Abstract
In both humans and animals, meat not only constitutes one of the sources of protein, but also includes various water-soluble bioactive substances such as imidazole peptides (carnosine and anserine) and taurine. Previous studies demonstrated that dietary meat species could differently affect physiological functions; however, the mechanisms of this remain unclear. To explore the physiological effects of dietary defatted meat species, especially on metabolism, we investigated their influence on the metabolomic profiles of the liver, gastrocnemius muscle, and cecal content in mice. Casein (control) or a defatted meat species (beef leg, pork leg, chicken leg, or chicken breast) was supplied as the major protein source in the diet for four weeks, and metabolism-related molecules were measured by gas chromatography–mass spectrometry. We found that various metabolite levels in tissues and cecal content differed according to the types of dietary protein consumed. Specifically, differences in carnosine, 1,5-anhydro-glucitol, inositol, butyric acid, and propionic acid were clearly observed. Among them, the highest carnosine intake by dietary pork leg was clearly related to the highest carnosine level in the liver. In addition, taurine intake was suggested to be linked to some metabolic pathways including taurine and hypotaurine metabolism in cecal content. These results provide additional knowledge of the effects of different dietary meat species on human and animal health.
1. Introduction
The intricate relationship between dietary habits and health is well known, including the role of diet in obesity and metabolic disorders in both humans and animals. Dietary proteins have various effects depending on their origin and quantity [1,2]. Especially in humans, meat is one of the major sources of dietary protein; as such, much attention has been paid to the relationship between meat consumption and health [3,4].
In addition to being a source of protein, meat also includes water-soluble bioactive substances such as imidazole peptides (carnosine and anserine) [5,6] and taurine [7]. Carnosine, a dipeptide composed of β-alanine and L-histidine, has antioxidant properties [8], buffering capacity [9], anti-inflammatory activity [10], anti-depressant-like effects [11], and ameliorative effects on the manifestations of metabolic syndrome [12]. Anserine, a dipeptide composed of β-alanine and 1-methyl-L-histidine, also has antioxidant properties [8] and buffering capacity [9]. Taurine is an antioxidant [13] and has anti-depressant properties [14].
The dietary effects of meat species have previously been investigated. Nagasawa et al. [15] compared the dietary effects of soy protein and defatted beef, pork, and chicken in mice, and found that monoamine metabolism in the brain was influenced by the type of dietary meat. They suggested that these effects were independent of the amino acid composition of dietary protein sources. Another study found that dietary chicken or mutton protein induced stronger thermogenesis than did beef, pork, or horse in rats [16]; this was also independent of the amino acid composition of the dietary protein sources. However, neither of these studies clarified the mechanisms involved in eliciting these observed differences. Known and/or unknown bioactive substances may be involved in these actions. Because dietary components can interact with various metabolic pathways in the body, we speculated that using a metabolomics-based approach would be effective in elucidating the various effects of dietary meat sources on bodily functions.
Therefore, we performed this study to investigate dietary effects of defatted meat species on the metabolomic profiles of the liver, gastrocnemius muscle, and cecal content of mice. For analytical samples, liver, gastrocnemius muscle, and cecal content were selected because they have various important roles in the metabolism and/or health of animals.
2. Results
2.1. Growth Parameters and Tissue Weights
Casein (control) or defatted meat species (beef leg, pork leg, chicken leg, or chicken breast) were fed to mice as their major dietary protein sources for four weeks. We found that the body weights, total food intake, and weights of the liver, brain, lung, gastrocnemius muscle, inguinal fat, epididymal fat, perirenal fat, and brown fat did not differ according to the ingested dietary protein type (Table S1).
2.2. Targeted and Non-Targeted Metabolomic Analysis
2.2.1. Quantitative Analysis of Free Amino Acids in the Liver and Gastrocnemius Muscle
Of the 25 amino acids quantified in the liver, the levels of six (alanine, 2-aminoadipic acid, 2-aminobutyric acid, glutamic acid, glycine, and proline) were influenced by the type of dietary protein (Table S2). These six amino acid levels clearly differed between mice fed casein and those fed meat from any source; however, they did not differ according to the type of ingested meat protein.
