Effects of Dietary Defatted Meat Species on Metabolomic Profiles of Murine Liver, Gastrocnemius Muscle, and Cecal Content
Abstract
1. Introduction
2. Results
2.1. Growth Parameters and Tissue Weights
2.2. Targeted and Non-Targeted Metabolomic Analysis
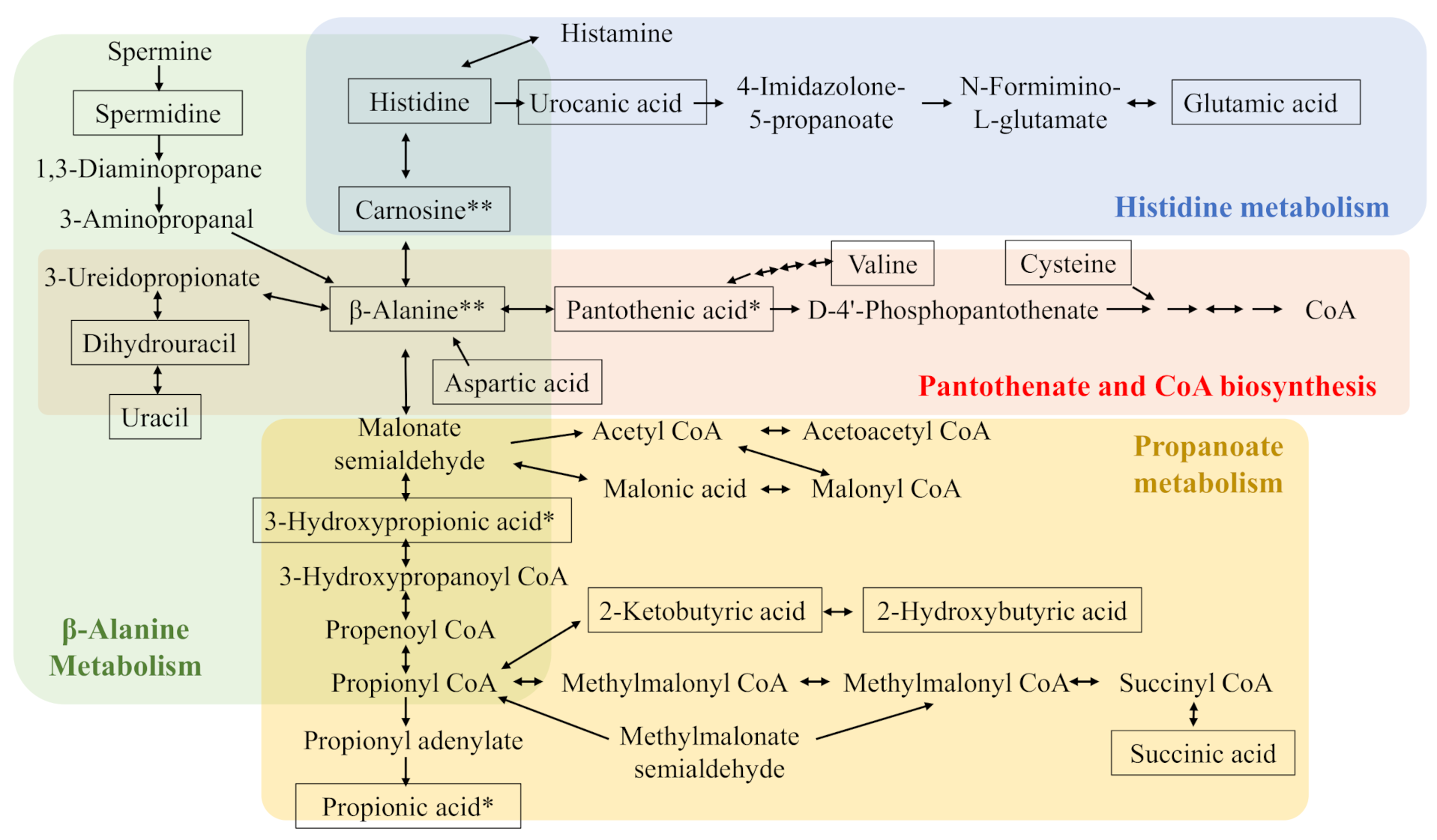
2.2.1. Quantitative Analysis of Free Amino Acids in the Liver and Gastrocnemius Muscle
2.2.2. Quantitative Analysis of Short-Chained Fatty Acids in Cecal Content
2.2.3. Non-Targeted and Semi-Quantified Analyses of Metabolites in the Liver, Gastrocnemius Muscle, and Cecal Content
2.2.4. Integrated Analyses of Quantified and Semi-Quantified Metabolites
2.2.5. Carnosine, Anserine, and Taurine Levels in Experimental Protein Sources
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Gas Chromatography/Mass Spectrometry (GC–MS/MS)-Based Non-Targeted Metabolomic Analysis
4.3. GC–MS-Based Free Amino Acid Analysis
4.4. GC–MS/MS-Based Short-Chain Fatty Acid Analysis
4.5. Amino Acid Composition, Imidazole Dipeptides, and Taurine Quantification in Protein Sources
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gilbert, J.-A.; Bendsen, N.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. S2), B16–B31. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of Dairy Proteins on Appetite, Energy Expenditure, Body Weight, and Composition: A Review of the Evidence from Controlled Clinical Trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Inoue, M.; Tsugane, S.; et al. Meat Consumption and Colorectal Cancer Risk: An Evaluation Based on a Systematic Review of Epidemiologic Evidence Among the Japanese Population. Jpn. J. Clin. Oncol. 2014, 44, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, A.M.A.; Toldrá, F. Hydrophilic Chromatographic Determination of Carnosine, Anserine, Balenine, Creatine, and Creatinine. J. Agric. Food Chem. 2007, 55, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, R.; Miyazaki, M.; Miyazaki, T.; Shigeno, Y.; Tokairin, Y.; Konno, H.; Yamashita, T. LC-ESI-MS/MS quantification of carnosine, anserine, and balenine in meat samples. J. Chromatogr. B 2019, 1132, 121826. [Google Scholar] [CrossRef] [PubMed]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Moscow) 2000, 65, 757–765. [Google Scholar]
- Stegen, S.; Stegen, B.; Aldini, G.; Altomare, A.; Cannizzaro, L.; Orioli, M.; Gerlo, S.; Deldicque, L.; Ramaekers, M.; Hespel, P.; et al. Plasma carnosine, but not muscle carnosine, attenuates high-fat diet-induced metabolic stress. Appl. Physiol. Nutr. Metab. 2015, 40, 868–876. [Google Scholar] [CrossRef]
