- Bentson, J.; Reza, M.; Winter, J.; Wilson, G. Steroids and Apparent Cerebral Atrophy on Computed Tomography Scans. J. Comput. Assist. Tomogr. 1978, 2, 16–23. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Maes, M.; Jacobs, M.P.; Suy, E.; Minner, B.; Leclercq, C.; Christiaens, F.; Raus, J. Suppressant Effects of Dexamethasone on the Availability of Plasma L-Tryptophan and Tyrosine in Healthy Controls and in Depressed-Patients. Acta Psychiatr. Scand. 1990, 81, 19–23. [Google Scholar] [CrossRef]
- Parvez, H.; Parvez, S. Control of Catecholamine Release and Degradation by Glucocorticoids. Experientia 1972, 28, 1330–1332. [Google Scholar] [CrossRef]
- Williams, L.R.; Sandquist, D.; Black, A.C.; Williams, T.H. Glucocorticoids Increase Tyrosine-Hydroxylase Activity in Cultured Murine Neuro-Blastoma. J. Neurochem. 1981, 36, 2057–2062. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Role of Precursor Availability in Control of Monoamine Biosynthesis in Brain. Physiol. Rev. 1983, 63, 484–546. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Klie, S.; Jurchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Carvalho, C.; Marinho, R.; Simoes, A.; Sena, C.M.; Matafome, P.; Santos, M.S.; Seica, R.M.; Moreira, P.I. Effects of methylglyoxal and pyridoxamine in rat brain mitochondria bioenergetics and oxidative status. J. Bioenerg. Biomembr. 2014, 46, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. 2005, 62, 1671–1681. [Google Scholar] [CrossRef]
- Fournet, M.; Bonte, F.; Desmouliere, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- McBean, G.J. Cysteine, Glutathione, and Thiol Redox Balance in Astrocytes. Antioxidants 2017, 6, 62. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Kompis, I.M.; Islam, K.; Then, R.L. DNA and RNA synthesis: Antifolates. Chem. Rev. 2005, 105, 593–620. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Nissim, A.; Horyn, O.; Luhovyy, B.; Lazarow, A.; Nissim, I. Brain amino acid requirements and toxicity: The example of leucine. J. Nutr. 2005, 135, 1531S–1538S. [Google Scholar] [CrossRef]
- Tyagi, N.; Lominadze, D.; Gillespie, W.; Moshal, K.S.; Sen, U.; Rosenberger, D.S.; Steed, M.; Tyagi, S.C. Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine. Clin. Chem. Lab. Med. 2007, 45, 1777–1784. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. Gamma-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and application. In Study in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 413–452. [Google Scholar]
