Comparative Analysis of Milk Triglycerides Profile between Jaffarabadi Buffalo and Holstein Friesian Cow
Abstract
1. Introduction
2. Results and Discussion
2.1. Cumulative Lipid Identifications
- Mass-identification (Mass-ID): only mass-resolved lipid molecular ion species.
- Group-ID: lipid IDs at sum composition level with only class-specific head group identification (e.g., PC 32:0).
- PA-ID (partial-acyl identification): For example, PC 14:0*_18:0 where the suffix * indicates that the MS/MS spectrum does not feature characteristic ion/s corresponding to the fatty acid chain 14:0.
- FA-ID (lipid IDs with full-acyl identification) [32]: For example, PC 14:0_18:0, indicating that the MS/MS spectrum features all the characteristic ion/s corresponding to the head group/neutral loss of head group along with the fatty acid chains.
2.2. Characterization of Novel DG and TG Lipid Species that Are Not Yet Cataloged in the LIPIDMAPS Database
2.3. LC-MS Peak Detection for Assignment of Low Abundance PS, PI, and PC Lipid Molecular Ions
2.4. Common Fatty Acid Chains of the Reported TG Species
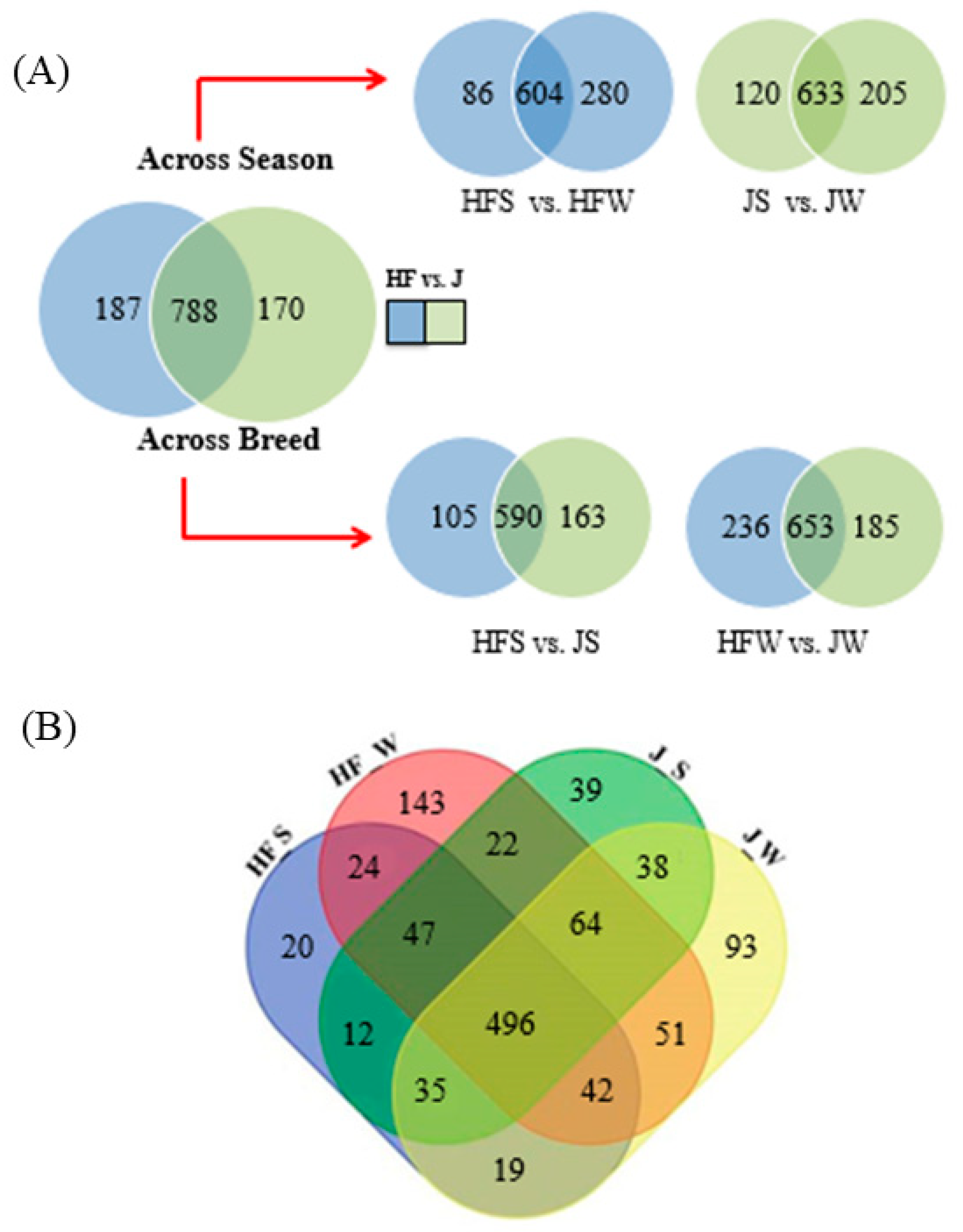
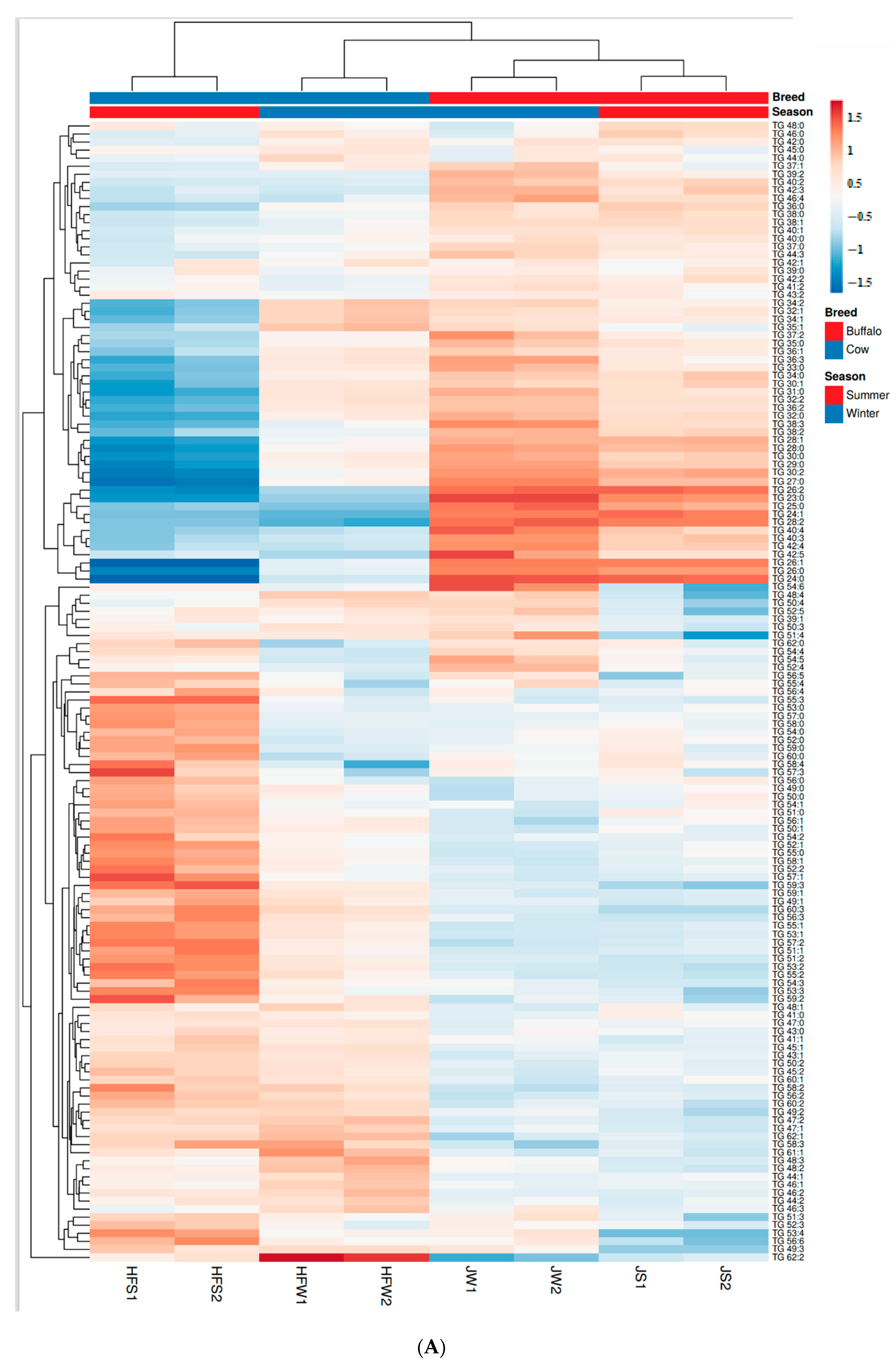
2.5. Comparative Analysis of Lipids between Holstein Friesian Cow and Jaffarabadi Buffalo across Season
2.6. Variation in Unsaturation between Holstein Friesian Cow and Jaffarabadi Buffalo
2.7. Relative Quantification of Lipids and Setting up of Hypothesis