Of the 24 amino acids quantified in the gastrocnemius muscle, the levels of eight (glycine, isoleucine, leucine, methionine, phenylalanine, proline, tyrosine, and valine) were influenced by the source of dietary protein (Table S3). The levels of all eight amino acids differed according to protein source (casein versus some of meat), although no differences were observed among the types of meat.
2.2.2. Quantitative Analysis of Short-Chained Fatty Acids in Cecal Content
The levels of three short-chained fatty acids were quantified in the cecal content (Table 1). Acetic acid levels were not influenced by the source of dietary protein; however, with propionic acid and butyric acid levels, some differences were confirmed in mice fed casein versus those fed meat. Moreover, propionic acid and butyric acid levels partly differed according to the type of meat ingested.

Table 1.
Effects of dietary protein sources on short-chain fatty acid levels in the cecal content of mice.
2.2.3. Non-Targeted and Semi-Quantified Analyses of Metabolites in the Liver, Gastrocnemius Muscle, and Cecal Content
Among the water-soluble bioactive substances in meat, we could not analyze anserine in all samples because the metabolome database we used did not contain analytical conditions for anserine.
Of the 148 metabolites semi-quantified in the liver, the levels of 25 were influenced by the dietary protein source (Table S5). The levels of 19 metabolites differed in mice fed casein versus those fed meat (Table S5); moreover, the levels of 11 metabolites differed according to the type of ingested meat (1,5-anhydro-glucitol, 2-hydroxyisovaleric acid, β-alanine, carnosine, dimethylglycine, glyoxylic acid, inositol, hydroxyproline, sorbitol, tartaric acid, and xylitol) (Table 2). Liver taurine levels were not altered by the diets (Table 2).

Table 2.
Effects of dietary protein sources on metabolite levels in the liver of mice.
Of the 133 metabolites semi-quantified in the gastrocnemius muscle, the levels of seven were influenced by the dietary protein source (Table S6). The levels of six metabolites differed in mice fed casein versus those fed meat (Table S6); moreover, the levels of two metabolites (1,5-anhydro-glucitol and inositol) differed according to the type of meat ingested (Table 3). Levels of β-alanine, carnosine, and taurine in the gastrocnemius muscle were not influenced by the type of diet (Table 3).

Table 3.
Effect of dietary protein sources on metabolite levels in the gastrocnemius muscle of mice.
Of the 141 metabolites semi-quantified in cecal content, 47 were influenced by the dietary protein source (Table S7). The levels of 35 metabolites differed in mice fed casein versus those fed meat (Table S7), whereas the levels of 18 metabolites were influenced by the type of meat ingested (2-aminobutyric acid, 3-hydroxypropionic acid, 3-methyl-2-oxovaleric acid, 3-sulfinoalanine, β-alanine, carnosine, galactose, glutaric acid, glycerol 3-phosphate, glycine, homocysteine, mannose 6-phosphate, N-acetylaspartic acid, N-acetylglutamine, nicotinamide, pantothenic acid, phenylacetic acid, and ribose) (Table 4). Taurine was undetectable in cecal content (Table 4).

Table 4.
Effect of dietary protein sources on metabolite levels in the cecal content of mice.
2.2.4. Integrated Analyses of Quantified and Semi-Quantified Metabolites
Next, data from the aforementioned analyses (Table 1, Table 2, Table 3 and Table 4, Table S2, Table S3, Tables S5–S7) were integrated and reanalyzed. The results were as follows:
Of 173 metabolites tested in the liver, 31 were influenced by the source of dietary protein. The levels of 25 metabolites differed in mice fed casein versus those fed meat; moreover, the levels of 11 metabolites differed according to the type of ingested meat (1,5-anhydro-glucitol, 2-hydroxyisovaleric acid, β-alanine, carnosine, dimethylglycine, glyoxylic acid, inositol, hydroxyproline, sorbitol, tartaric acid, and xylitol).