- Ngo, D.H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Lin, P.P.; Hsieh, Y.M.; Kuo, W.W.; Lin, C.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Tsai, C.C. Inhibition of cardiac hypertrophy by probiotic-fermented purple sweet potato yogurt in spontaneously hypertensive rat hearts. Int. J. Mol. Med. 2012, 30, 1365–1375. [Google Scholar] [CrossRef][Green Version]
- Ren, R.Q.; Oakley, R.H.; Cruz-Topete, D.; Cidlowski, J.A. Dual Role for Glucocorticoids in Cardiomyocyte Hypertrophy and Apoptosis. Endocrinology 2012, 153, 5346–5360. [Google Scholar] [CrossRef]
- Van de Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Renal arginine metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef]
- Newsholme, P.; Lima, M.M.R.; Porcopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Jacob, M.; Gu, X.; Luo, X.; Al-Mousa, H.; Arnaout, R.; Al-Saud, B.; Lopata, A.L.; Li, L.; Dasouki, M.; Rahman, A.M.A. Metabolomics Distinguishes DOCK8 Deficiency from Atopic Dermatitis: Towards a Biomarker Discovery. Metabolites 2019, 9, 274. [Google Scholar] [CrossRef]
- Stumvoll, M.; Perriello, G.; Meyer, C.; Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999, 55, 778–792. [Google Scholar] [CrossRef]
- Thunhorst, R.L.; Beltz, T.G.; Johnson, A.K. Glucocorticoids increase salt appetite by promoting water and sodium excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1444–R1451. [Google Scholar] [CrossRef]
- Shetewy, A.; Shimada-Takaura, K.; Warner, D.; Jong, C.J.; Al Mehdi, A.; Alexeyev, M.; Takahashi, K.; Schaffer, S.W. Mitochondrial defects associated with beta-alanine toxicity: Relevance to hyper-beta-alaninemia. Mol. Cell. Biochem. 2016, 416, 11–22. [Google Scholar] [CrossRef]
- Vairetti, M.; Carini, R.; De Cesaris, M.G.; Splendore, R.; Richelmi, P.; Berte, F.; Albano, E. Beta-alanine protection against hypoxic liver injury in the rat. Biochim. Biophys. Acta 2002, 1587, 83–91. [Google Scholar] [CrossRef]
- Le, T.T.; Ziemba, A.; Urasaki, Y.; Hayes, E.; Brotman, S.; Pizzorno, G. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J. Lipid Res. 2013, 54, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.J.; Cao, L.P.; Du, J.L.; Jia, R.; Kitazawa, T.; Kubota, A.; Teraoka, H. Dexamethasone-induced hepatomegaly and steatosis in larval zebrafish. J. Toxicol. Sci. 2017, 42, 455–459. [Google Scholar] [CrossRef]