2.7.1. Collating Lipid Ion Species as Sum Compositions and Measuring Ion Abundances for Relative Quantitation
2.7.2. Measurement of Ion Abundances of Analytes from Extracted Ion Chromatograms
2.7.3. Data Normalization and Data Transformation
2.7.4. Two-Way Analysis of Variance Analysis (ANOVA)
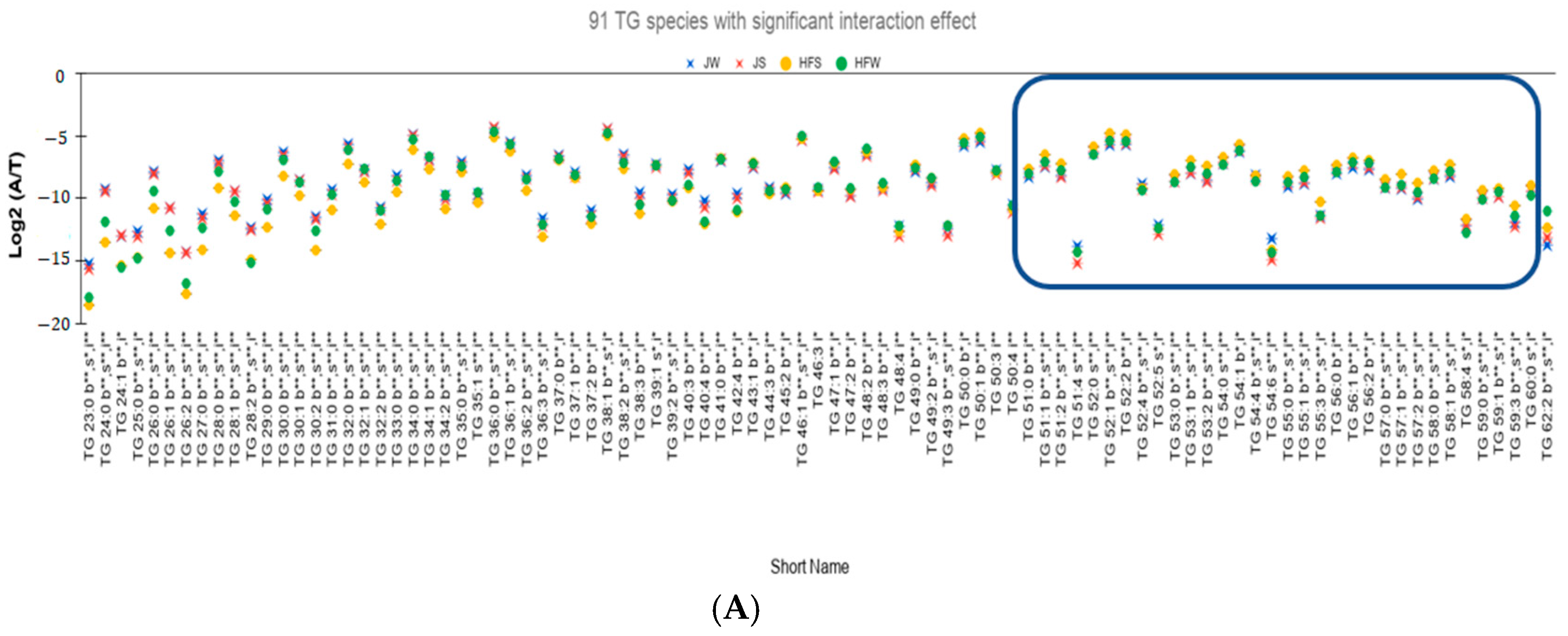
2.8. Quantitative Comparison of TG Profiles between Holstein Friesian Cow and Jaffarabadi Buffalo
- (i)
- Interaction effect is significant for 91 TG species (Figure 3A).
- (ii)
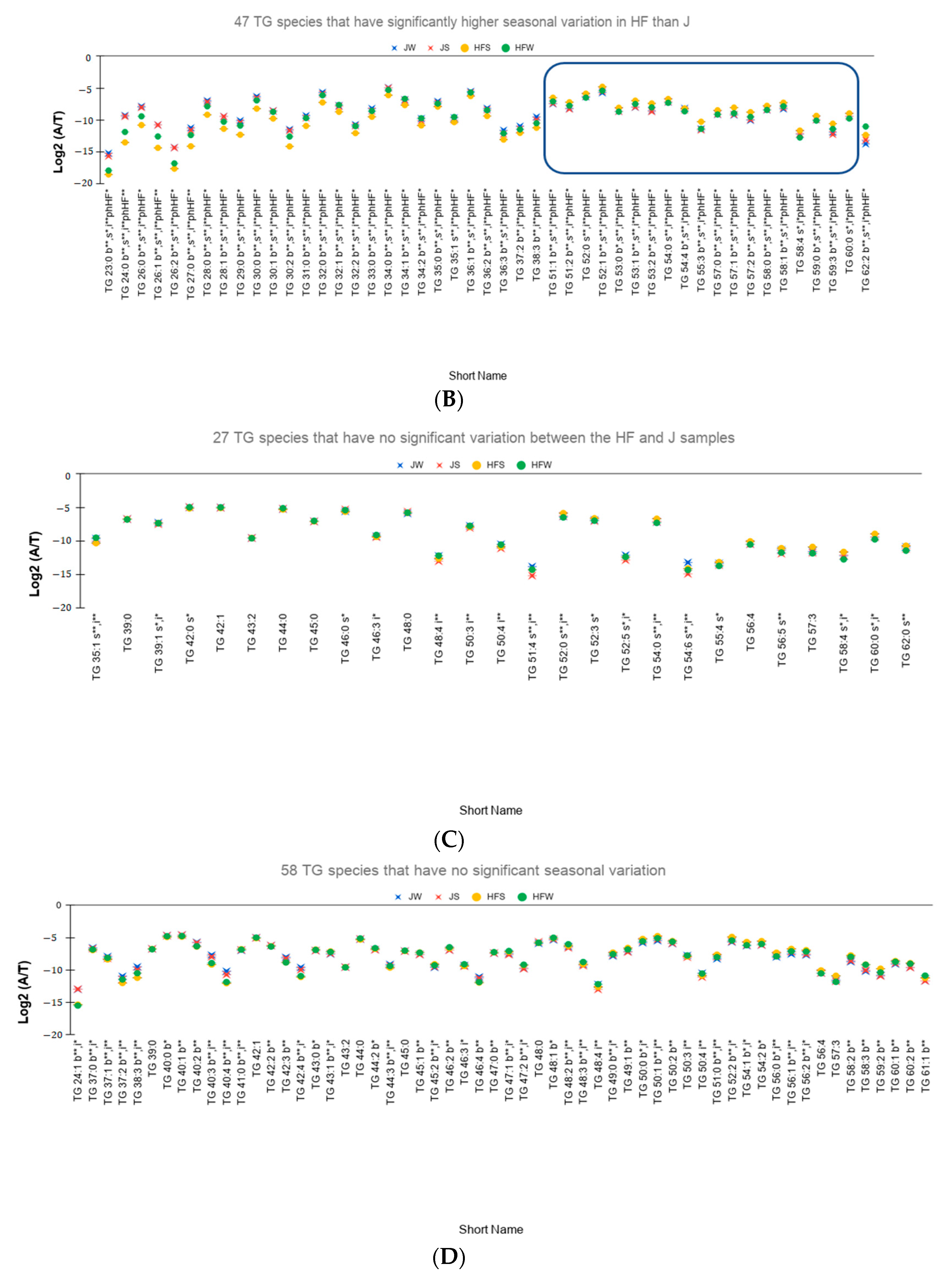
- Out of the 91 species, 71 TG species have higher seasonal variation—defined as the absolute difference of relative abundances between the summer season and the winter season—for the HF samples than the J samples. On performing Tukey’s HSD test to check whether there is a significant variation between the seasons, 47 TG species have statistically significant seasonal variations (Figure 3B).
- (iii)
- Twenty TG species have higher seasonal variation in J samples than in HF samples. However, only four TG species, namely TG 48:4, TG 50:4, TG 51:4, and TG 54:6, vary significantly based on Tukey’s HSD Test.
- (a)
- The relative abundances of 81% of the total reported 114 TG species have significant variation between the breeds. Figure 3C showed the 27 TG species that do not have significant variation between the breeds.
