Using an Untargeted Metabolomics Approach to Identify Salivary Metabolites in Women with Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Quality Control for Untargeted Metabolomics
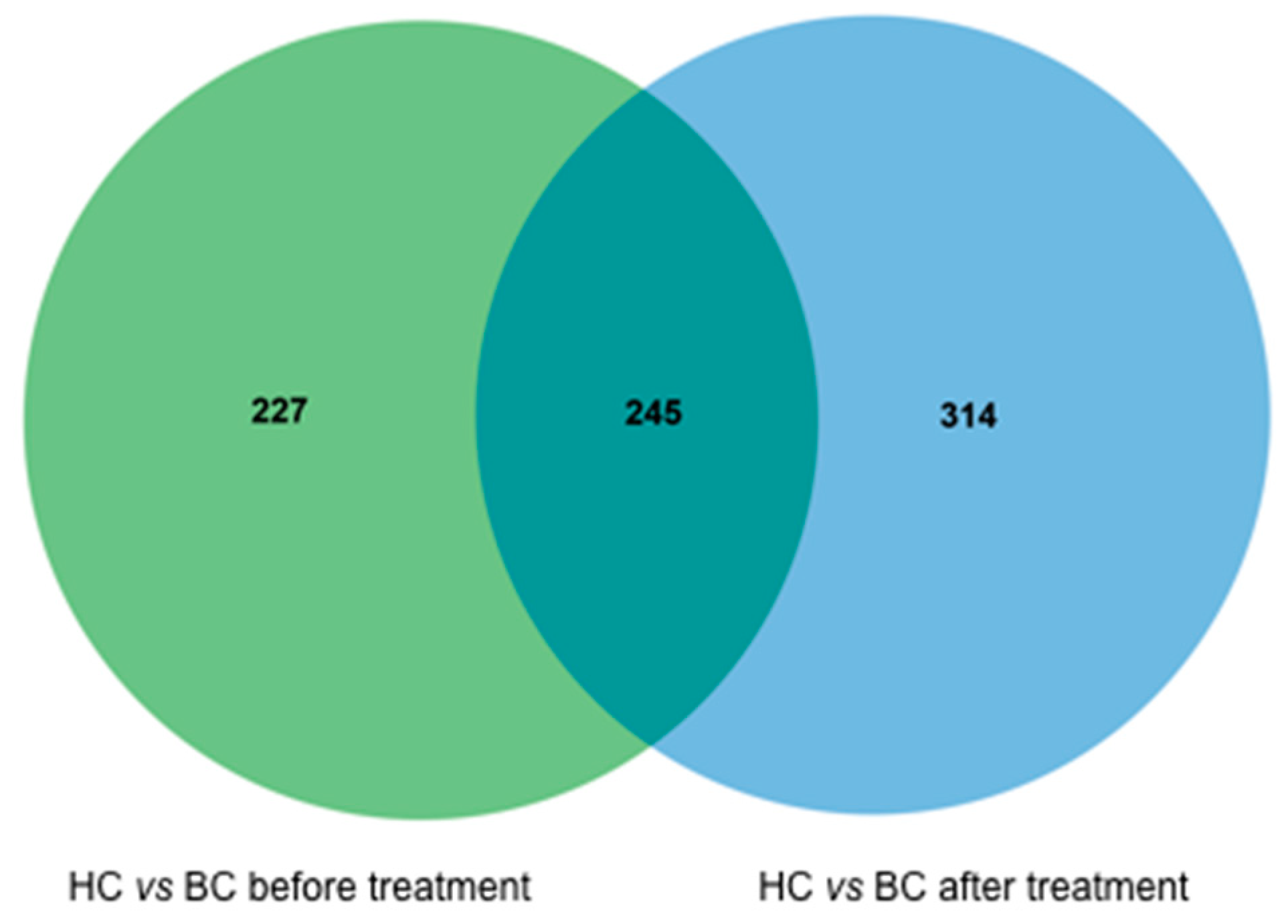
2.3. The LC/MS Profiles of the Healthy Controls and BC Patients
2.4. Comparing the LC/MS Profiles before and after Systemic Treatments
2.5. The Receiver Operating Characteristic Curve Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Study Participants
4.3. Specimen Collection, Transportation, and Preparation
4.4. Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (LC-Q-TOF/MS)
4.5. Metabolic Pathways
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Material
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Cho, K.; Uritboonthai, W.; Zhengijiang, Z.; Patti, G.; Siuzdak, G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012, 30, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault- Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.; Bhargavan, M.; Lewis, R.; Ioffe, O. Diagnostic accuracy of mammography, clinical examination, US, and MRI imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef]
- Silva, C.L.; Olival, A.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Untargeted Urinary 1H NMR-Based Metabolomic Pattern as a Potential Platform in Breast Cancer Detection. Metabolites 2019, 9, 269. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wei, L.; Li, J.; Status qu Zheng, Y.; Li, X. Status quo and development trend of breast biopsy technology. Gland Surg. 2013, 2, 15–24. [Google Scholar]
- Chiappin, S.; Antonelli, G.; Gatti, R.; Palo, E. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- De Almeida Pdel, V.; Gregio, A.M.; Machado, M.A.; Lima, A.; Azevedo, L. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Porto-Mascarenhas, E.C.; Assad, D.X.; Chardin, H.; Gozal, D.; Canto, G.; Acevedo, A.; Guerra, E. Salivary biomarkers in the diagnosis of breast cancer: A review. Crit. Rev. Oncol. Hematol. 2017, 110, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Benton, H.P.; Siuzdak, G. Bioinformatics: The next frontier of metabolomics. Anal. Chem. 2015, 87, 147–156. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef]