- Wang, R.X.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Lin, H. L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells. Oxidative Med. Cell. Longev. 2018, 2018, 7569127. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbe, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Salehian, B.; Mahabadi, V.; Bilas, J.; Taylor, W.E.; Ma, K. The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 2006, 55, 1239–1247. [Google Scholar] [CrossRef]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain—Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Klein, G.L. The effect of glucocorticoids on bone and muscle. Osteoporos. Sarcopenia 2015, 1, 39–45. [Google Scholar] [CrossRef]
- Peters, V.; Klessens, C.Q.F.; Baelde, H.J.; Singler, B.; Veraar, K.A.M.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; de Heer, E. Intrinsic carnosine metabolism in the human kidney. Amino Acids 2015, 47, 2541–2550. [Google Scholar] [CrossRef]
- Jessen, H. Taurine And Beta-Alanine Transport In An Established Human Kidney-Cell Line Derived From The Proximal Tubule. Biochim. Biophys. Acta 1994, 1194, 44–52. [Google Scholar] [CrossRef]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Orioli, M.; Pedretti, A.; Regazzoni, L.; Canevotti, R.; Negrisoli, G.; Carini, M.; Aldini, G. Design, Synthesis, and Evaluation of Carnosine Derivatives as Selective and Efficient Sequestering Agents of Cytotoxic Reactive Carbonyl Species. Chemmedchem 2009, 4, 967–975. [Google Scholar] [CrossRef]
- Alhamdani, M.S.S.; Al-Kassir, A.; Abbas, F.K.H.; Jaleel, N.A.; Al-Taee, M.F. Antiglycation and antioxidant effect of carnosine against glucose degradation products in peritoneal mesothelial cells. Nephron Clin. Pract. 2007, 107, C26–C34. [Google Scholar] [CrossRef]
- Jia, H.J.; Qi, X.D.; Fang, S.H.; Jin, Y.H.; Han, X.Y.; Wang, Y.; Wang, A.M.; Zhou, H.B. Carnosine inhibits high glucose-induced mesangial cell proliferation through mediating cell cycle progression. Regul. Pept. 2009, 154, 69–76. [Google Scholar] [CrossRef]
- Zhao, K.X.; Li, Y.; Wang, Z.Q.; Han, N.; Wang, Y. Carnosine Protects Mouse Podocytes from High Glucose Induced Apoptosis through PI3K/AKT and Nrf2 Pathways. Biomed. Res. Int. 2019, 2019, 4348973. [Google Scholar] [CrossRef]
- Riedl, E.; Koeppel, H.; Brinkkoetter, P.; Sternik, P.; Steinbeisser, H.; Sauerhoefer, S.; Janssen, B.; van der Woude, F.J.; Yard, B.A. A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in cos-7-transfected cells. Diabetes 2007, 56, 2410–2413. [Google Scholar] [CrossRef]