- (b)
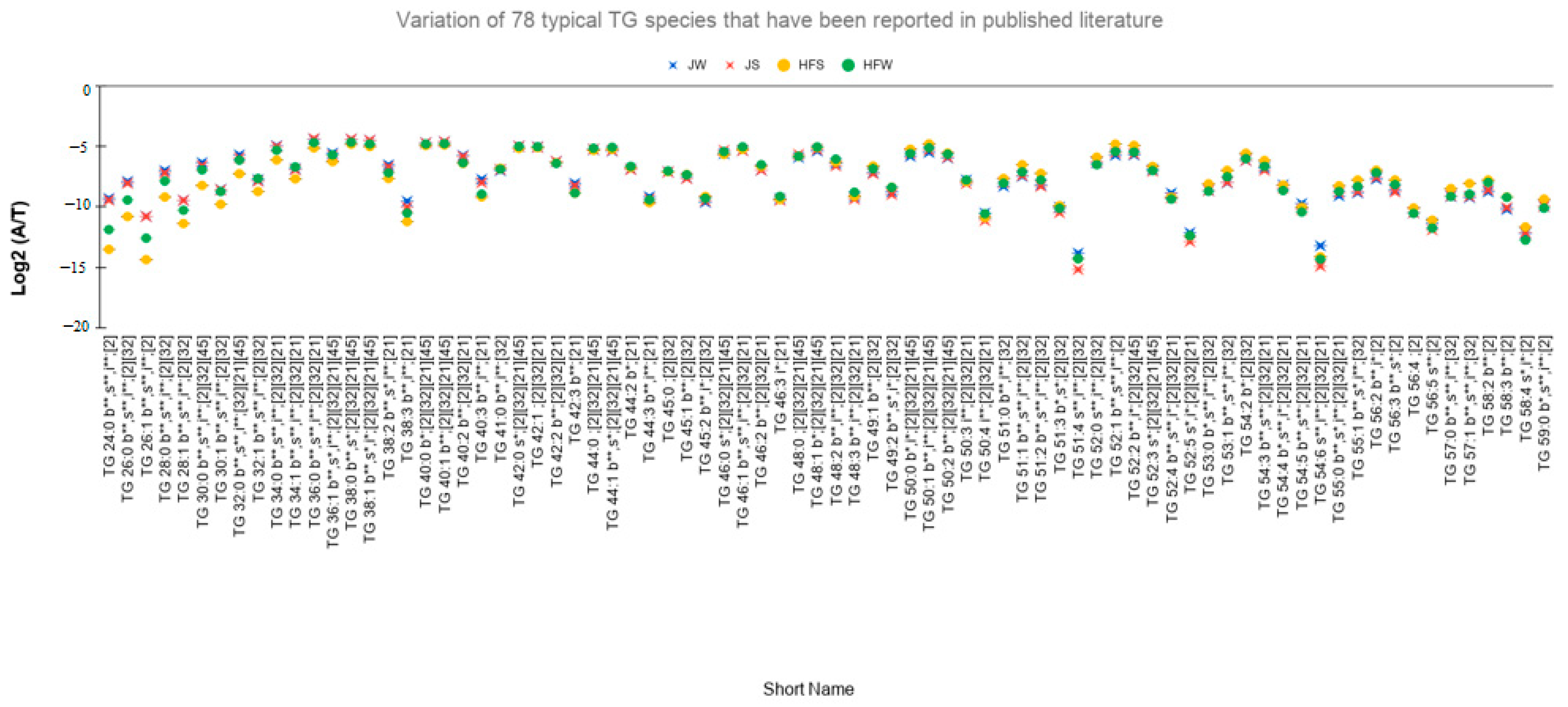
- Relative abundances of 83 TG species, i.e., 59% vary significantly between the seasons. Figure 3D shows the relative abundances of the 58 TG species that do not vary significantly between the season.
- (c)
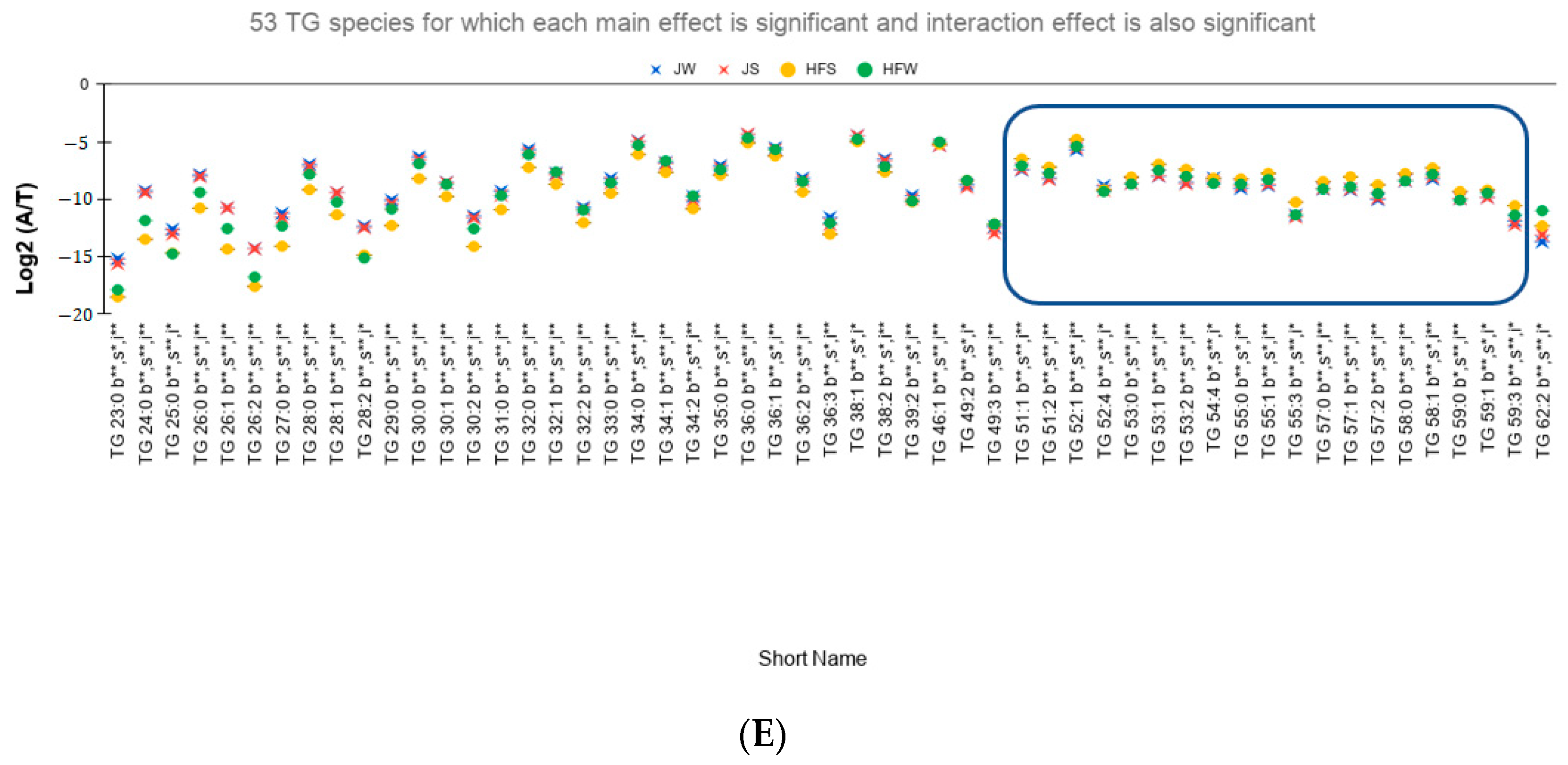
- A total of 53 TG species have not only significant interaction effect but also significant main effects. Out of these 53 TG species, 48 TG species have higher seasonal variation in HF samples than the J samples of which 40 species are found to be statistically significant (Figure 3E). Only 5 TG species have higher seasonal variation in J samples than the HF samples. However, Tukey’s HSD test showed that none of the TG species is statistically significant.
- Different effect of season on different breeds: J samples showed lesser seasonal variation than HF samples. On conducting Tukey’s HSD test for Post Hoc analysis of the 53 TG species with significant interaction effect, only one species, TG 23:0, has significant variation between the summer and winter seasons whereas for the HF samples, 40 TG species showed significant variation between the seasons.
- Potential seasonal effect based on the length of the fatty acids: For the HF milk samples, 34 TG species with TC—where TC is total number of carbons in Sn1, Sn2, and Sn3 chains—ranging from 23 to 49 have higher relative abundance in winter season than the summer season while 19 TG species with TC ranging between 51 to 59 have higher relative abundances in summer season than the winter season (rectangle in Figure 3B shows these 19 TG species). Of note, TG 62:2 has higher relative abundance in winter than in summer.