- Huan, T.; Forsberg, E.; Rinehart, D.; Jonhson, C.; Ivanisevich, J.; Benton, H.; Fang, M.; Aisporna, A.; Hilmers, B.; Poole, F.; et al. Systems biology guided by XCMS Online metabolomics. Nat. Methods 2017, 14, 461–462. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Zhang, A.; Song, J.; Chang, J.; Wang, K.; Zhang, Y.; Li, S.; Liu, H.; Alfranca, G.; et al. Salivary analysis based on surface enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. J. Biomed. Nanotechnol. 2018, 14, 1773–1784. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Tu, M.; Sugano, A.; Yamamori, I.; Iba, A.; Yusa, K.; Kaneko, M.; Ota, S.; et al. Effect of timing of collection of salivary metabolomic biomarkers on oral cancer detection. Amino Acids 2017, 49, 761–770. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Lin, J. Spectral analysis of human saliva for detection of lung cancer using surface-enhanced Raman spectroscopy. J. Biomed. Opt. 2012, 17, 037003. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.; Sherlin, H.J.; Ramani, P.; Premkumar, P.; Natesan, A.; Chandrasekar, T. Amino acid profile of saliva from patients with oral squamous cell carcinoma using high performance liquid chromatography. J. Oral Sci. 2012, 54, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Tsutsui, H.; Shimizu, I.; Toyama, T.; Yoshimoto, N.; Endo, Y.; Inoue, K.; Todoroki, K.; Min, J.; Mizuno, H.; et al. Diagnostic approach to breast cancer patients based on target metabolomics in saliva by liquid chromatography with tandem mass spectrometry. Clin. Chim. Acta 2016, 452, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, P.; Cheng, F.; Wang, X.; Duan, Y. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra-performance liquid chromatography-mass spectrometry. Talanta 2014, 119, 299–305. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef]
- Achalli, S.; Madi, M.; Babu, S.G.; Shetty, S.; Kumari, S.; Bhat, S. Sialic acid as a biomarker of oral potentially malignant disorders and oral cancer. Indian J. Dent. Res. 2017, 28, 395–399. [Google Scholar] [CrossRef]
- Almadori, G.; Bussu, F.; Galli, J.; Limongelli, A.; Persichilli, S.; Zappacosta, B.; Minucci, A.; Paludetti, G.; Giardina, B. Salivary glutathione and uric acid levels in patients with head and neck squamous cell carcinoma. Head Neck 2007, 29, 648–654. [Google Scholar] [CrossRef]
- Aps, J.K.; Martens, L.C. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef]
- Choi, B.H.; Coloff, J.L. The diverse functions of non-essential amino acids in cancer. Cancers 2019, 11, 675. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Z.; Huang, Y.; Duan, Y.; Wang, X. Investigation of salivary free amino acid profile for early diagnosis of breast cancer with ultra performance liquid chromatography-mass spectrometry. Clin. Chim. Acta 2015, 447, 23–31. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Dolan, M.E. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 2009, 15, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Cheng, F.; Lu, X.; Duan, Y.; Wang, X. Untargeted saliva metabonomics study of breast cancer based on ultra performance liquid chromatography coupled to mass spectrometry with HILIC and RPLC separations. Talanta 2016, 158, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.; Moore, M.; McLachlan, A.; Piquette-Miller, M.; Xu, H.; Clarke, S. Etnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin. Drug Metab. Toxicol. 2009, 3, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, J.X. Direct visualization of de novo lipogenesis in single living cells. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.; Vigh, L.; Sanchez-Magraner, L.; Fernandez, A.; Busquets, X.; Horvath, I.; Barcelo-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.; Liang, K.; Vasquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.; Simns, K.; Schevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef]
- Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual 2017. Available online: https://cancerstaging.org/Pages/default.aspx (accessed on 26 September 2020).
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
| Characteristic * | Breast Cancers (n = 23) | Healthy Controls (n = 35) | p-Value # |
|---|---|---|---|
| Mean age range (years) | 47.52 ± 9.79 | 42.00 ± 13.83 | 0.1028 |
| Menopause status | 0.7072 | ||
| Premenopause | 14 (60.87) | 23 (65.71) | |
| Menopause | 9 (39.13) | 12 (34.29) | |
| Tobacco use | 0.2019 | ||
| No | 19 (82.61) | 33 (94.29) | |
| Yes | 4 (17.39) | 2 (5.71) | |
| Use of medication | 0.2446 | ||
| No | 16 (69.57) | 19 (54.29) | |
| Yes | 7 (30.43) | 16 (45.71) | |
| Childbearing | 0.2663 | ||
| No | 8 (36.36) | 18 (51.43) | |
| Yes | 14 (63.64) | 17 (48.57) |
| Characteristic * | Breast Cancer Cases (n = 23) |
|---|---|
| Grade | |
| Grade 1 | 2 |
| Grade 2 | 13 |
| Grade 3 | 8 |
| Node status | |
| Node negative | 13 |
| Node positive | 10 |
| Stage | |
| Stage 1 | 2 |
| Stage 2 | 12 |
| Stage 3 | 5 |
| Stage 4 | 4 |
| ER status | |
| ER positivity ≥ 10% | 14 |
| ER positivity < 10% | 9 |
| PR status | |
| PR positivity ≥ 10% | 13 |
| PR positivity < 10% | 10 |
| HER2 status | |
| HER2 positive | 11 |
| HER2 negative | 12 |
| KI67 status | |
| KI67 ≤ 20% | 9 |
| KI67 > 20% | 14 |
| Molecular profile | |
| Luminal A-like | 5 |
| Luminal B HER2 negative-like | 8 |
| Luminal B HER2 positive-like | 3 |