- Yadav, A.K.; Sinha, N.; Kumar, V.; Bhansali, A.; Dutta, P.; Jha, V. Association of CTG repeat polymorphism in carnosine dipeptidase 1 (CNDP1) gene with diabetic nephropathy in north Indians. Indian J. Med. Res. 2016, 143, 32–37. [Google Scholar] [CrossRef]
- Peters, V.; Yard, B.; Schmitt, C. Carnosine and diabetic nephropathy. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Rezzani, R.; Favero, G.; Ferroni, M.; Lonati, C.; Moghadasian, M.H. A carnosine analog with therapeutic potentials in the treatment of disorders related to oxidative stress. PLoS ONE 2019, 14, e0215170. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Schmitt, C.P.; Weigand, T.; Klingbeil, K.; Thiel, C.; van den Berg, A.; Calabrese, V.; Nawroth, P.; Fleming, T.; Forsberg, E.; et al. Allosteric inhibition of carnosinase (CN1) by inducing a conformational shift. J. Enzym. Inhib. Med. Chem. 2017, 32, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Fantauzzi, C.B.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharmacol. 2018, 175, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G.; Kovacs, P. Imprinting effects of proline containing dipeptides (proline glycine, proline-leucine, proline-valine and their retro variants) in Tetrahymena. Evolutionary conclusions. Biosci. Rep. 1997, 17, 537–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.M.; Li, Y.G.; Yang, W.H. Preventive effects of nitroglycerine on glucocorticoid-induced osteoporosis in growing rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, L. Differential C-12/C-13-Isotope Dansylation Labeling and Fast Liquid Chromatography/Mass Spectrometry for Absolute and Relative Quantification of the Metabolome. Anal. Chem. 2009, 81, 3919–3932. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L. Determination of Total Concentration of Chemically Labeled Metabolites as a Means of Metabolome Sample Normalization and Sample Loading Optimization in Mass Spectrometry-Based Metabolomics. Anal. Chem 2012, 84, 10723–10731. [Google Scholar] [CrossRef]
- Zhou, R.; Tseng, C.L.; Huan, T.; Li, L. IsoMS: Automated Processing of LC-MS Data Generated by a Chemical Isotope Labeling Metabolomics Platform. Anal. Chem. 2014, 86, 4675–4679. [Google Scholar] [CrossRef]
- Huan, T.; Li, L. Counting Missing Values in a Metabolite-Intensity Data Set for Measuring the Analytical Performance of a Metabolomics Platform. Anal. Chem. 2015, 87, 1306–1313. [Google Scholar] [CrossRef]