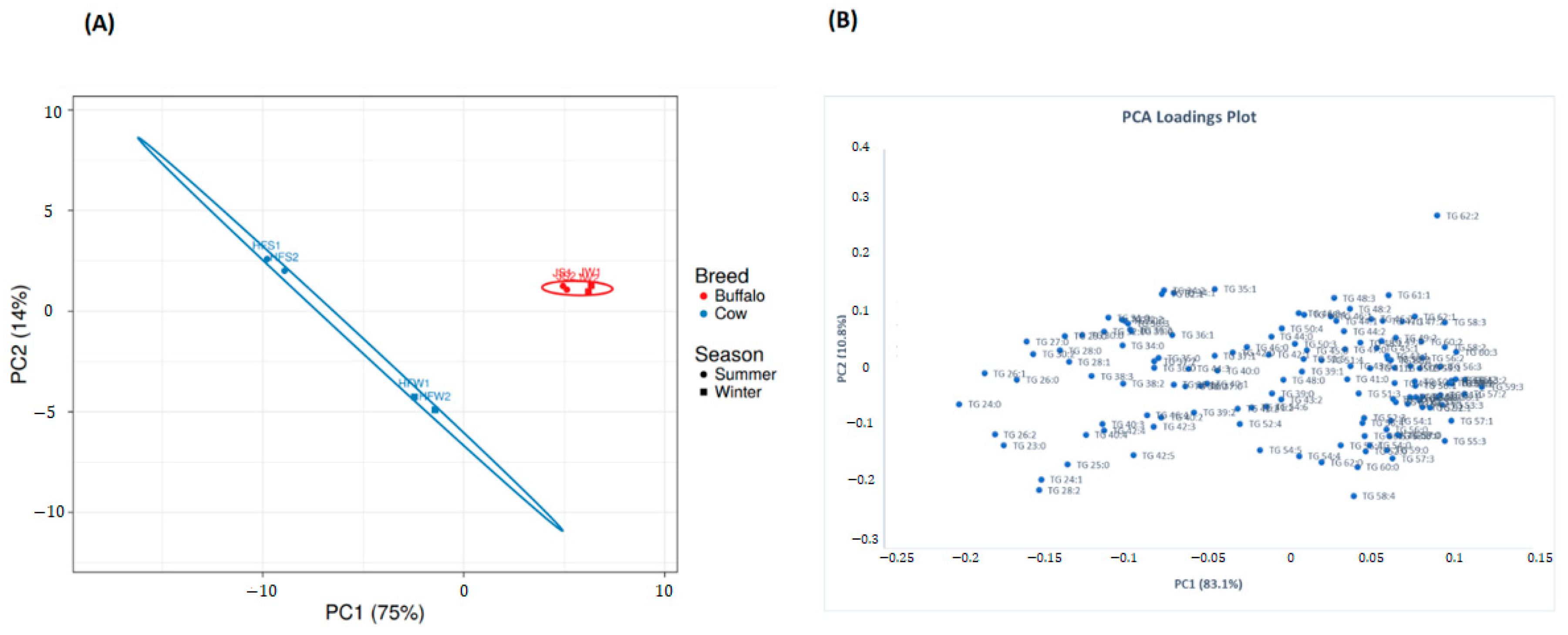
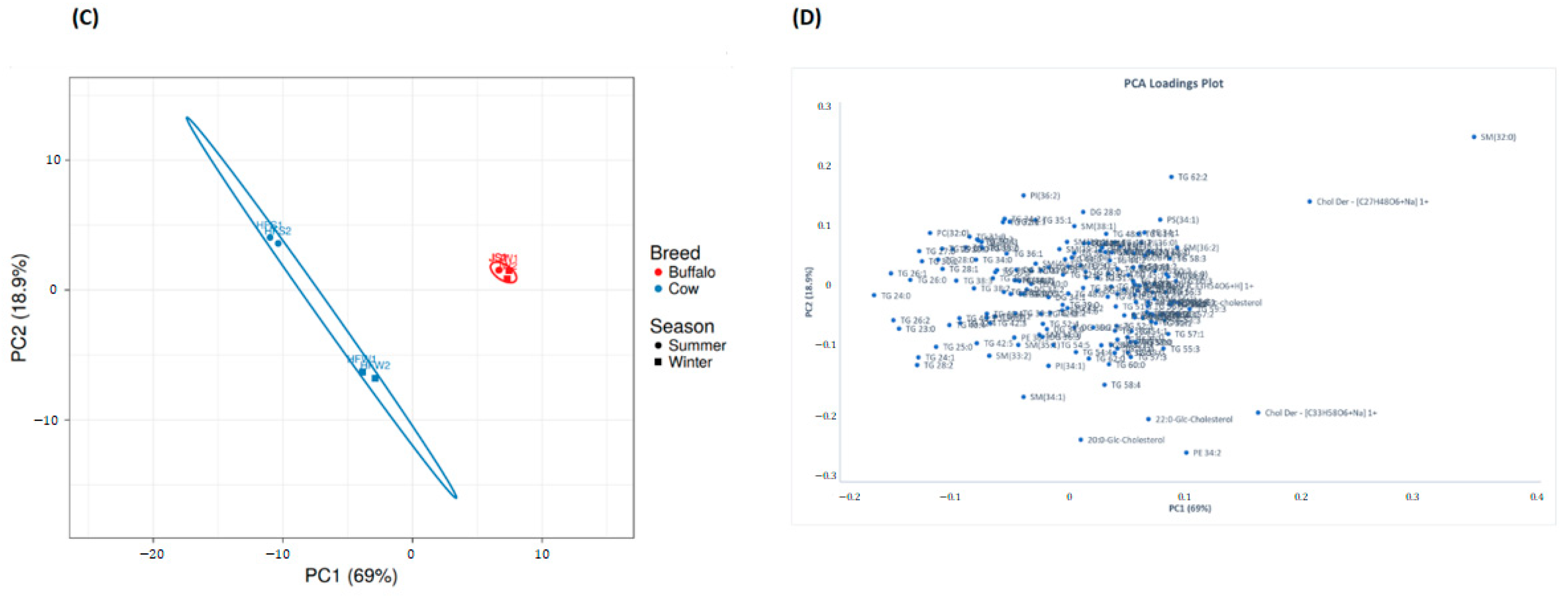
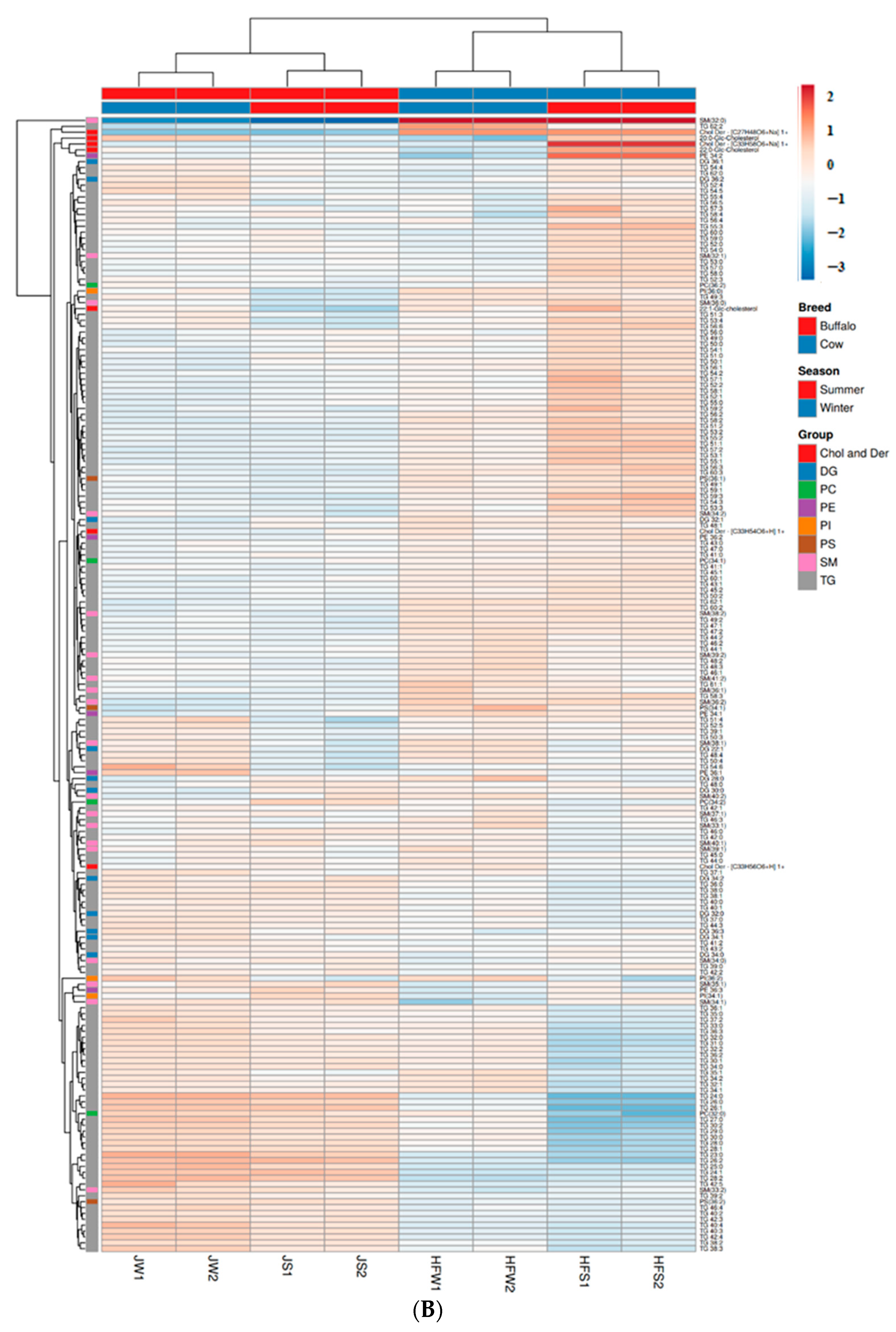
2.9. Multivariate Analysis of Lipid Profiles between Holstein Friesian Cow and Jaffarabadi Buffalo
3. Methods
3.1. Sample Collection
3.2. Isolation of Lipids from Milk Samples
3.3. Ultra-Performance Liquid Chromatography Mass Spectrometry
3.4. Data Analysis Software Tools
3.4.1. Tandem Mass Spectrometry Data Analysis for Lipid Identification
3.4.2. Relative Quantification of Lipids and Statistical Analyses
3.4.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Automated Assignment of MS/MS Spectra with of Probable Lipid Species Using SimLipid Software Supplementary Material
Appendix A.2. Verification and Grading of the Lipid Ion Species Reported by SimLipid Database Search
- Mass-ID (only mass-resolved lipid molecular ion species);
- Group-ID (lipid IDs at sum composition level with only class-specific head group identification);
- PA-ID (lipid IDs with Partial-Acyl Identification);
- FA-ID (lipid IDs with Full-Acyl Identification) [32].