Of 157 metabolites tested in the gastrocnemius muscle, the levels of 15 were influenced by the dietary protein source. The levels of 14 metabolites differed in mice fed casein versus those fed meat, while the levels of two differed according to the type of meat (1,5-anhydro-glucitol and inositol).
Of the total 144 metabolites tested in cecal content, 49 were influenced by the dietary protein source. The levels of 37 metabolites difference in mice fed casein versus those fed meat; moreover, the levels of 20 metabolites differed according to the type of meat ingested (2-aminobutyric acid, 3-hydroxypropionic acid, 3-methyl-2-oxovaleric acid, 3-sulfinoalanine, β-alanine, butyric acid, carnosine, galactose, glutaric acid, glycerol 3-phosphate, glycine, homocysteine, mannose 6-phosphate, N-acetylaspartic acid, N-acetylglutamine, nicotinamide, pantothenic acid, phenylacetic acid, propionic acid, and ribose).
Analyses of the pathways associated with the significantly affected metabolites revealed that 12, 6, and 14 pathways in the liver, gastrocnemius muscle, and cecal content, respectively, were potentially affected by diet (Table 5). Metabolic pathway number (Metab. No., Table S4) was added to each pathway in Table 5. Metab. Nos. 6, 7, 15, and 20 are related to carnosine metabolism while the Nos. 5 and 22 are related to taurine metabolism. These were added to their corresponding metabolites shown in Table 1, Table 2, Table 3 and Table 4. As a result, the effects of dietary meat species on carnosine-related metabolism, not taurine-related metabolism, were clearly confirmed. They were summarized in Figure 1.

Table 5.
Potential metabolic pathways affected by dietary protein sources.
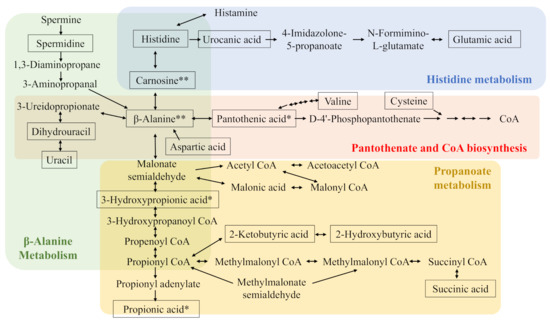
Figure 1.
Carnosine-related metabolism. Boxed metabolites are quantified or semi-quantified metabolites in the liver, gastrocnemius muscle, or cecal content. * indicates metabolites affected by meat species in cecal content. ** indicates metabolites affected by meat species in both cecal content and liver. CoA, coenzyme A.
To explore differences among dietary meat species further, partial least squares discriminant analysis (PLS-DA) was tried for four different meat groups using metabolite levels in liver, gastrocnemius muscle, or cecal content. However, no good discriminant model could be made (data not shown).
2.2.5. Carnosine, Anserine, and Taurine Levels in Experimental Protein Sources
Among the water-soluble bioactive substances in meat, carnosine levels were clearly affected by the type of dietary meat ingested (Table 1, Table 2, Table 3, Table 4 and Table 5, Figure 1). Therefore, we quantified water-soluble bioactive substances in meat including carnosine in all protein sources (Table 6). Carnosine, anserine, and taurine were not detected in casein, but were detected at markedly different levels in meat species.

Table 6.
Carnosine, anserine, and taurine levels in experimental protein sources.
Next, intake of carnosine, anserine, and taurine (Table 7) were calculated and analyzed by their levels in experimental protein sources (Table 6), diet composition (Table S8), and food intake (Table S1). The highest intake of carnosine was detected in pork leg and the lowest in chicken leg. Interestingly, the same patterns were observed in the liver and cecal content (Table 2 and Table 4) but not in muscle (Table 3). Then, to explore metabolic effects by intake of carnosine, anserine, or taurine, PLS regression (PLS-R) was tried using metabolite levels in liver, gastrocnemius muscle, or cecal content. Consequently, only one good regression model could be made, which can predict taurine intake by metabolite levels of cecal content (R2Y = 0.996, Q2 = 0.82). Analysis of pathways associated with the significant metabolites revealed that 13 metabolic pathways were potentially affected by taurine intake (Table 8).