- Xia, J.G.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Li, L.; Li, R.H.; Zhou, J.J.; Zuniga, A.; Stanislaus, A.E.; Wu, Y.M.; Huan, T.; Zheng, J.M.; Shi, Y.; Wishart, D.S.; et al. MyCompoundID: Using an Evidence-Based Metabolome Library for Metabolite Identification. Anal. Chem. 2013, 85, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
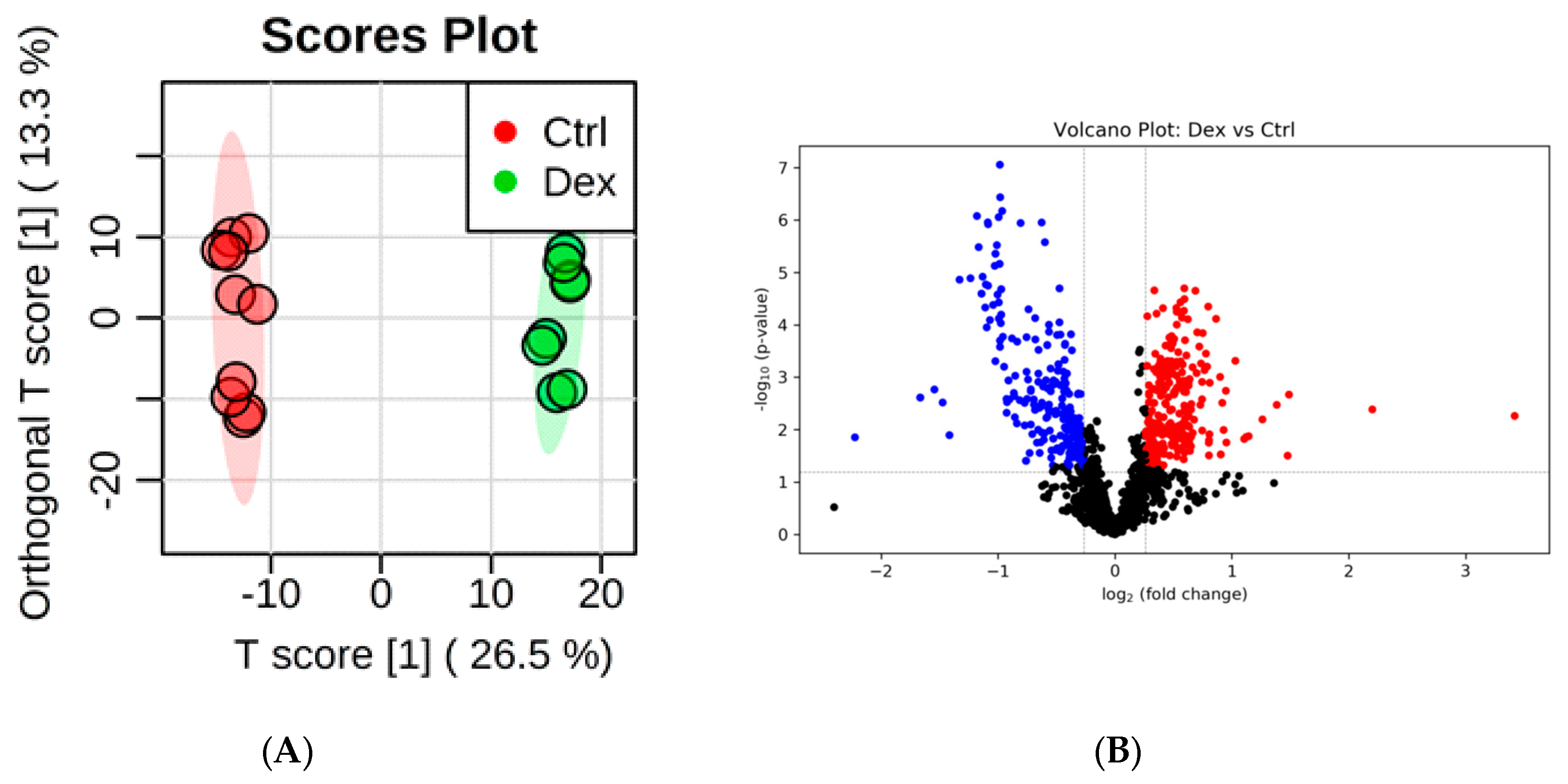
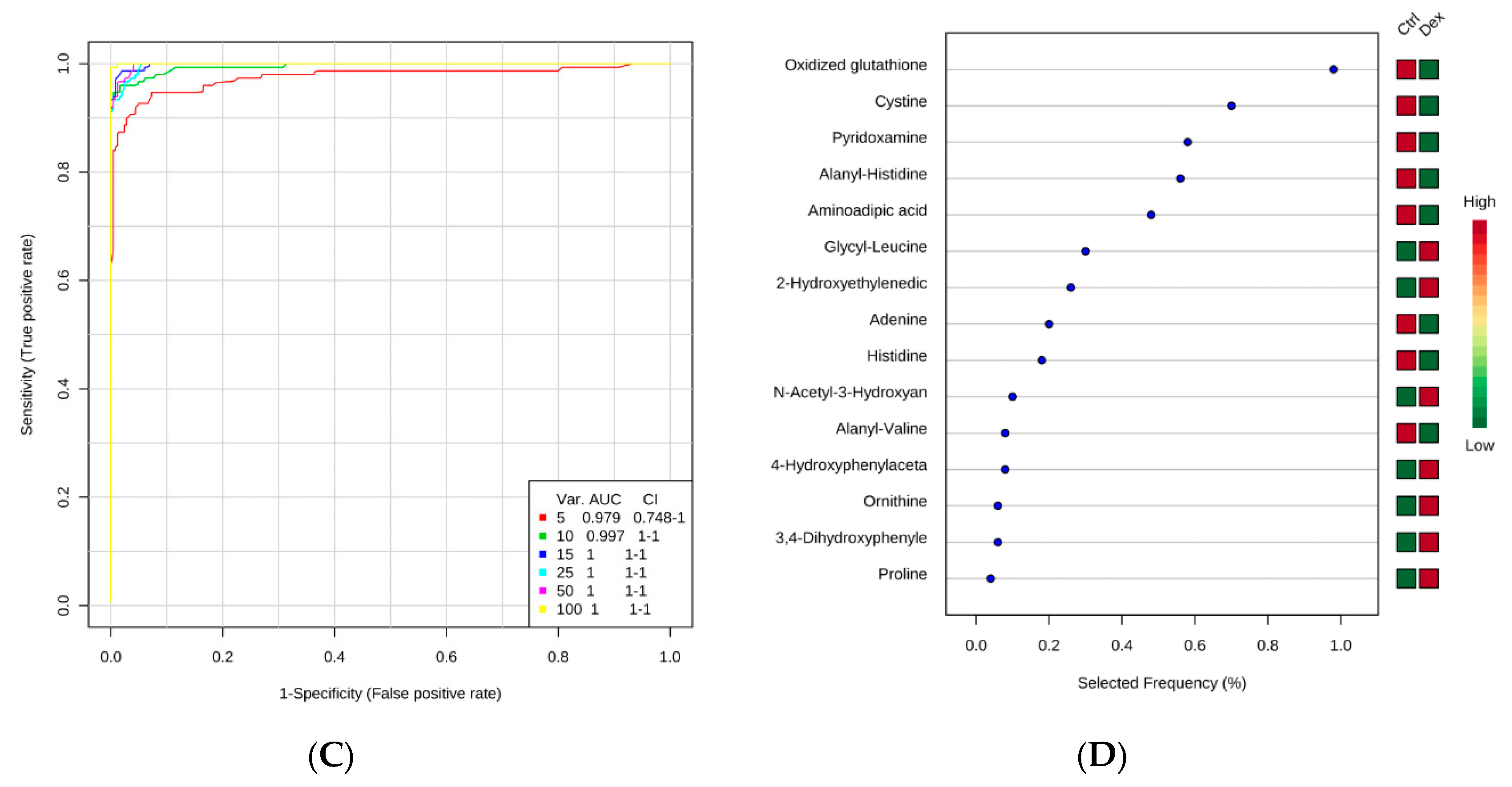

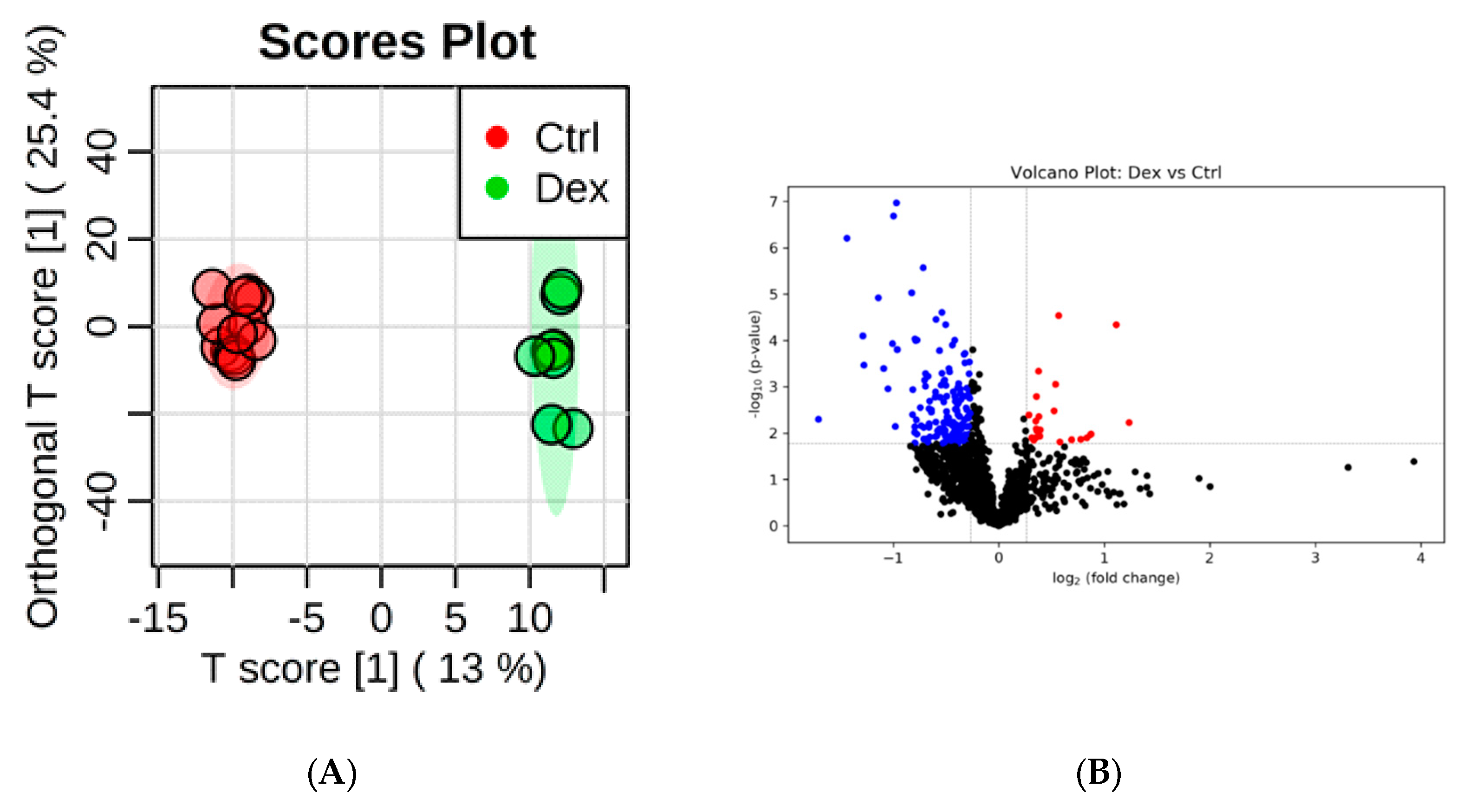

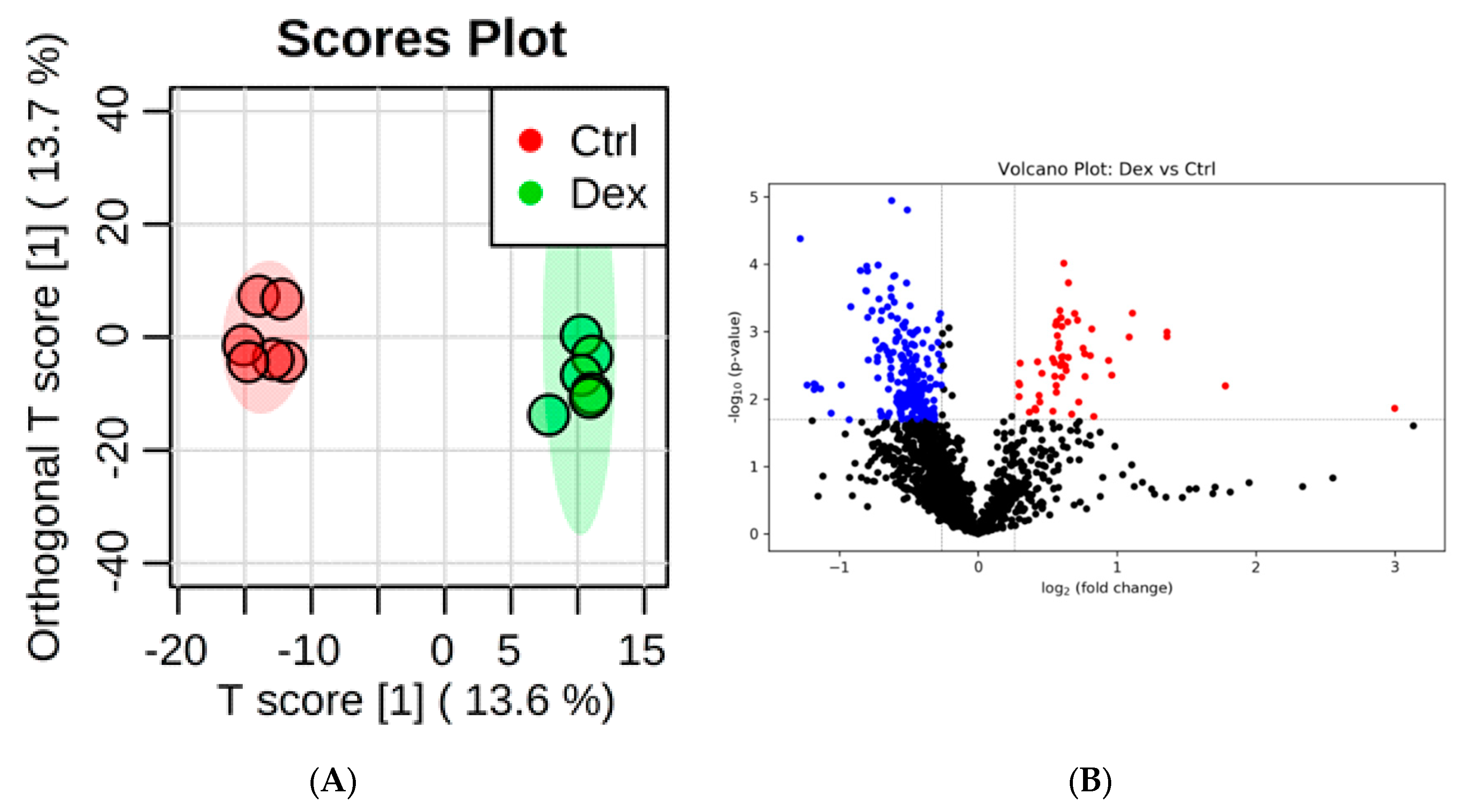
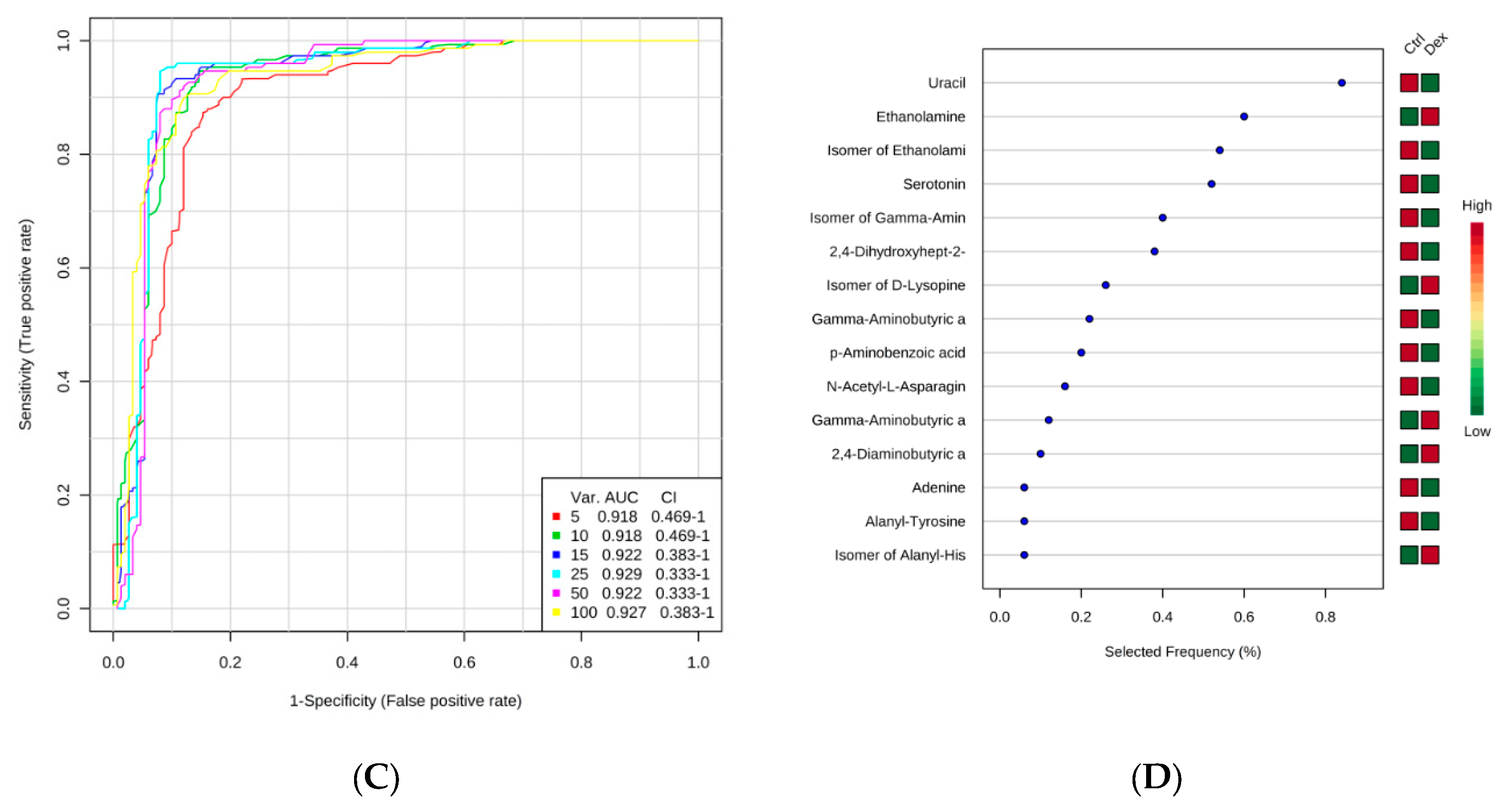


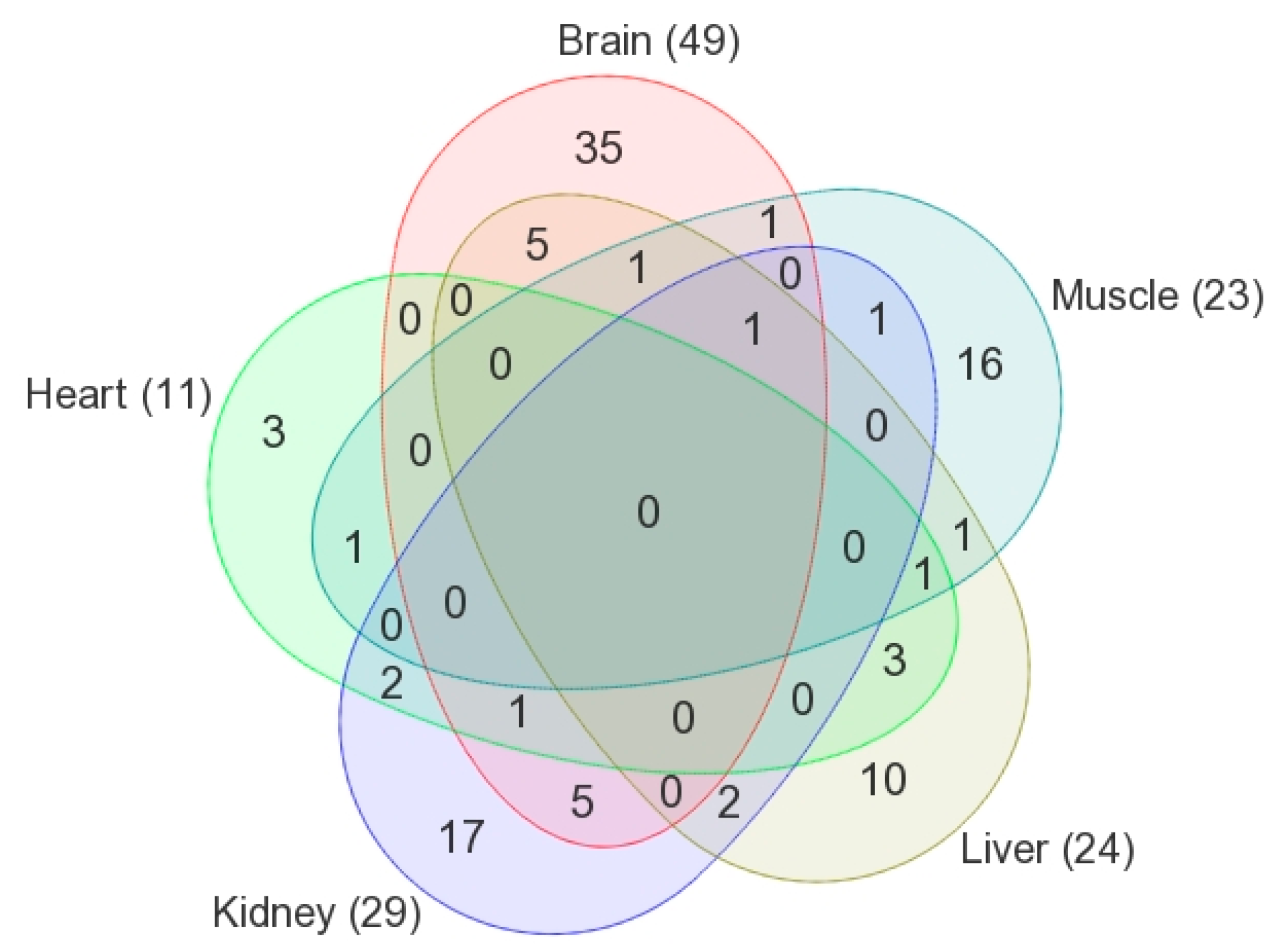
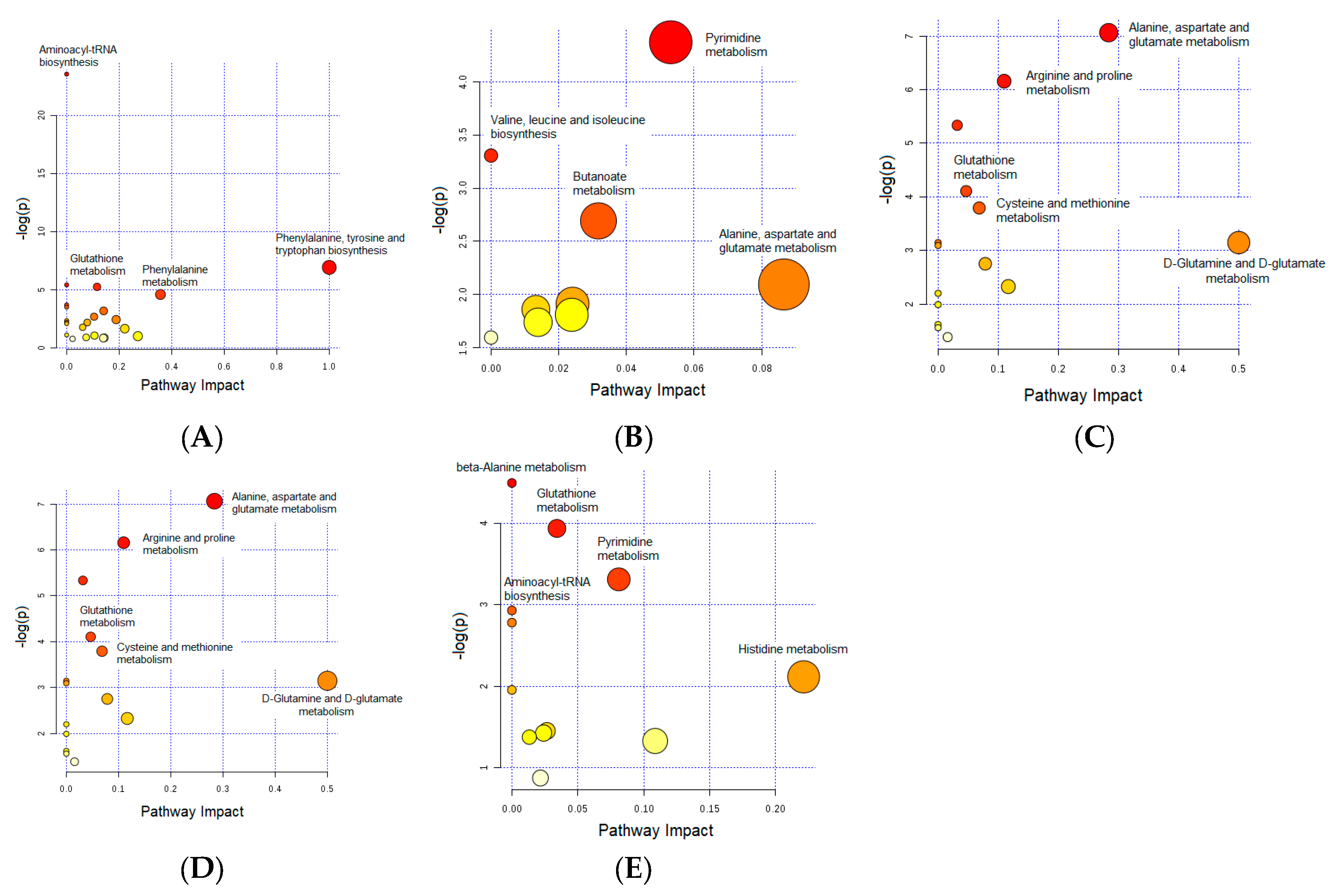
| Metabolic Identification | Brian | Heart | Kidney | Liver | Skeletal Muscle |
|---|---|---|---|---|---|
| Unique peak pairs detected (n) | 1421 | 1555 | 1538 | 1821 | 1793 |
| Positively identified or putatively matched (n) (%) | 1272 (89.5%) | 1378 (88.6%) | 1386 (90.1%) | 1586 (87.1%) | 1575 (87.8%) |
| Tier 1 (n) | 104 | 114 | 136 | 124 | 123 |
| Tier 2 (n) | 45 | 39 | 52 | 45 | 49 |
| Tier 3 (Zero-reaction) (n) | 328 | 325 | 325 | 360 | 356 |
| Tier 3 (One-reaction) (n) | 572 | 675 | 631 | 826 | 786 |
| Tier 3 (Two-reaction) (n) | 223 | 225 | 242 | 231 | 261 |
| Differentially expressed metabolites | |||||
| Up-regulated (n) | 257 | 16 | 24 | 55 | 268 |
| Down-regulated (n) | 235 | 89 | 162 | 245 | 174 |
| HMDB | Name | Neutral Mass (Da) * | Normalized RT (min) ** | Fold Change | p-Value |
|---|---|---|---|---|---|
| HMDB00192 | Cystine | 240.0252 | 14.14 | 2.210 | 1.34 × 10−2 |
| HMDB00177 | Histidine | 155.0697 | 18.24 | 1.727 | 6.28 × 10−4 |
| HMDB00162 | Proline | 115.0636 | 10.19 | 1.611 | 2.24 × 10−5 |
| HMDB00214 | Ornithine | 132.0911 | 16.38 | 1.554 | 8.83 × 10−3 |
| HMDB00159 | Phenylalanine | 165.0792 | 12.76 | 1.541 | 7.83 × 10−5 |
| HMDB0029016 | Prolyl-Glutamate | 244.1059 | 7.02 | 1.527 | 3.85 × 10−4 |
| HMDB0029022 | Prolyl-Lysine | 243.1599 | 17.97 | 1.514 | 4.01 × 10−3 |
| HMDB00696 | Methionine | 149.0512 | 10.91 | 1.488 | 7.29 × 10−5 |
| HMDB00929 | Tryptophan | 204.09 | 11.49 | 1.475 | 3.71 × 10−5 |
| HMDB0029030 | Prolyl-Valine | 214.1317 | 12.06 | 1.462 | 8.43 × 10−3 |
| HMDB00172 | Isoleucine | 131.0949 | 13.02 | 1.442 | 5.62 × 10−5 |
| HMDB00557 | Alloisoleucine | 131.0949 | 13.22 | 1.440 | 4.78 × 10−5 |
| HMDB01645 | Norleucine | 131.0947 | 13.93 | 1.409 | 2.27 × 10−4 |
| HMDB0028823 | Glutamyl-Leucine | 260.1386 | 9.92 | 1.404 | 2.03 × 10−2 |
| HMDB0029026 | Prolyl-Serine | 202.0952 | 6.47 | 1.397 | 4.95 × 10−4 |
| HMDB0011178 | Prolyl-Glycine | 172.0847 | 7.77 | 1.390 | 5.99 × 10−4 |
| HMDB00158 | Tyrosine | 181.0754 | 22.65 | 1.366 | 4.90 × 10−4 |