- Mass-ID (only mass-resolved lipid molecular ion species): This is the case when a lipid molecular ion species is assigned to an MS/MS spectrum because its precursor m/z value is within a specified mass tolerance e.g., 5 ppm and MS/MS spectrum features ions corresponding to the parent ion mass e.g., [M+NH4]+, [M+H]+. Since no peak corresponding to any diagnostic ion corresponding to class-specific head groups or FA chains was observed, we use a naming convention that includes the subclass name with the formula of the lipid molecular ion. The MS/MS spectrum (See Supplementary Figure S2) with precursor m/z 549.4128 featuring peaks at m/z 549.4123 (relative intensity (RI): 100%) was annotated with the lipid species bearing LM ID LMST01010173 belonging to lipid class: Sterols, subclass: Cholesterol and derivatives (Cho Der). We assign a Mass-ID Cho Der-[C33H56O6+H] 1+ (See Supplementary Figure S2). Of note, this assignment was purely on the exact mass database search. There was no diagnostic ion corresponding to the Cholesterol e.g., peak at m/z 369.352. Nevertheless, we still use the Cho Der- because LMST01010173 is the lipid molecular ion species that has the minimum mass deviation (3.5326 ppm) from the observed precursor m/z value in the SimLipid database.
- Group-ID (lipid IDs with only class-specific head group identification): We assign Group-ID for an MS/MS spectrum that features peaks corresponding to diagnostic ions of class-specific head groups e.g., peak at m/z 184.073 for lipids with phosphocholine head group. However, the MS/MS spectrum does not have peaks that facilitate the identification of fatty acyl composition of the lipid species. For example, for an MS/MS spectrum with precursor m/z 760.5854 and product ions at m/z 86.0969(RI: 20%), m/z 104.1075(RI: 2%), and m/z 184.0736(RI: 100%), SimLipid annotates the lipid species bearing LM ID LMGP01010005, Common Name: PC (16:0/18:1(9Z)). Additionally, SimLipid also reports “Name: PC (16:0_18:1)” and “Short Name: PC (34:1)”. Since there was no observed peak that corresponds to the diagnostic ion of either of the probable fatty acid chains reported by SimLipid, we assign PC (34:1) (See Supplementary Figure S3). Furthermore, label-free quantitative analysis of low abundant phospholipids was performed.
- PA-ID (lipid IDs with Partial-Acyl Identification): In this case, not all the FA chains in a lipid species were identified by peaks in the MS/MS spectrum. For example, SimLipid annotated an MS/MS spectrum (See Supplementary Figure S4) with precursor m/z 782.723 at 21.9502 min with a ammonium adducted TG lipid molecular ion that has the Common Name: TG(13:0/14:0/18:0), LM ID: LMGL03013680, Name: TG(13:0_14:0_18:0), Short name: TG(45:0). Peaks observed in the MS/MS spectrum were annotated by SimLipid as follows (Fragment name Observed m/z(RI)): m/z M-18:0-NH3_481.4257(RI: 95.3%), M-14:0-NH3_537.4888(RI: 8.3%). The diagnostic ions corresponding to the FA chains 14:0 and 18:0 were observed in the spectrum. However, there was no ion corresponding to 13:0. Hence, we assign a graded ID TG 13:0@_14:0_18:0; the suffix @ indicating that the diagnostic ion of this FA chain was not observed in the spectrum.
- If the MS/MS spectrum features only the peaks corresponding to diagnostic ions of one of the three FA chains of the TG lipid molecular species e.g., only the peak at m/z 481.4257, then we use the Group-ID, TG 45:0, as the graded ID instead of using the notation TG 13:0@_14:0@_18:0 (See Supplementary Figure S4).
- FA-ID (lipid IDs with Full-Acyl Identification) [54]: In this case, an MS/MS spectrum features m/z peaks corresponding to all the FA chains. For example, SimLipid assigns lipid species with the Common Name: TG (16:0/18:0/18:1(9Z)), Name: TG (16:0_18:0_18:1), Short Name: TG (52:1), LM ID: LMGL03010085 for the MS/MS spectrum with precursor m/z 878.819 at 22.5484 min and product ions annotated as follows (Fragment name Observed m/z (RI)): 15:0 C=O+_239.2374(4.4786), 17:1 C=O+_265.2541(8.0049), 17:0 C=O+_267.2693(1.3489), M-18:0_577.5211(75.1463), M-18:1_579.5364(84.6283), M-16:0_605.5515(59.7802). We use the graded ID TG 16:0_18:0_18:1 for analysis (See Supplementary Figure S5).
Appendix A.3. Characterization of Novel DG and TG Lipid Species that Are Not Yet Cataloged in the LIPIDMAPS Database
- (i)
- The observed peak (Supplementary Figure S6) at m/z 215.1280 indicating the neutral loss of 15:0 from the [TG 23:0 + NH4]+ ion, i.e., 474.3788 − 215.1280 − 18.033823 = 241.217 which matches the theoretical mass of 15:0 with 0.9 (approx.) ppm mass deviation.
- (ii)
- Similarly, the observed peak at m/z 369.3001 indicates the neutral loss of the fatty acid chain 4:0 from the [TG 23:0 + NH4]+ ion (Supplementary Figure S6).
- (iii)
- Finally, TG 23:0 could have the possible lipid species TG 4:0_4:0_15:0 (we use the underscore, “_”, between the fatty acid chains to indicate non-specificity of the Sn1/Sn2/Sn3 positions of these reported fatty acid chains).
- (iv)
- Once we establish the probable lipid species for an MS/MS spectrum, we create structures using LM and create a glycerolipid structure from LM Abbreviation”.
- (v)
- (http://www.lipidmaps.org/tools/structuredrawing/StrDraw.pl?Mode=SetupGLStrDraw) wherein we enter the lipid species abbreviation and selecting the option of “Allow arbitrary chain abbreviation” as YES.
- (vi)
- The generated SDF files are imported into the SimLipid server database for automated assignment of lipid species on the MS/MS spectra across all the raw data files.
- (vii)
- The process (i)–(v) is repeated for all the MS/MS spectra with annotated TG compositions.
Appendix A.4. LC-MS Peak Detection for Assignment of Low Abundance PS, PI, and PC Lipid Molecular Ions
- (i)
- If it has an LC-peak with a minimum peak width of 0.1 min when plotted the XIC with 0.005 Da mass tolerance.
- (ii)
- The averaged spectrum of all the MS1 data across the start and end time of the XIC features isotopic cluster with the ion as the monoisotopic peak.
Appendix A.5. Measurement of Ion Abundances of Analytes from Extracted Ion Chromatograms
Appendix A.6. Verification of the Assumptions of the Two-Way ANOVA
- Measurement of the dependent variable at the continuous level: This condition is satisfied.
- Two or more than two categorical independent groups in two factors: This condition is satisfied with our goal to test the variation in average relative ion abundances of analytes between different breeds and seasons.
- Categorical independent groups should have the same size: Our data satisfy this condition.
- Independence of observations: Our data were collected independently.
- Normal distribution of the population from which each sample is drawn.
- Homoscedasticity or Homogeneity of the variance.
Appendix A.6.1. Test of Normality of the Population from Which the Data Is Drawn
Appendix A.6.2. Homoscedasticity or Homogeneity of the Variance
- Zij = |Xij-| where is the mean of the ith sample. This choice provides the best power for symmetric, moderate-tailed, distributions.
- Zij = |Xij-Median(i.)| where Median(i.) is the median of the ith sample. The choice of median performed best when the underlying data followed a skewed distribution.
- Zij = |Xij-trimMean(i.)| where trimMean(i.) is the 10% trimmed mean of the ith sample. The choice of using the trimmed mean performed best when the underlying data followed a Cauchy distribution (i.e., heavy-tailed).
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| JW difference | 195 | 503.1354 | 2.580182 | 3.279944 | ||
| JS Difference | 195 | 519.703 | 2.665144 | 3.756559 | ||
| HFS Difference | 195 | 554.1781 | 2.841939 | 4.800262 | ||
| HFW Difference | 195 | 540.3826 | 2.771193 | 4.352645 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
| Between Groups | 7.78679 | 3 | 2.595597 | 0.641307 | 0.588588 | 2.616378 |
| Within Groups | 3140.746 | 776 | 4.047352 | |||
| Total | 3148.532 | 779 | ||||
Appendix A.7. Tukey’s HSD (Honestly Significant Difference)
- Observations are independent within and among groups.
- The groups for each mean in the test are normally distributed.
- There is equal within-group variance across the groups associated with each mean in the test (homogeneity of variance).
- Mi − Mj is the difference between the pair of means with Mi > Mj.
- S2 is the pooled sample variance is the Mean Square Within, and n is the number in the group or treatment.