- Tomonaga, S.; Yamane, H.; Onitsuka, E.; Yamada, S.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Carnosine-induced antidepressant-like activity in rats. Pharmacol. Biochem. Behav. 2008, 89, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawalha, N.A.; Alshogran, O.Y.; Awawdeh, M.S.; Almomani, B.A. The effects of l-Carnosine on development of metabolic syndrome in rats. Life Sci. 2019, 237, 116905. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Nan, C.; Tian, J.; Jean-Charles, P.; Li, Y.; Weissbach, H.; Huang, X.-P. Protective effects of taurine against oxidative stress in the heart of MsrA knockout mice. J. Cell. Biochem. 2012, 113, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Furuse, M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: Antidepressant versus anxiolytic-like effects. Amino Acids 2010, 39, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Murakami, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Dietary animal proteins alter monoamine metabolism in the brain. Anim. Sci. J. 2011, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, M.; Wakamatsu, J.-I.; Takahata, Y.; Hasegawa, T.; Morimatsu, F.; Nishimura, T. Diet-Induced Thermogenesis and Expression Levels of Thyroid Hormone Target Genes and Their Products in Rats Differ between Meat Proteins. J. Nutr. Sci. Vitaminol. 2016, 62, 93–100. [Google Scholar] [CrossRef]
- Nakata, R.; Sato, M.; Tomonaga, S. The effect of dietary carnosine supplementation on metabolite levels in the liver and cecal content of mice. Trace Nutr. Res. 2019, 36, 66–72. [Google Scholar]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamada, K.-I.; Uchida, K. 2-Oxo-histidine–containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef]
- Nakamura, S.; Tanabe, K.; Yoshinaga, K.; Shimura, F.; Oku, T. Effects of 1,5-anhydroglucitol on postprandial blood glucose and insulin levels and hydrogen excretion in rats and healthy humans. Br. J. Nutr. 2017, 118, 81–91. [Google Scholar] [CrossRef][Green Version]
- Shimada, M.; Hibino, M.; Takeshita, A. Dietary supplementation with myo -inositol reduces hepatic triglyceride accumulation and expression of both fructolytic and lipogenic genes in rats fed a high-fructose diet. Nutr. Res. 2017, 47, 21–27. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, J.-I.; Fujii, R.; Yamaguchi, K.; Miyoshi, S.; Nishimura, T.; Hattori, A. Effects of meat species on the postprandial thermic effect in rats. Anim. Sci. J. 2012, 84, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kanazawa, M.; Ogiwara, A.; Arita, M. MRMPROBS suite for metabolomics using large-scale MRM assays. Bioinformatics 2014, 30, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