| HMDB0029015 | Prolyl-Glutamine | 243.1214 | 5.75 | 1.361 | 1.10 × 10−2 |
| HMDB0029013 | Prolyl-Aspartate | 230.0902 | 6.85 | 1.355 | 1.56 × 10−3 |
| HMDB0028928 | Leucyl-Glutamate | 260.1372 | 8.89 | 1.333 | 1.82 × 10−3 |
| HMDB00883 | Valine | 117.0792 | 10.92 | 1.333 | 7.48 × 10−4 |
| HMDB0029010 | Prolyl-Alanine | 186.1004 | 8.64 | 1.307 | 9.74 × 10−4 |
| HMDB00300 | Uracil | 112.0275 | 11.28 | 1.299 | 1.28 × 10−3 |
| HMDB0028736 | Asparaginyl-Lysine | 260.1438 | 12.10 | 1.285 | 4.11 × 10−2 |
| HMDB0029136 | Valyl-Serine | 204.1107 | 6.28 | 1.282 | 3.62 × 10−2 |
| HMDB00750 | 3-Hydroxymandelic acid | 122.0371 | 21.21 | 1.278 | 8.05 × 10−4 |
| HMDB00500 | 4-Hydroxybenzoic acid | 138.0319 | 17.55 | 1.263 | 3.10 × 10−3 |
| HMDB00182 | Lysine | 146.1068 | 17.50 | 1.259 | 7.52 × 10−3 |
| HMDB0029101 | Tyrosyl-Aspartate | 296.1022 | 18.41 | 1.248 | 7.35 × 10−3 |
| HMDB00123 | Glycine | 75.0322 | 6.61 | 1.235 | 3.83 × 10−2 |
| HMDB00228 | Phenol | 94.042 | 23.15 | 1.212 | 1.33 × 10−2 |
| HMDB28699 | Alanyl-Tyrosine | 252.1119 | 21.69 | 0.788 | 6.04 × 10−3 |
| HMDB0000759 | Glycyl-Leucine | 188.1162 | 11.13 | 0.704 | 1.23 × 10−3 |
| HMDB00510 | Aminoadipic acid | 161.0687 | 5.88 | 0.670 | 5.70 × 10−3 |
| HMDB01431 | Pyridoxamine | 168.091 | 19.52 | 0.670 | 2.46 × 10−4 |
| HMDB28689 | Alanyl-Histidine | 226.1068 | 17.44 | 0.607 | 8.03 × 10−3 |
| HMDB03337 | Oxidized glutathione | 612.1526 | 7.99 | 0.497 | 3.02 × 10−6 |
| HMDB | Name | Neutral Mass (Da) * | Normalized RT (min) ** | Fold Change | p-Value |
|---|---|---|---|---|---|
| HMDB00296 | Uridine | 244.0695 | 7.68 | 1.289 | 7.48× 10−3 |
| HMDB00157 | Hypoxanthine | 136.0387 | 9.54 | 0.832 | 5.14× 10−3 |
| HMDB00149 | Ethanolamine | 61.0528 | 6.11 | 0.829 | 7.16× 10−3 |
| HMDB00755 | Hydroxyphenyllactic acid | 182.0579 | 14.27 | 0.786 | 5.65× 10−3 |
| HMDB00167 | Threonine | 119.0584 | 5.80 | 0.704 | 3.02× 10−3 |
| HMDB00763 | 5-Hydroxyindoleacetic acid | 191.0584 | 14.80 | 0.584 | 3.95× 10−3 |
| HMDB00262 | Thymine | 126.0431 | 13.10 | 0.538 | 3.50× 10−3 |
| HMDB00112 | Gamma-aminobutyric acid | 103.0634 | 7.72 | 0.344 | 4.41× 10−4 |
| HMDB | Name | Neutral Mass (Da) * | Normalized RT (min) ** | Fold Change | p-Value |
|---|---|---|---|---|---|
| HMDB00148 | Glutamic Acid | 129.0428 | 9.33 | 1.484 | 2.94 × 10−5 |
| HMDB0029016 | Prolyl-Glutamate | 244.1058 | 7.03 | 1.300 | 4.63 × 10−4 |
| HMDB03911 | 3-Aminoisobutanoic acid | 85.0524 | 16.11 | 1.258 | 1.42 × 10−2 |