References
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rochfort, S.; Cocks, B. Milk lipidomics: What we know and what we don’t. Prog. Lipid Res. 2018, 71, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Invited review: Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef] [PubMed]
- Samková, E.; Spicka, J.; Pesek, M.; Pelikánová, T.; Hanus, O. Animal factors affecting fatty acid composition of cow milk fat: A review. S. Afr. J. Anim. Sci. 2012, 42, 83–100. [Google Scholar]
- Griffiths, M. Improving the Safety and Quality of Milk; Woodhead Publishing: Sawston, UK, 2010. [Google Scholar]
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L.; Denise Beaulieu, A.; Barbano, D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Hellenius, M.L.; Rosell, M.; Zdravkovic, S.; de Faire, U.; Vessby, B.; Hamsten, A.; Skoglund-Andersson, C.; Fisher, R.M.; Sjogren, P. Milk-Derived Fatty Acids Are Associated with a More Favorable LDL Particle Size Distribution in Healthy Men. J. Nutr. 2004, 134, 1729–1735. [Google Scholar]
- Fox, P.F.; Singh, T.K.; McSweeney, P.L. Biogenesis of flavour compounds in cheese. Adv. Exp. Med. Biol. 1995, 367, 59–98. [Google Scholar]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. PLoS ONE 2014, 9, e83764. [Google Scholar] [CrossRef]
- Ebbesson, S.O.E.; Voruganti, V.S.; Higgins, P.B.; Fabsitz, R.R.; Ebbesson, L.O.; Laston, S.; Harris, W.S.; Kennish, J.; Umans, B.D.; Wang, H.; et al. Fatty acids linked to cardiovascular mortality are associated with risk factors. Int. J. Circumpolar Health 2015, 74, 28055. [Google Scholar] [CrossRef] [PubMed]
- Praagman, J.; de Jonge, E.A.; Kiefte-de Jong, J.C.; Beulens, J.W.; Sluijs, I.; Schoufour, J.D.; Hofman, A.; van der Schouw, Y.T.; Franco, O.H. Dietary Saturated Fatty Acids and Coronary Heart Disease Risk in a Dutch Middle-Aged and Elderly Population. Arter. Thromb Vasc. Biol. 2016, 36, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Chardigny, J.M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, M.A.; Mensink, R.P. Fatty acids and atherosclerotic risk. Handb. Exp. Pharm. 2005, 170, 165–194. [Google Scholar]
- Chen, C.L.; Tetri, L.H.; Neuschwander-Tetri, B.A.; Huang, S.S.; Huang, J.S. A mechanism by which dietary trans fats cause atherosclerosis. J. Nutr. Biochem. 2011, 22, 649–655. [Google Scholar] [CrossRef][Green Version]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in milk fat: Composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Liu, Z.; Moate, P.; Cocks, B.; Rochfort, S. Comprehensive polar lipid identification and quantification in milk by liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 978–979, 95–102. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-Depth Lipidomic Analysis of Molecular Species of Triacylglycerides, Diacylglycerides, Glycerophospholipids, and Sphingolipids of Buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef]
- Simionato, J.I.; Garcia, J.C.; Santos, G.T.d.; Oliveira, C.C.; Visentainer, J.V.; Souza, N.E.D. Validation of the determination of fatty acids in milk by gas chromatography. J. Braz. Chem. Soc. 2010, 21, 520–524. [Google Scholar] [CrossRef]
- Sokol, E.; Ulven, T.; Færgeman, N.J.; Ejsing, C.S. Comprehensive and quantitative profiling of lipid species in human milk, cow milk and a phospholipid-enriched milk formula by GC and MS/MS(ALL). Eur. J. Lipid Sci. Technol. 2015, 117, 751–759. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. B 2014, 951–952, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zhu, D.; Pang, X.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Amores, G.; Virto, M. Total and Free Fatty Acids Analysis in Milk and Dairy Fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef]
- Jensen, R.G.; Newburg, D.S. Bovine milk lipids. In Handbook of Milk Composition; Academic Press: London, UK, 1995. [Google Scholar]
- Parodi, P. Milk fat in human nutrition. Aust. J. Dairy Technol. 2004, 59, 3–59. [Google Scholar]
- Pimentel, L.; Gomes, A.; Pintado, M.; Rodríguez-Alcalá, L. Isolation and Analysis of Phospholipids in Dairy Foods. J. Anal. Methods Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Schwudke, D.; Shevchenko, A.; Hoffmann, N.; Ahrends, R. Lipidomics informatics for life-science. J. Biotechnol. 2017, 261, 131–136. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Cocks, B.G.; Rochfort, S. Seasonal Variation of Triacylglycerol Profile of Bovine Milk. Metabolites 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J. The composition of milk fat. J. Dairy Sci. 1991, 74, 3228–3243. [Google Scholar] [CrossRef]
- Saroj, M.B.; Van Tran, L.; Sharma, A.N.; Kumar, S.; Tyagi, A.K. Seasonal variation in fatty acid profile in the milk of different species under popularly followed feeding system in India. Indian J. Anim. Sci. 2017, 87, 484–489. [Google Scholar]
- Cortes, C.; da Silva-Kazama, D.C.; Kazama, R.; Gagnon, N.; Benchaar, C.; Santos, G.T.; Zeoula, L.M.; Petit, H.V. Milk composition, milk fatty acid profile, digestion, and ruminal fermentation in dairy cows fed whole flaxseed and calcium salts of flaxseed oil. J. Dairy Sci. 2010, 93, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Precht, D.; Molkentin, J. Analysis and seasonal variation of conjugated linoleic acid and further cis-/trans-isomers of C18:1 and C18:2 in bovine milk fat. Milchwirtsch. Forsch. 1999, 51, 63–77. [Google Scholar]
- El Hamdani, M.; Benali, A.; El Antari, A.; El Housni, A.; Douaik, A.; Khadija, O.; Bouksaim, M. The Season Effect on the Milk Fatty Acid Profile of Oulmes Breed. Cattle in Moroccan Oulmes Zone. J. Biotechnol. Biochem. 2017, 3, 13–17. [Google Scholar] [CrossRef]
- Larsen, M.; Andersen, K.K.; Kaufmann, N.; Lars, W. Seasonal variation in the composition and melting behavior of milk fat. J. Diary Sci. 2014, 97, 4703–4712. [Google Scholar] [CrossRef]
- Anankanbil, S.; Larsen, M.K.; Weisbjerg, M.R.; Wiking, L. Effects of variation in fatty acids and triglyceride composition on melting behavior in milk fat. Milk Sci. Int. 2018, 71, 4–9. [Google Scholar]
- Carrara, E.R.; Gaya, L.G.; Mourão, G.B. Fatty acid profile in bovine milk: Its role in human health and modification by selection. Arch. Zootec. 2017, 66, 151–158. [Google Scholar] [CrossRef]
- Jorjong, S.; van Knegsel, A.T.; Verwaeren, J.; Bruckmaier, R.M.; De Baets, B.; Kemp, B.; Fievez, V. Milk fatty acids as possible biomarkers to diagnose hyperketonemia in early lactation. J. Dairy Sci. 2015, 98, 5211–5221. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Zhang, C.; Jones, A.D.; Sordillo, L.M. Differences in the Oxylipid Profiles of Bovine Milk and Plasma at Different Stages of Lactation. J. Agric. Food Chem. 2017, 65, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.E.; Zhang, X.; Weitz, K.K.; Monroe, M.E.; Ibrahim, Y.M.; Moore, R.J.; Cha, J.; Sun, X.; Lovelace, E.S.; Wagoner, J.; et al. Uncovering biologically significant lipid isomers with liquid chromatography, ion mobility spectrometry and mass spectrometry. Analyst 2016, 141, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.K.; Singh, R.J. LC-MS/MS in the Clinical Laboratory-Where to From Here? Clin. Biochem. Rev. 2011, 32, 5–31. [Google Scholar] [PubMed]
- Liu, Z.; Logan, A.; Cocks, B.G.; Rochfort, S. Seasonal variation of polar lipid content in bovine milk. Food Chem. 2017, 237, 865–869. [Google Scholar] [CrossRef]
- Parodi, P.W. Some aspects of milk fat triglyceride structure. In Proceedings of the Developments in Milk Fat Technology Seminar; Rich, B.B., Ed.; Department for Environment, Food & Rural Affairs: Victoria, Australia, 1989; p. 1. [Google Scholar]
- Brevik, A.; Veierod, M.B.; Drevon, C.A.; Andersen, L.F. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur. J. Clin. Nutr. 2005, 59, 1417–1422. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef]
- Statistics Kingdom. Shapiro-Wilk Test Calculator. 2017. Available online: https://www.statskingdom.com/320ShapiroWilk.html (accessed on 10 October 2019).