Table 7.
Intake of carnosine, anserine, and taurine in mice fed various meat protein species.

Table 8.
Potential metabolic pathways linked to taurine intake in cecal content.
3. Discussion
There were no obvious detrimental effects of meat-based diets on growth parameters (food intake, body weight, and tissue weights) when compared to a casein-based diet (the control). On the other hand, the levels of numerous metabolites were influenced by the source of the dietary protein. Moreover, pathway analyses suggested that certain metabolic pathways were significantly affected by the type of protein in the diet. Significant differences in the levels of numerous metabolites were observed in mice fed casein versus meat; such differences may be a result of the amino acid composition of the proteins (Table S9) and/or the existence of meat-specific bioactive substances. While we mainly focused on the metabolic effects of various dietary meat species, the intra-meat differences did not appear to be as marked as those between casein and meat given that the compositions of amino acids among the various meat species were almost identical (Table S9). Therefore, we investigated meat-derived bioactive substances that were associated with obviously different metabolite levels (Table 6).
Carnosine-related metabolism was clearly affected by the dietary meat species. Higher dietary carnosine intake resulted in both higher carnosine and β-alanine levels in the liver and cecal content. These results are consistent with our previous study that showed that dietary carnosine increased both carnosine and β-alanine levels in the liver and cecal content of mice in a dose-dependent manner [17]. The increased carnosine levels may have a positive effect on animal health as stated in the Introduction. On the other hand, a limitation of the present study is that we could not fully evaluate the effects of dietary anserine levels on anserine-related metabolism because present analytical methods could not confirm the presence of anserine and its metabolite 1-methylhistidine in tissues and cecal content. Because anserine also has various actions (some in common with carnosine) [8,9], its role should be evaluated in future studies. On the other hand, 2-oxo-imidazole dipeptides could be produced from imidazole peptides in mouse tissue and have strong antioxidant activity [18]. Therefore, to evaluate dietary effects obtained in the present study more precisely, not only carnosine and anserine, but also 2-oxo-carnosine and 2-oxo-anserine should be investigated in the future in this mouse model.
We observed no influence of dietary taurine on taurine levels in tissues and cecal content, indicating that dietary taurine levels may not be sufficient to elicit an increase in free taurine expression. On the other hand, effects of taurine intake on some metabolic pathways including taurine and hypotaurine metabolism were speculated by the pathway analysis in cecal content. To evaluate metabolic effects of dietary taurine further, focusing on not only taurine metabolic pathway but also other metabolic pathways suggested in the present study may be effective.
In terms of tissues, 1,5-anhydro-glucitol and inositol were affected by dietary meat species in both liver and muscle. Because dietary 1,5-anhydro-glucitol can modulate glucose homeostasis [19] while dietary inositol can regulate lipid metabolism [20], our current results may provide useful information with which to understand the effects of dietary meat species on metabolic syndrome, given that it is closely linked to glucose and lipid metabolism.
Butyric and propionic acid levels in cecal content were affected by the type of dietary meat species. These short-chain fatty acids have important roles in animal health, including metabolic syndrome [21]; therefore, our results ought to be informative for understanding the different effects of dietary meat species. Propionic acid can be derived from β-alanine (Figure 1), and its highest levels were observed in mice fed chicken breast, which contains the highest levels of imidazole dipeptides (the combination of carnosine and anserine). Therefore, propionic acid levels may be, in part, derived from β-alanine resulting from the degradation of dietary carnosine and/or anserine. To better investigate this, additional studies such as metabolic flux analysis should be performed.
In conclusion, we identified some metabolites whose levels in tissues or cecal content differed according to the type of dietary meat species. These included some bioactive substances such as carnosine, 1,5-anhydro-glucitol, inositol, butyric acid, and propionic acid. In addition, some metabolic pathways linked to taurine intake could be suggested. Focusing on these results will be useful to clarify the different effects of dietary meat species on metabolism and/or health in humans and animals.