| HMDB0028922 | Leucyl-Alanine | 202.1319 | 10.47 | 0.826 | 1.26 × 10−3 |
| HMDB0029010 | Prolyl-Alanine | 186.1005 | 8.64 | 0.813 | 1.07 × 10−3 |
| HMDB00161 | Beta-Alanine | 89.0477 | 7.59 | 0.802 | 1.89 × 10−4 |
| HMDB00161 | Alanine | 89.0475 | 7.92 | 0.801 | 3.04 × 10−4 |
| HMDB29098 | Tyrosyl-Alanine | 252.1125 | 20.76 | 0.800 | 5.20 × 10−3 |
| NA | Glycyl-Alanine | 146.0691 | 6.20 | 0.779 | 1.41 × 10−3 |
| HMDB00452 | Alpha-aminobutyric acid | 103.0634 | 9.16 | 0.778 | 1.13 × 10−3 |
| HMDB00939 | S-Adenosylhomocysteine | 384.1222 | 10.87 | 0.766 | 1.66 × 10−2 |
| HMDB01431 | Pyridoxamine | 168.0912 | 19.53 | 0.743 | 1.29 × 10−3 |
| HMDB03337 | Oxidized glutathione | 612.1519 | 8.20 | 0.735 | 1.27 × 10−2 |
| HMDB28680 | Alanyl-Alanine | 160.0846 | 6.34 | 0.722 | 4.04 × 10−4 |
| HMDB00296 | Uridine | 244.0693 | 7.69 | 0.698 | 7.52 × 10−4 |
| HMDB03464 | 4-Guanidinobutanoic acid | 127.0737 | 10.74 | 0.642 | 3.55 × 10−3 |
| HMDB02362 | 2,4-Diaminobutyric acid | 118.0755 | 15.73 | 0.618 | 5.19 × 10−4 |
| HMDB02393 | N-Methyl aspartic acid | 147.0532 | 7.27 | 0.609 | 2.71 × 10−6 |
| HMDB00112 | Gamma-aminobutyric acid | 103.0633 | 7.72 | 0.576 | 7.33 × 10−3 |
| HMDB | Name | Neutral Mass (Da) * | Normalized RT (min) ** | Fold Change | p-Value |
|---|---|---|---|---|---|
| HMDB00300 | Uracil | 112.0275 | 11.28 | 2.565 | 1.19 × 10−3 |
| HMDB01414 | 1,4-Diaminobutane | 88.1015 | 20.97 | 1.476 | 6.32 × 10−3 |
| HMDB00149 | Ethanolamine | 61.0528 | 6.12 | 1.472 | 8.01 × 10−4 |
| HMDB00500 | 4-Hydroxybenzoic acid | 138.032 | 17.54 | 1.232 | 2.95 × 10−3 |
| HMDB00089 | Cytidine | 243.0854 | 5.59 | 1.225 | 5.82 × 10−3 |
| HMDB00177 | Histidine | 155.0698 | 18.23 | 0.822 | 6.65 × 10−4 |
| HMDB0028823 | Glutamyl-Leucine | 260.1386 | 9.92 | 0.802 | 1.36 × 10−2 |
| HMDB28848 | Glycyl-Phenylalanine | 222.1006 | 11.70 | 0.790 | 1.47 × 10−2 |
| HMDB0029126 | Valyl-Glutamate | 246.1214 | 6.88 | 0.787 | 6.60 × 10−3 |
| HMDB0029041 | Seryl-Histidine | 242.1019 | 15.36 | 0.761 | 1.42 × 10−2 |
| HMDB0028907 | Isoleucyl-Glycine | 188.1153 | 8.84 | 0.753 | 1.62 × 10−2 |
| HMDB0000759 | Glycyl-Leucine | 188.1162 | 11.11 | 0.751 | 6.20 × 10−3 |
| HMDB00939 | S-Adenosylhomocysteine | 384.1228 | 10.87 | 0.736 | 1.30 × 10−2 |
| HMDB00157 | Hypoxanthine | 136.0387 | 9.54 | 0.736 | 1.99 × 10−2 |
| HMDB03337 | Oxidized glutathione | 612.1532 | 7.93 | 0.718 | 6.37 × 10−3 |
| HMDB0029125 | Valyl-Glutamine | 245.1369 | 5.68 | 0.703 | 9.21 × 10−3 |