- Henao, J.J.A.; Meitei, N.S.; Chalil, D.; Smith, R.W.; Stark, K.D. Comparing Automatic Identifcations in the Macrolipidomic Profles of Human Whole Blood Across UHPLC-MS/MS Platforms and Acquisition Modes. In Proceedings of the 66th ASMS Conference on Mass Spectrometry and Allied Topics, San Diego, CA, USA, 3–7 June 2018. [Google Scholar]
- Mottram, H.R.; Evershed, R.P. Elucidation of the composition of bovine milk fat triacylglycerols using high-performance liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A 2001, 926, 239–253. [Google Scholar] [CrossRef]
- Kramer, J.K.; Fellner, V.; Dugan, M.E.; Sauer, F.D.; Mossoba, M.M.; Yurawecz, M.P. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 1997, 32, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Olkin, I. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Stanford, CA, USA, 1960. [Google Scholar]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Amer. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
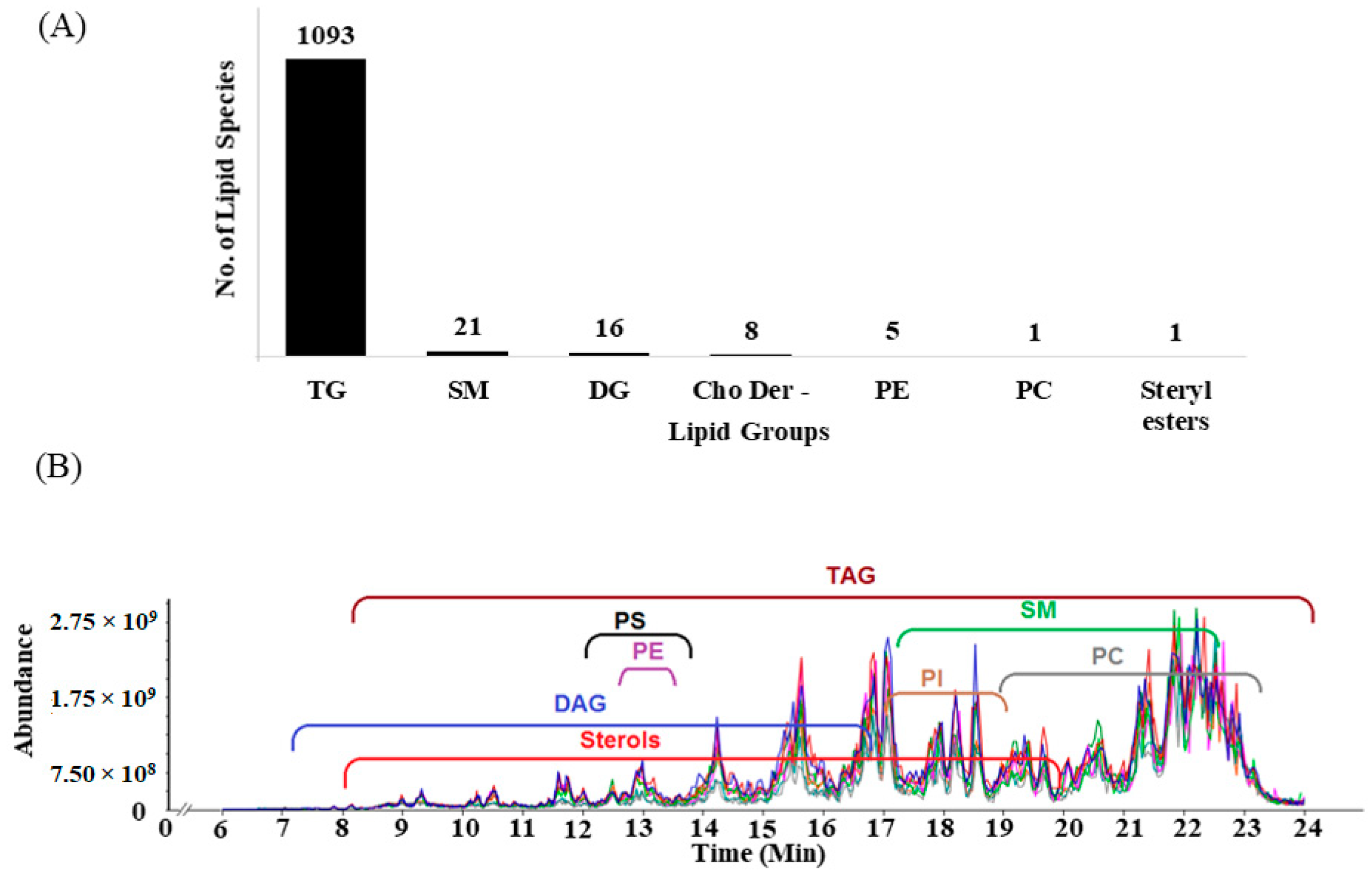
| 1 | Total No. of MS/MS Spectra Subjected to SimLipid MS/MS Database Search | 24,312 |
| 2 | No. of MS/MS spectra annotated with possible lipid molecular ions | 6710 |
| 3 | No. of unique graded IDs e.g., PC(16:0_18:1) or PC 34:1 From LIPIDMAPS | 882 |
| 4 | The number of unique DAG and TAG lipid species identified by manually annotating m/z peaks in the MS/MS spectra with neutral loss of all the possible fatty acyls for each lipid. LIPID MAPS has not yet catalogued these lipids species | 261 |
| 5 | No. of unique lipid compositions at Group-ID/Mass-ID levels for which relative quantitation was performed | 196 |
| Group | Mass-ID | Group-ID | PA-ID | FA-ID | Class Wise Total Lipids |
|---|---|---|---|---|---|
| TG | N/C | N/C | 243 * | 833 | 1076 |
| DG | N/C | N/C | 7 * | 14 | 21 |
| PC | N/C | 1 | 0 | 0 | 1 |
| PE | N/C | 3 | 1 | 0 | 4 |
| Chol & Der | 8 # | 0 | 0 | 0 | 9 |
| SM | 0 | 21 | 0 | 0 | 21 |
| Total | 1132 − 243 * − 7 * = 882 |
| TG Lipid | Ours | [2] | [33] | [23] | [34] | TG Lipid | Ours | [2] | [33] | [23] | [34] | TG Lipid | Ours | [2] | [33] | [23] | [34] | TG Lipid | Ours | [2] | [33] | [23] | [34] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TG 23:0 | 1 | 0 | 0 | 0 | 0 | TG 39:0 | 1 | 0 | 0 | 0 | 0 | TG 48:1 | 1 | 1 | 1 | 1 | 1 | TG 54:5 | 1 | 1 | 1 | 1 | 0 |
| TG 24:0 | 1 | 1 | 0 | 0 | 0 | TG 39:1 | 1 | 0 | 0 | 0 | 0 | TG 48:2 | 1 | 1 | 1 | 1 | 0 | TG 54:6 | 1 | 0 | 1 | 0 | 0 |
| TG 24:1 | 1 | 0 | 0 | 0 | 0 | TG 39:2 | 1 | 0 | 0 | 0 | 0 | TG 48:3 | 1 | 1 | 1 | 1 | 0 | TG 55:0 | 1 | 0 | 0 | 0 | 0 |
| TG 25:0 | 1 | 0 | 0 | 0 | 0 | TG 40:0 | 1 | 1 | 1 | 1 | 1 | TG 48:4 | 1 | 0 | 0 | 0 | 0 | TG 55:1 | 1 | 0 | 0 | 0 | 0 |
| TG 26:0 | 1 | 1 | 1 | 0 | 0 | TG 40:1 | 1 | 1 | 1 | 1 | 1 | TG 49:0 | 1 | 0 | 0 | 0 | 0 | TG 55:2 | 1 | 0 | 0 | 0 | 0 |
| TG 26:1 | 1 | 1 | 0 | 0 | 0 | TG 40:2 | 1 | 1 | 1 | 1 | 0 | TG 49:1 | 1 | 1 | 1 | 0 | 0 | TG 55:3 | 1 | 0 | 0 | 0 | 0 |
| TG 26:2 | 1 | 0 | 0 | 0 | 0 | TG 40:3 | 1 | 0 | 0 | 1 | 0 | TG 49:2 | 1 | 1 | 1 | 0 | 0 | TG 55:4 | 1 | 0 | 0 | 0 | 0 |
| TG 27:0 | 1 | 0 | 0 | 0 | 0 | TG 40:4 | 1 | 0 | 0 | 0 | 0 | TG 49:3 | 1 | 0 | 0 | 0 | 0 | TG 56:0 | 1 | 1 | 0 | 0 | 0 |
| TG 28:0 | 1 | 1 | 1 | 0 | 0 | TG 41:0 | 1 | 0 | 1 | 0 | 0 | TG 50:0 | 1 | 1 | 1 | 1 | 1 | TG 56:1 | 1 | 1 | 0 | 0 | 0 |
| TG 28:1 | 1 | 1 | 1 | 0 | 0 | TG 41:1 | 1 | 0 | 0 | 0 | 0 | TG 50:1 | 1 | 1 | 1 | 1 | 1 | TG 56:2 | 1 | 1 | 0 | 0 | 0 |
| TG 28:2 | 1 | 0 | 0 | 0 | 0 | TG 41:2 | 1 | 0 | 0 | 0 | 0 | TG 50:2 | 1 | 1 | 1 | 1 | 1 | TG 56:3 | 1 | 1 | 0 | 0 | 0 |
| TG 29:0 | 1 | 0 | 0 | 0 | 0 | TG 42:0 | 1 | 1 | 1 | 1 | 1 | TG 50:3 | 1 | 1 | 1 | 1 | 0 | TG 56:4 | 1 | 0 | 0 | 0 | 0 |
| TG 30:0 | 1 | 1 | 1 | 0 | 1 | TG 42:1 | 1 | 1 | 1 | 1 | 0 | TG 50:4 | 1 | 1 | 1 | 1 | 0 | TG 56:5 | 1 | 0 | 1 | 0 | 0 |
| TG 30:1 | 1 | 1 | 1 | 0 | 0 | TG 42:2 | 1 | 1 | 1 | 1 | 0 | TG 50:5 | 0 | 0 | 1 | 0 | 0 | TG 56:6 | 1 | 0 | 1 | 0 | 0 |
| TG 30:2 | 1 | 0 | 0 | 0 | 0 | TG 42:3 | 1 | 0 | 0 | 1 | 0 | TG 51:0 | 1 | 1 | 1 | 0 | 0 | TG 57:0 | 1 | 0 | 0 | 0 | 0 |
| TG 31:0 | 1 | 0 | 0 | 0 | 0 | TG 42:4 | 1 | 0 | 0 | 0 | 0 | TG 51:1 | 1 | 1 | 1 | 0 | 0 | TG 57:1 | 1 | 0 | 0 | 0 | 0 |
| TG 32:0 | 1 | 0 | 1 | 1 | 1 | TG 42:5 | 1 | 0 | 0 | 0 | 0 | TG 51:2 | 1 | 1 | 1 | 0 | 0 | TG 57:2 | 1 | 0 | 0 | 0 | 0 |