4. Materials and Methods
4.1. Animals and Diets
Thirty male C57BL/6J mice (three weeks old, SLC Japan) were used in this experiment. The animals were housed individually in plastic cages in a room maintained at 24 ± 1 °C with a 14 h–10 h light–dark cycle (the lights were on from 5:00 to 19:00). All animals were allowed free access to water and were fed a normal diet (AIN-93G) for one week before the experiment. Institutional ethical approval was obtained (code number 29–87).
The ingredients of the AIN-93G diet used in this study are shown in Table S8; this diet uses casein (CLEA Japan Inc., Tokyo, Japan) as the protein source. Casein was replaced with powdered and defatted meat including from beef leg, pork leg, chicken leg, or chicken breast for use as experimental diets. Animal meats (beef leg, pork leg, chicken leg, and chicken breast) were prepared at NH Foods Ltd. (Osaka, Japan). After removing the fat, tendons, and peels, each type of meat was packed in vacuo and heated for 60 min at 75 °C in a convection oven. After confirming that the temperature at the center of the pack was over 72 °C, meats were cooled in iced water. The powder of each meat was produced according to the method of Wakamatsu et al. [22]; in brief, all the meats were minced and freeze-dried, and fat was removed using n-hexane. Table S9 shows the amino acid components of the experimental protein sources. Other food materials were purchased from CLEA Japan Inc. (Tokyo, Japan). We prepared each diet by mixing these food materials.
After acclimation, mice were divided into five groups based on body weight and were fed their assigned diets for four weeks. Daily food intake and weekly body weight were recorded during the experimental period. At the end of the experiment, blood was drawn under anesthesia and plasma was isolated by centrifugation at 10,000× g and 4 °C for 10 min. The brain, liver, gastrocnemius muscle, inguinal fat, epididymal fat, perirenal fat, and brown fat were removed and their wet weights were measured. Additionally, cecal content was removed and all samples were immediately frozen in liquid nitrogen and stored at −80 °C. The liver and gastrocnemius muscle were crushed with liquid nitrogen and powdered before the analysis.
4.2. Gas Chromatography/Mass Spectrometry (GC–MS/MS)-Based Non-Targeted Metabolomic Analysis
Cecal content were diluted five-fold in distilled water and centrifuged at 20,000× g and 4 °C for 5 min. The resulting supernatant (50 μL), along with liver (10 mg) and gastrocnemius muscle (10 mg), underwent metabolomic analysis. The samples were treated according to Goto et al. [23]; briefly, the hydrophilic fraction was extracted and derivatized by methoxyamine hydrochloride (MP Biomedicals, California, USA) and N-methyl-N-(trimethylsilyl)trifluoroacetamide (Funakoshi, Tokyo, Japan). Moreover, 2-isopropylmalic acid was used as an internal standard. GC–MS/MS analyses were performed using a GCMS-TQ8050 instrument (Shimadzu, Kyoto, Japan). According to Smart Metabolites Database (Shimadzu, Kyoto, Japan), a DB-5 column (length 30 m, inner diameter 0.25 mm, and film thickness 1.00 μm) (Agilent Technologies, Santa Clara, CA, USA) was used for the liver and gastrocnemius muscle, while a BPX-5 column (length 30 m, inner diameter 0.25 mm, and film thickness 0.25 μm) (SGE, Melbourne, Australia) was used for cecal content.
Data processing was performed using the Smart Metabolites Database, MS-DIAL version 3.04 [24] and MRMPROBS program version 2.50 [25]. An annotation of metabolites was conducted using thresholds as follows: −10 < retention index < +10, a mass spectrum similarity of >50 in the SCAN data, and the presence of a second ion peak in the Multiple Reaction Monitoring (MRM) data according to the analysis using the Smart Metabolites Database and MS-DIAL. The relative level of a metabolite was calculated using the peak area of each metabolite relative to that of the internal standard (2-isopropylmalic acid) using the MRMPROBS program version 2.50. The value of each metabolite was then corrected so that the average value of all samples was 100.