| HMDB0028939 | Leucyl-Threonine | 232.1423 | 8.71 | 0.698 | 3.33 × 10−3 |
| HMDB0029127 | Valyl-Glycine | 174.1004 | 7.32 | 0.688 | 8.67 × 10−3 |
| HMDB00167 | Threonine | 119.0584 | 5.80 | 0.650 | 1.14 × 10−5 |
| HMDB00446 | N-Alpha-acetyllysine | 188.116 | 6.85 | 0.576 | 1.27 × 10−4 |
| HMDB | Name | Neutral Mass (Da) * | Normalized RT (min) ** | Fold Change | p-Value |
|---|---|---|---|---|---|
| HMDB01414 | 1,4-Diaminobutane | 88.1015 | 20.97 | 2.160 | 2.22 × 10−6 |
| HMDB00099 | Cystathionine | 222.0686 | 13.69 | 1.867 | 5.93 × 10−4 |
| HMDB01257 | Spermidine | 145.1594 | 10.34 | 1.478 | 1.18 × 10−2 |
| HMDB00157 | Hypoxanthine | 136.0387 | 8.69 | 1.472 | 2.43 × 10−3 |
| HMDB02390 | 3-Cresotinic acid | 152.0477 | 16.80 | 1.369 | 2.98 × 10−4 |
| HMDB00669 | Ortho-Hydroxyphenylacetic acid | 152.0479 | 16.49 | 1.368 | 3.03 × 10−4 |
| HMDB00206 | N6-Acetyl-Lysine | 188.1161 | 5.66 | 1.288 | 1.03 × 10−2 |
| HMDB00719 | Homoserine | 101.0478 | 9.01 | 1.268 | 6.07 × 10−4 |
| HMDB00164 | Methylamine | 31.0423 | 9.73 | 0.831 | 1.58 × 10−4 |
| HMDB00148 | Glutamic Acid | 129.0429 | 9.34 | 0.800 | 9.10 × 10−3 |
| HMDB01370 | Diaminopimelic acid | 190.0967 | 12.97 | 0.689 | 2.05 × 10−2 |
| HMDB00182 | Lysine | 146.1068 | 17.51 | 0.650 | 2.96 × 10−4 |
| HMDB03337 | Oxidized glutathione | 612.1527 | 7.90 | 0.577 | 1.35 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahabiyeh, L.A.; Malkawi, A.K.; Wang, X.; Colak, D.; Mujamammi, A.H.; Sabi, E.M.; Li, L.; Dasouki, M.; Abdel Rahman, A.M. Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS Analysis. Metabolites 2020, 10, 42. https://doi.org/10.3390/metabo10020042
Dahabiyeh LA, Malkawi AK, Wang X, Colak D, Mujamammi AH, Sabi EM, Li L, Dasouki M, Abdel Rahman AM. Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS Analysis. Metabolites. 2020; 10(2):42. https://doi.org/10.3390/metabo10020042
Chicago/Turabian StyleDahabiyeh, Lina A., Abeer K. Malkawi, Xiaohang Wang, Dilek Colak, Ahmed H. Mujamammi, Essa M. Sabi, Liang Li, Majed Dasouki, and Anas M. Abdel Rahman. 2020. "Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS Analysis" Metabolites 10, no. 2: 42. https://doi.org/10.3390/metabo10020042
APA StyleDahabiyeh, L. A., Malkawi, A. K., Wang, X., Colak, D., Mujamammi, A. H., Sabi, E. M., Li, L., Dasouki, M., & Abdel Rahman, A. M. (2020). Dexamethasone-Induced Perturbations in Tissue Metabolomics Revealed by Chemical Isotope Labeling LC-MS Analysis. Metabolites, 10(2), 42. https://doi.org/10.3390/metabo10020042