| TG 32:1 | 1 | 1 | 1 | 0 | 0 | TG 43:0 | 1 | 0 | 0 | 0 | 0 | TG 51:3 | 1 | 1 | 1 | 0 | 0 | TG 57:3 | 1 | 0 | 0 | 0 | 0 |
| TG 32:2 | 1 | 0 | 0 | 0 | 0 | TG 43:1 | 1 | 0 | 0 | 0 | 0 | TG 51:4 | 1 | 1 | 1 | 0 | 0 | TG 58:0 | 1 | 1 | 0 | 0 | 0 |
| TG 33:0 | 1 | 0 | 0 | 0 | 0 | TG 43:2 | 1 | 0 | 0 | 0 | 0 | TG 52:0 | 1 | 1 | 0 | 0 | 0 | TG 58:1 | 1 | 1 | 0 | 0 | 0 |
| TG 34:0 | 1 | 1 | 1 | 1 | 1 | TG 44:0 | 1 | 1 | 1 | 1 | 1 | TG 52:1 | 1 | 1 | 1 | 0 | 1 | TG 58:2 | 1 | 1 | 0 | 0 | 0 |
| TG 34:1 | 1 | 1 | 1 | 1 | 0 | TG 44:1 | 1 | 1 | 1 | 1 | 1 | TG 52:2 | 1 | 1 | 1 | 1 | 1 | TG 58:3 | 1 | 1 | 0 | 0 | 0 |
| TG 34:2 | 1 | 0 | 0 | 0 | 0 | TG 44:2 | 1 | 0 | 0 | 1 | 0 | TG 52:3 | 1 | 1 | 1 | 1 | 0 | TG 58:4 | 1 | 0 | 0 | 0 | 0 |
| TG 35:0 | 1 | 0 | 0 | 0 | 0 | TG 44:3 | 1 | 0 | 0 | 1 | 0 | TG 52:4 | 1 | 1 | 1 | 1 | 0 | TG 59:0 | 1 | 0 | 0 | 0 | 0 |
| TG 35:1 | 1 | 0 | 0 | 0 | 0 | TG 45:0 | 1 | 1 | 1 | 0 | 0 | TG 52:5 | 1 | 1 | 1 | 0 | 0 | TG 59:1 | 1 | 0 | 0 | 0 | 0 |
| TG 36:0 | 1 | 1 | 1 | 1 | 1 | TG 45:1 | 1 | 1 | 1 | 0 | 0 | TG 52:6 | 0 | 0 | 1 | 0 | 0 | TG 59:2 | 1 | 0 | 0 | 0 | 0 |
| TG 36:1 | 1 | 1 | 1 | 1 | 1 | TG 45:2 | 1 | 1 | 1 | 0 | 0 | TG 53:0 | 1 | 0 | 0 | 0 | 0 | TG 59:3 | 1 | 0 | 0 | 0 | 0 |
| TG 36:2 | 1 | 0 | 0 | 0 | 0 | TG 46:0 | 1 | 1 | 1 | 1 | 1 | TG 53:1 | 1 | 0 | 0 | 0 | 0 | TG 60:0 | 1 | 0 | 0 | 0 | 0 |
| TG 36:3 | 1 | 0 | 0 | 0 | 0 | TG 46:1 | 1 | 1 | 1 | 1 | 1 | TG 53:2 | 1 | 0 | 0 | 0 | 0 | TG 60:1 | 1 | 0 | 0 | 0 | 0 |
| TG 37:0 | 1 | 0 | 0 | 0 | 0 | TG 46:2 | 1 | 1 | 1 | 1 | 0 | TG 53:3 | 1 | 0 | 0 | 0 | 0 | TG 60:2 | 1 | 0 | 0 | 0 | 0 |
| TG 37:1 | 1 | 0 | 0 | 0 | 0 | TG 46:3 | 1 | 0 | 0 | 1 | 0 | TG 53:4 | 1 | 0 | 0 | 0 | 0 | TG 60:3 | 1 | 0 | 0 | 0 | 0 |
| TG 37:2 | 1 | 0 | 0 | 0 | 0 | TG 46:4 | 1 | 0 | 0 | 0 | 0 | TG 54:0 | 1 | 1 | 1 | 0 | 0 | TG 61:1 | 1 | 0 | 0 | 0 | 0 |
| TG 38:0 | 1 | 1 | 1 | 1 | 1 | TG 47:0 | 1 | 0 | 0 | 0 | 0 | TG 54:1 | 1 | 1 | 1 | 1 | 0 | TG 62:0 | 1 | 0 | 0 | 0 | 0 |
| TG 38:1 | 1 | 1 | 1 | 1 | 1 | TG 47:1 | 1 | 0 | 0 | 0 | 0 | TG 54:2 | 1 | 1 | 1 | 1 | 0 | TG 62:1 | 1 | 0 | 0 | 0 | 0 |
| TG 38:2 | 1 | 0 | 0 | 1 | 0 | TG 47:2 | 1 | 0 | 0 | 0 | 0 | TG 54:3 | 1 | 1 | 1 | 1 | 0 | TG 62:2 | 1 | 0 | 0 | 0 | 0 |
| TG 38:3 | 1 | 0 | 0 | 1 | 0 | TG 48:0 | 1 | 1 | 1 | 1 | 1 | TG 54:4 | 1 | 1 | 1 | 1 | 0 | Total | 141 | 64 | 60 | 42 | 21 |
| Rank (1 Being the Most Abundant) | TG Composition | Total Content (mol/100 mol) | Rank in J | Rank in HF |
|---|---|---|---|---|
| 1 | TG 36:0 | 7.2 | 1 | 4 |
| 2 | TG 38:0 | 5 | 2 | 1 |
| 3 | TG 34:0 | 4.8 | 6 | 17 |
| 4 | TG 50:1 | 3.7 | 13 | 6 |
| 5 | TG 48:1 | 2.9 | 10 | 8 |
| 6 | TG 40:0 | 2.2 | 5 | 3 |
| 7 | TG 38:1 | 2.2 | 3 | 5 |
| 8 | TG 32:0 | 1.9 | 20 | 29 |
| 9 | TG 52:2 | 1.8 | 17 | 13 |
| 10 | TG 48:0 | 1.6 | 18 | 20 |
| 11 | TG 40:1 | 1.4 | 4 | 2 |
| 12 | TG 46:1 | 1.3 | 11 | 11 |
| 13 | TG 46:0 | 1.3 | 14 | 15 |
| 14 | TG 52:1 | 1.1 | 15 | 10 |
| 15 | TG 44:0 | 1.1 | 9 | 14 |
| 16 | TG 42:0 | 1.1 | 7 | 9 |
| 17 | TG 36:1 | 1.1 | 16 | 22 |
| 18 | TG 50:0 | 1 | 22 | 19 |
| 19 | TG 30:0 | 0.9 | 27 | 32 |
| 20 | TG 50:2 | 0.8 | 21 | 16 |
| 21 | TG 44:1 | 0.7 | 12 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Meitei, N.S.; Gajbhiye, P.U.; Raftery, M.J.; Ambatipudi, K. Comparative Analysis of Milk Triglycerides Profile between Jaffarabadi Buffalo and Holstein Friesian Cow. Metabolites 2020, 10, 507. https://doi.org/10.3390/metabo10120507
Verma A, Meitei NS, Gajbhiye PU, Raftery MJ, Ambatipudi K. Comparative Analysis of Milk Triglycerides Profile between Jaffarabadi Buffalo and Holstein Friesian Cow. Metabolites. 2020; 10(12):507. https://doi.org/10.3390/metabo10120507
Chicago/Turabian StyleVerma, Aparna, Ningombam Sanjib Meitei, Prakash U. Gajbhiye, Mark J. Raftery, and Kiran Ambatipudi. 2020. "Comparative Analysis of Milk Triglycerides Profile between Jaffarabadi Buffalo and Holstein Friesian Cow" Metabolites 10, no. 12: 507. https://doi.org/10.3390/metabo10120507
APA StyleVerma, A., Meitei, N. S., Gajbhiye, P. U., Raftery, M. J., & Ambatipudi, K. (2020). Comparative Analysis of Milk Triglycerides Profile between Jaffarabadi Buffalo and Holstein Friesian Cow. Metabolites, 10(12), 507. https://doi.org/10.3390/metabo10120507