4.3. GC–MS-Based Free Amino Acid Analysis
For amino acid analysis, 300 μL of 0.1 M perchloric acid was added to liver, gastrocnemius muscle, and cecal content (30 mg each) and homogenized and placed on ice in a dark place for 30 min. These were then centrifugated (20,000× g, 0 °C) for 15 min to obtain the supernatant. Norvaline as internal standard was added to this supernatant and mixed amino acids standard. After further treatment and derivatization using the EZfaast kit (Phenomenex, CA, USA), these samples were analyzed in the SIM mode using the GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan) with a Zebron ZB-AAA capillary GC column (length 10 m, inner diameter 0.25 mm) (Phenomenex, Torrance, CA, USA) according to the Smart Metabolites Database. Amino acid levels were calculated using the peak area of each metabolite relative to that of the internal standard (norvaline).
4.4. GC–MS/MS-Based Short-Chain Fatty Acid Analysis
Cecal content (50 mg) was diluted in distilled water (200 μL) and centrifuged at 20,000× g and 4 °C for 5 min. For an internal standard, acetic acid-d4 was added to this supernatant (50 μL) and to mixed short-chain fatty acids (acetic acid, propionic acid, and butyric acid). After adding methanol and shaking for 30 s, the solution was centrifuged at 16,000× g and 25 °C for 3 min. The supernatant (180 μL) was obtained and derivatized using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride methanol solution and n-octylamine methanol solution for 9 h. These samples were then analyzed in MRM mode using the GCMS-TQ8050 with a BPX-5 column (length 30 m, inner diameter 0.25 mm, and film thickness 0.25 μm). Helium was used as the carrier gas at a pressure of 68.4 kPa, with a total flow rate of 40.4 mL/min and purge flow rate of 5.0 mL. The splitless injection mode was used. The column oven temperature program was to hold at 60 °C for 2 min, then increase at 15 °C per min from 60 to 330 °C. The vaporization room and ion source temperatures were 250 °C and 200 °C, respectively. Each metabolite was quantified using its peak area relative to that of the internal standard (acetic acid-d4).
4.5. Amino Acid Composition, Imidazole Dipeptides, and Taurine Quantification in Protein Sources
The amino acid content in hydrochloric-acid-hydrolyzed protein sources was quantified using an amino acid analyzer JLC-500/V (JEOL, Tokyo, Japan). Imidazole dipeptides (carnosine and anserine) and taurine levels in sulfosalicylic-acid-deproteinized protein sources were also quantified using the JLC-500/V analyzer.
4.6. Statistical Analyses
Data are expressed as means ± standard error of the mean. Data comparisons were performed using one-way analysis of variance with Tukey’s post-hoc multiple comparison test. The JMP software version 13.0.0 (SAS Institute, Cary, NC, USA) was used to perform these analyses. Metabolites significantly affected were subjected to pathway analysis using MetaboAnalyst 4.0 [26]. “Mus musculus (Kyoto Encyclopedia of Genes and Genomes)” was used for the pathway library. In these analyses, p < 0.05 was considered statistically significant. For PLS-DA and PLS-R, SIMCA 13.0.3 (Umetrics, Umeå, AB, Sweden) was used. In PLS-R, significant metabolite was determined as its variable importance in projection (VIP) score is larger than one and larger than the cross-validation standard error.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/10/12/503/s1, Table S1: Growth parameters, Table S2: Liver amino acids, Table S3: Muscle amino acids, Table S4: Number and name of metabolic pathway, Table S5: Liver metabolites, Table S6: Muscle metabolites, Table S7: Cecal content metabolites, Table S8: Diet composition, and Table S9: Amino acid composition.
Author Contributions
Conceptualization, S.T. and M.S.; methodology, S.T.; formal analysis, R.N. and S.T.; investigation, R.N.; resources, M.S.; data curation, R.N.; writing—original draft preparation, R.N.; writing—review and editing, S.T.; visualization, R.N. and S.T.; project administration, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
NH Foods funded this study and provided support for the author S.T. The author M.S. is a regular employee of NH Foods. The author R.N. states no conflict of interest.