| Casein | Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | ANOVA | Metab. No. | |
|---|---|---|---|---|---|---|---|
| Acetic acid | 20.42 ± 0.97 | 17.24 ± 1.85 | 18.00 ± 1.98 | 23.29 ± 1.42 | 21.19 ± 1.38 | NS | - |
| Propionic acid | 3.48 ± 0.28 a | 1.96 ± 0.30 b | 1.97 ± 0.32 b | 3.76 ± 0.24 a | 3.44 ± 0.58 a,b | <0.05 | 6 |
| Butyric acid | 7.09 ± 0.42 b | 8.7 ± 0.93 a,b | 8.94 ± 0.37 a,b | 7.83 ± 0.36 b | 10.73 ± 0.91 a | <0.05 | - |
| Casein | Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | ANOVA | Metab. No. | |
|---|---|---|---|---|---|---|---|
| 1,5-Anhydro-glucitol | 111 ± 4 a,b | 97 ± 5 b,c | 129 ± 8 a | 87 ± 4 c | 76 ± 5 c | <0.0001 | - |
| 2-Hydroxyisovaleric acid | 128 ± 9 a | 80 ± 3 c | 105 ± 5 a,b | 99 ± 2 b,c | 88 ± 6 b,c | <0.0001 | - |
| β-Alanine | 85 ± 5 c | 88 ± 3 b,c | 117 ± 4 a | 100 ± 5 a,b,c | 111 ± 9 a,b | <0.05 | 6, 7, 15, 20 |
| Carnosine | 7 ± 1 d | 125 ± 21 b | 242 ±30 a | 39 ± 5 c,d | 86 ± 19 b,c | <0.0001 | 7, 15 |
| Dimethylglycine | 90 ± 10 a,b | 115 ± 14 a,b | 83 ± 10 a,b | 76 ± 4 b | 136 ± 24 a | <0.05 | - |
| Glyoxylic acid | 91 ± 10 a,b | 114 ± 14 a,b | 83 ± 9 a,b | 76 ± 3 b | 136 ± 25 a | <0.05 | - |
| Inositol | 82 ± 4 b | 96 ± 4 b | 101 ± 2 a,b | 121 ± 4 a | 101 ± 8 a,b | <0.05 | - |
| Hydroxyproline | 98 ± 4 b | 91 ± 2 b | 104 ± 4 a,b | 120 ± 7 a | 87 ± 5 b | <0.05 | - |
| Sorbitol | 86 ± 13 b | 86 ± 14 b | 92 ± 11 a,b | 137 ± 11 a | 98 ± 9 a,b | <0.05 | - |
| Tartaric acid | 61 ± 8 c | 95 ± 14 a,b,c | 129 ±21 a,b | 143 ± 21 a | 72 ± 7 b,c | <0.05 | - |
| Taurine | 95 ± 6 | 94 ± 5 | 105 ± 3 | 110 ± 4 | 97 ± 7 | NS | 22 |
| Xylitol | 95 ± 4 a,b | 93 ± 6 b | 100 ± 2 a,b | 115 ± 4 a | 96 ± 7 a,b | <0.05 | - |
| Casein | Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | ANOVA | Metab. No. | |
|---|---|---|---|---|---|---|---|
| 1,5-Anhydro-glucitol | 110 ± 9 a,b | 94 ± 2 b | 123 ± 5 a | 88 ± 5 b | 86 ± 7 b | <0.05 | - |
| β-Alanine | 100 ± 10 | 96 ± 10 | 104 ± 10 | 98 ± 6 | 102 ± 10 | NS | 6, 7, 15, 20 |
| Carnosine | 119 ± 21 | 81 ± 17 | 86 ± 14 | 105 ± 22 | 108 ± 26 | NS | 7, 15 |
| Inositol | 83 ± 9 b | 104 ± 4 a,b | 97 ± 4 b | 123 ± 6 a | 93 ± 3 b | <0.05 | - |
| Taurine | 99 ± 3 | 99 ± 1 | 101 ± 2 | 102 ± 4 | 100 ± 1 | NS | 22 |
| Casein | Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | ANOVA | Metab. No. | |
|---|---|---|---|---|---|---|---|
| 2-Aminobutyric acid | 114 ± 12 a,b | 72 ± 9 b | 65 ± 13 b | 147 ± 26 a | 103 ± 23 a,b | <0.05 | 5 |