| HER2 positive (non-luminal)-like | 3 |
| Triple negative | 3 |
| -Metabolite Number | m/z | Retention Time | Fold Change (a) | p-Value | Raw Formula | Putative METLIN Identification (b) | Mw g/mole (Error in ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 380.22 | 3.0 | 1.8 | 0.03 | C24H29NO3 | Donazepil | 379.2147 (1) |
| 2 | 457.23 | 2.5 | 3.1 | 0.003 | C45H72O16 | Dioscin or Desglucomusenin | 868.482 (1) |
| 3 | 622.33 | 3.1 | 2.3 | 0.04 | C32H40N6O6 | Dilazep or H-Thr-Trp-Trp-(Ile/Leu)-OH or H-Pro-Arg-Arg-Arg-OH | 604.3009 (1) |
| 4 | 633.34 | 3.0 | 2.2 | 0.02 | C38H48O8 | Tetrahydrogambogic acid | 632.3349 (1) |
| 5 | 440.23 | 3.4 | 3.7 | 0.03 | C15H31N9O5 | H-Arg-Arg-Ser-OH | 417.2448 (3) |
| 6 | 442.24 | 3.1 | 2.1 | 0.01 | C18H31N7O6 | H-His-Lys-(Ala-Ser)-OH or (Gly-Thr)-OH | 441.2335 (1) |
| 7 | 543.23 | 54.8 | 2.2 | 0.03 | C26H31N5O5S | H-Ala-Lys-Phe-Trp-OH or H-Gly-Lys-Thr-Ser-OH or H-Arg-Arg-Ser-Ser-OH | 525.2045 (1) |
| 8 | 585.31 | 15.7 | 3.1 | 0.02 | C26H42N8O6 | H-Phe-Ile-Gln-Arg-OH | 562.3227 (2) |
| 9 | 596.31 | 3.0 | 2.7 | 0.03 | C25H38N8O8 | H-Glu-Phe-Gln-Arg-OH or H-Ile-Lys-Gln-Trp-OH | 578.2812 (1) |
| 10 | 630.33 | 3.1 | 1.9 | 0.04 | C32H45N7O5 | H-Phe-Lys-Lys-Trp-OH or H-Phe-Gln-Arg-Tyr-OH | 607.3482 (1) |
| 11 | 644.31 | 3.0 | 3.0 | 0.02 | C34H38N6O6 | H-Phe-Phe-Gln-Trp-OH | 626.2852 (1) |
| 12 | 669.42 | 36.9 | 2.1 | 0.04 | C35H67O7P | PA 32:1 (c) | 630.4624 (5) |
| 13 | 718.39 | 19.4 | 6.3 | 0.01 | C34H66NO10P | PS 28:0 (c) | 679.4423 (9) |
| 14 | 817.46 | 2.4 | 3.5 | 0.04 | C40H75O12P | PI 31:1 (c) | 778.4996 (9) |
| 15 | 874.49 | 2.5 | 2.9 | 0.03 | C46H78NO10P | PS 40:6 (c) | 835.5362 (7) |
| 16 | 919.47 | 3.0 | 2.5 | 0.02 | C47H77O13P | PI 38:7 (c) | 880.5101 (1) |
| 17 | 533.22 | 2.5 | 2.4 | 0.01 | C23H43O9P | PG 14:2 (c) | 494.2644 (1) |
| Metabolites | AUC | CI 95% | Optimal Cutoff Value | Sensitivity | CI 95% | Specificity | CI 95% |
|---|---|---|---|---|---|---|---|
| PG14:2 | 0.7329 | 0.5962–0.8697 | 19,325.80 | 65.22% | 42.77–83.62% | 77.14% | 59.86–89.58% |
| PA 32:1 | 0.5988 | 0.4319–0.7656 | 1915.30 | 60.87% | 38.54–80.29% | 60.00% | 42.11–76.23% |
| PS 28:0 | 0.6273 | 0.4644–0.7902 | 25,012.90 | 47.83% | 26.82–69.41% | 88.57% | 73.26–96.80% |
| PI 31:1 | 0.5876 | 0.4250–0.7502 | 37,403.88 | 43.48% | 23.19–65.51% | 82.86% | 66.35–93.44% |
| PS 40:6 | 0.5950 | 0.4357–0.7544 | 12,784.24 | 56.52% | 34.49–76.81% | 57.14% | 39.35–73.68% |
| PI 38:7 | 0.6609 | 0.5132–0.8085 | 1951.16 | 60.87% | 38.54–80.29% | 71.43% | 53.70–85.36% |