| 3-Hydroxypropionic acid | 83 ± 6 b | 127 ± 8 a | 90 ± 3 b | 104 ± 9 a,b | 97 ± 9 a,b | <0.05 | 6, 7 |
| 3-Methyl-2-oxovaleric acid | 80 ± 8 b | 82 ± 3 b | 99 ± 14 ab | 138 ± 12 a | 101 ± 21 a,b | <0.05 | - |
| 3-Sulfinoalanine | 55 ± 8 b | 88 ± 14 b | 103 ± 15 a,b | 167 ± 27 a | 87 ± 14 b | <0.05 | 5, 22 |
| β-Alanine | 20 ± 3 b | 268 ± 30 a | 86 ± 14 b | 42 ± 4 b | 83 ± 18 b | <0.0001 | 6, 7, 15, 20 |
| Carnosine | 0 ± 0 b | 289 ± 53 a | 180 ± 33 a | 4 ± 1 b | 27 ± 11 b | <0.0001 | 7, 15 |
| Galactose | 148 ± 39 a | 48 ± 7 b | 77 ± 10 a,b | 153 ± 29 a | 74 ± 8 a,b | <0.05 | - |
| Glutaric acid | 85 ± 16 b | 154 ± 20 a | 104 ± 10 a,b | 83 ± 5 b | 74 ± 5 b | <0.05 | - |
| Glycerol 3-phosphate | 74 ± 13 a,b | 116 ± 17 a,b | 129 ± 14 a | 62 ± 14 b | 118 ± 18 a,b | <0.05 | - |
| Glycine | 106 ± 13 a,b | 97 ± 16 a,b | 72 ± 13 b | 137 ± 13 a | 88 ± 9 ab | <0.05 | - |
| Homocysteine | 134 ± 16 a | 87 ± 12 a,b | 100 ± 9 a,b | 61 ± 4 b | 117 ± 15 a | <0.05 | 5 |
| Mannose 6-phosphate | 64 ± 14 b | 94 ± 14 a,b | 124 ± 21 a,b | 68 ± 19 b | 151 ± 26 a | <0.05 | - |
| N-Acetylaspartic acid | 33 ± 8 c | 56 ± 11 b,c | 142 ± 28 a,b | 163 ± 22 a | 107 ± 36 a,b,c | <0.05 | - |
| N-Acetylglutamine | 32 ± 7 c | 30 ± 4 c | 150 ± 32 a,b | 200 ± 13 a | 89 ± 40 b,c | <0.0001 | - |
| Nicotinamide | 16 ± 1 d | 106 ± 6 b | 226 ± 13 a | 86 ± 5 bc | 65 ± 12 c | <0.0001 | - |
| Pantothenic acid | 120 ± 18 a,b | 77 ± 8 b | 70 ± 7 b | 137 ± 18 a | 95 ± 13 a,b | <0.05 | 20 |
| Phenylacetic acid | 42 ± 5 b | 97 ± 10 b | 100 ± 18 b | 174 ± 17 a | 86 ± 16 b | <0.0001 | - |
| Ribose | 102 ± 10 a,b | 74 ± 8 b | 120 ± 5 a | 109 ± 8 a,b | 95 ± 13 a,b | <0.05 | - |
| Taurine | ND | ND | ND | ND | ND | - | 22 |
| Metab. No. | Pathway Name | Liver | Gastrocnemius Muscle | Cecal Content |
|---|---|---|---|---|
| 1 | Aminoacyl-tRNA biosynthesis | * | ** | ** |
| 2 | Glycine, serine, and threonine metabolism | * | * | * |
| 3 | Valine, leucine, and isoleucine biosynthesis | ** | ** | |
| 4 | Valine, leucine, and isoleucine degradation | * | * | |
| 5 | Cysteine and methionine metabolism | * | * | |
| 6 | Propanoate metabolism | * | * | |
| 7 | β-Alanine metabolism | * | * | |
| 8 | Fructose and mannose metabolism | * | * | |
| 9 | Glycerolipid metabolism | * | * | |
| 10 | Alanine, aspartate, and glutamate metabolism | * | ||
| 11 | Arginine and proline metabolism | * | ||
| 12 | Butanoate metabolism | * | ||
| 13 | Glutamate metabolism | * | ||
| 14 | Glyoxylate and dicarboxylate metabolism | * | ||
| 15 | Histidine metabolism | * | ||