| H-His-Lys-(Ala-Ser)-OH or (Gly-Thr)-OH | 0.7280 | 0.5962–0.8597 | 6274.70 | 73.91% | 55.97–91.86% | 62.86% | 46.85–78.86% |
| H-Phe-Phe-Gln-Trp-OH | 0.7478 | 0.6113–0.8844 | 1961.38 | 73.91% | 55.97–91.86% | 74.29% | 59.81–88.77% |
| H-Phe-Ile-Gln-Arg-OH | 0.6795 | 0.5373–0.8218 | 2406.38 | 69.57% | 50.76–88.37% | 68.57% | 53.19–83.95% |
| H-Arg-Arg-Ser-OH | 0.6621 | 0.5137–0.8105 | 4842.48 | 73.91% | 55.97–91.86% | 51.43% | 34.87–67.99% |
| H-Glu-Phe-Gln-Arg-OH or H-Ile-Lys-Gln-Trp-OH | 0.6565 | 0.4961–0.8170 | 5926.30 | 60.87% | 40.92–80.81% | 80.00% | 66.75–93.25% |
| H-Phe-Lys-Lys-Trp-OH or H-Phe-Gln-Arg-Tyr-OH | 0.6783 | 0.5356–0.8209 | 6499.44 | 56.52% | 36.26–76.78% | 74.29% | 59.81–88.77% |
| H-Ala-Lys-Phe-Trp_OH or H-Gly-Lys-Thr-Ser-OH or H-Arg-Arg-Ser-Ser-OH | 0.6497 | 0.5028–0.7966 | 3266.77 | 60.87% | 40.92–80.81% | 62.86% | 46.85–78.86% |
| m/z 602.96 | 0.6584 | 0.5005–0.8163 | 6006.47 | 56.52% | 36.26–76.78% | 71.43% | 56.46–86.39% |
| m/z 749.4 | 0.6696 | 0.5186–0.8205 | 3542.90 | 65.22% | 45.75–84.68% | 65.71% | 49.99–81.44% |
| m/z 456.72 | 0.7441 | 0.6080–0.8802 | 101,578.83 | 69.57% | 50.76–88.37% | 74.29% | 59.81–88.77% |
| m/z 475.70 | 0.7391 | 0.5979–0.8803 | 7027.85 | 65.22% | 45.75–84.68% | 74.29% | 59.81–8877% |
| m/z 467.71 | 0.7702 | 0.6473–0.8931 | 8138.34 | 73.91% | 55.97–91.86% | 65.71% | 49.99–81.44% |
| m/z 614.32 | 0.6665 | 0.5135–0.8194 | 11,030.99 | 52.17% | 31.76–72.59% | 77.14% | 63.23–91.05% |
| m/z 457.73 | 0.6497 | 0.5132–0.8085 | 11,204.51 | 60.87% | 40.92–80.81% | 60.00% | 43.77–76.23% |
| m/z 356.69 | 0.6845 | 0.5384–0.8306 | 2956.67 | 47.83% | 27.41–68.24% | 82.86% | 70.37–95.34% |
| m/z 467.71 | 0.6857 | 0.5452–0.8262 | 12,496.76 | 73.91% | 55.97–91.86% | 60.00% | 43.77–76.23% |
| m/z 534.23 | 0.6826 | 0.5389–0.8263 | 3642.90 | 65.22% | 45.75–84.68% | 65.71% | 49.99–81.44% |
| m/z 1015.04 | 0.6609 | 0.5103–0.8114 | 9239.95 | 65.22% | 45.75–84.68% | 68.57% | 53.19–83.95% |
| m/z 413.22 | 0.6783 | 0.5365–0.8200 | 2335.12 | 73.91% | 55.97–91.86% | 57.14% | 40.75–73.54% |
| m/z 602.32 | 0.6646 | 0.5182–0.8110 | 4543.59 | 60.87% | 40.92–80.81% | 65.71% | 49.99–81.44% |
| m/z 661.34 | 0.7025 | 0.5670–0.8380 | 1690.58 | 69.57% | 50.76–88.37% | 65.71% | 49.99–81.44% |
| m/z 875.49 | 0.5652 | 0.4024–0.7281 | 14,840.39 | 39.13% | 19.19–59.08% | 77.14% | 63.23–91.05% |
| m/z 594.82 | 0.6857 | 0.4024–0.7281 | 619.06 | 86.96% | 73.19–100.00% | 51.43% | 34.87–67.99% |
| m/z 614.82 | 0.8124 | 0.4024–0.7281 | 2749.30 | 82.61% | 67.12–98.10% | 77.14% | 63.23–91.05% |