| 16 | Phenylalanine, tyrosine, and tryptophan biosynthesis | * | ||
| 17 | Phenylalanine metabolism | * | ||
| 18 | Amino sugar and nucleotide sugar metabolism | * | ||
| 19 | Galactose metabolism | * | ||
| 20 | Pantothenate and CoA biosynthesis | * | ||
| 21 | Pentose phosphate pathway | * | ||
| 22 | Taurine and hypotaurine metabolism | * |
| Casein | Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | |
|---|---|---|---|---|---|
| Carnosine | ND | 11,245 | 25,742 | 4763 | 9021 |
| Anserine | ND | 2846 | 1040 | 10,615 | 26,601 |
| Taurine | ND | 1246 | 2034 | 6421 | 1123 |
| Beef Leg | Pork Leg | Chicken Leg | Chicken Breast | ANOVA | |
|---|---|---|---|---|---|
| Carnosine | 451 ± 12 b | 963 ± 20 a | 179 ± 3 d | 342 ± 7 c | <0.0001 |
| Anserine | 114 ± 3 c | 39 ± 1 d | 400 ± 7 b | 1009 ± 21 a | <0.0001 |
| Taurine | 50 ± 1 c | 76 ± 2 b | 242 ± 4 a | 43 ± 1 c | <0.0001 |
| Metab. No. | Pathway Name | Cecal Content |
|---|---|---|
| 1 | Aminoacyl-tRNA biosynthesis | ** |
| 2 | Glycine, serine and threonine metabolism | * |
| 3 | Valine, leucine and isoleucine biosynthesis | ** |
| 5 | Cysteine and methionine metabolism | * |
| 6 | Propanoate metabolism | * |
| 8 | Fructose and mannose metabolism | * |
| 10 | Alanine, aspartate and glutamate metabolism | * |
| 14 | Glyoxylate and dicarboxylate metabolism | * |
| 16 | Phenylalanine, tyrosine and tryptophan biosynthesis | * |
| 17 | Phenylalanine metabolism | * |
| 18 | Amino sugar and nucleotide sugar metabolism | * |
| 20 | Pantothenate and CoA biosynthesis | * |
| 22 | Taurine and hypotaurine metabolism | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, R.; Sato, M.; Tomonaga, S. Effects of Dietary Defatted Meat Species on Metabolomic Profiles of Murine Liver, Gastrocnemius Muscle, and Cecal Content. Metabolites 2020, 10, 503. https://doi.org/10.3390/metabo10120503
Nakata R, Sato M, Tomonaga S. Effects of Dietary Defatted Meat Species on Metabolomic Profiles of Murine Liver, Gastrocnemius Muscle, and Cecal Content. Metabolites. 2020; 10(12):503. https://doi.org/10.3390/metabo10120503
Chicago/Turabian StyleNakata, Rise, Mikako Sato, and Shozo Tomonaga. 2020. "Effects of Dietary Defatted Meat Species on Metabolomic Profiles of Murine Liver, Gastrocnemius Muscle, and Cecal Content" Metabolites 10, no. 12: 503. https://doi.org/10.3390/metabo10120503
APA StyleNakata, R., Sato, M., & Tomonaga, S. (2020). Effects of Dietary Defatted Meat Species on Metabolomic Profiles of Murine Liver, Gastrocnemius Muscle, and Cecal Content. Metabolites, 10(12), 503. https://doi.org/10.3390/metabo10120503