| m/z 818.47 | 0.6186 | 0.4622–0.7751 | 7969.76 | 60.87% | 40.92–80.81% | 62.86% | 46.85–78.86% |
| METLIN Identification | Related Pathway |
|---|---|
| H-Arg-Arg-Ser-OH | N/S |
| H-His-Lys-(Ala-Ser)-OH or (Gly-Thr)-OH | N/S |
| H-Ala-Lys-Phe-Trp-OH Or H-Gly-Lys-Thr-Ser-OH or H-Arg-Arg-Ser-Ser-OH | N/S |
| H-Phe-Ile-Gln-Arg-OH | N/S |
| H-Glu-Phe-Gln-Arg-OH or H-Ile-Lys-Gln-Trp-OH | N/S |
| H-Phe-Lys-Lys-Trp-OH or H-Phe-Gln-Arg-Tyr-OH | N/S |
| H-Phe-Phe-Gln-Trp-OH | N/S |
| PA 32:1 | Triacylglycerol Biosynthesis; Cardiolipin biosynthesis; Glycerophospholipid metabolism |
| PS 28:0 | Phosphatidylcholine biosynthesis, Phosphatidylethanolamine biosynthesis, Glycerophospholipid metabolism and Lipid metabolism pathway |
| PI 31:1 | N/F |
| PS 40:6 | Phosphatidylcholine biosynthesis, Phosphatidylethanolamine biosynthesis, Glycerophospholipid metabolism and Lipid metabolism pathway |
| PI 38:7 | Lysolipid incorporation into ER pathway, Phosphatidylcholine biosynthesis, Phosphatidylethanolamine biosynthesis, Glycerophospholipid metabolism and Lipid metabolism pathway |
| PG 14:2 | Glycerophospholipid metabolism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier Assad, D.; Acevedo, A.C.; Cançado Porto Mascarenhas, E.; Costa Normando, A.G.; Pichon, V.; Chardin, H.; Neves Silva Guerra, E.; Combes, A. Using an Untargeted Metabolomics Approach to Identify Salivary Metabolites in Women with Breast Cancer. Metabolites 2020, 10, 506. https://doi.org/10.3390/metabo10120506
Xavier Assad D, Acevedo AC, Cançado Porto Mascarenhas E, Costa Normando AG, Pichon V, Chardin H, Neves Silva Guerra E, Combes A. Using an Untargeted Metabolomics Approach to Identify Salivary Metabolites in Women with Breast Cancer. Metabolites. 2020; 10(12):506. https://doi.org/10.3390/metabo10120506
Chicago/Turabian StyleXavier Assad, Daniele, Ana Carolina Acevedo, Elisa Cançado Porto Mascarenhas, Ana Gabriela Costa Normando, Valérie Pichon, Helene Chardin, Eliete Neves Silva Guerra, and Audrey Combes. 2020. "Using an Untargeted Metabolomics Approach to Identify Salivary Metabolites in Women with Breast Cancer" Metabolites 10, no. 12: 506. https://doi.org/10.3390/metabo10120506
APA StyleXavier Assad, D., Acevedo, A. C., Cançado Porto Mascarenhas, E., Costa Normando, A. G., Pichon, V., Chardin, H., Neves Silva Guerra, E., & Combes, A. (2020). Using an Untargeted Metabolomics Approach to Identify Salivary Metabolites in Women with Breast Cancer. Metabolites, 10(12), 506. https://doi.org/10.3390/metabo10120506