References
- Gilbert, J.-A.; Bendsen, N.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. S2), B16–B31. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of Dairy Proteins on Appetite, Energy Expenditure, Body Weight, and Composition: A Review of the Evidence from Controlled Clinical Trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Inoue, M.; Tsugane, S.; et al. Meat Consumption and Colorectal Cancer Risk: An Evaluation Based on a Systematic Review of Epidemiologic Evidence Among the Japanese Population. Jpn. J. Clin. Oncol. 2014, 44, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, A.M.A.; Toldrá, F. Hydrophilic Chromatographic Determination of Carnosine, Anserine, Balenine, Creatine, and Creatinine. J. Agric. Food Chem. 2007, 55, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, R.; Miyazaki, M.; Miyazaki, T.; Shigeno, Y.; Tokairin, Y.; Konno, H.; Yamashita, T. LC-ESI-MS/MS quantification of carnosine, anserine, and balenine in meat samples. J. Chromatogr. B 2019, 1132, 121826. [Google Scholar] [CrossRef] [PubMed]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Moscow) 2000, 65, 757–765. [Google Scholar]
- Stegen, S.; Stegen, B.; Aldini, G.; Altomare, A.; Cannizzaro, L.; Orioli, M.; Gerlo, S.; Deldicque, L.; Ramaekers, M.; Hespel, P.; et al. Plasma carnosine, but not muscle carnosine, attenuates high-fat diet-induced metabolic stress. Appl. Physiol. Nutr. Metab. 2015, 40, 868–876. [Google Scholar] [CrossRef]
- Tomonaga, S.; Yamane, H.; Onitsuka, E.; Yamada, S.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Carnosine-induced antidepressant-like activity in rats. Pharmacol. Biochem. Behav. 2008, 89, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawalha, N.A.; Alshogran, O.Y.; Awawdeh, M.S.; Almomani, B.A. The effects of l-Carnosine on development of metabolic syndrome in rats. Life Sci. 2019, 237, 116905. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Nan, C.; Tian, J.; Jean-Charles, P.; Li, Y.; Weissbach, H.; Huang, X.-P. Protective effects of taurine against oxidative stress in the heart of MsrA knockout mice. J. Cell. Biochem. 2012, 113, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Furuse, M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: Antidepressant versus anxiolytic-like effects. Amino Acids 2010, 39, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Murakami, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Dietary animal proteins alter monoamine metabolism in the brain. Anim. Sci. J. 2011, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, M.; Wakamatsu, J.-I.; Takahata, Y.; Hasegawa, T.; Morimatsu, F.; Nishimura, T. Diet-Induced Thermogenesis and Expression Levels of Thyroid Hormone Target Genes and Their Products in Rats Differ between Meat Proteins. J. Nutr. Sci. Vitaminol. 2016, 62, 93–100. [Google Scholar] [CrossRef]
- Nakata, R.; Sato, M.; Tomonaga, S. The effect of dietary carnosine supplementation on metabolite levels in the liver and cecal content of mice. Trace Nutr. Res. 2019, 36, 66–72. [Google Scholar]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamada, K.-I.; Uchida, K. 2-Oxo-histidine–containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef]
- Nakamura, S.; Tanabe, K.; Yoshinaga, K.; Shimura, F.; Oku, T. Effects of 1,5-anhydroglucitol on postprandial blood glucose and insulin levels and hydrogen excretion in rats and healthy humans. Br. J. Nutr. 2017, 118, 81–91. [Google Scholar] [CrossRef][Green Version]
- Shimada, M.; Hibino, M.; Takeshita, A. Dietary supplementation with myo -inositol reduces hepatic triglyceride accumulation and expression of both fructolytic and lipogenic genes in rats fed a high-fructose diet. Nutr. Res. 2017, 47, 21–27. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, J.-I.; Fujii, R.; Yamaguchi, K.; Miyoshi, S.; Nishimura, T.; Hattori, A. Effects of meat species on the postprandial thermic effect in rats. Anim. Sci. J. 2012, 84, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kanazawa, M.; Ogiwara, A.; Arita, M. MRMPROBS suite for metabolomics using large-scale MRM assays. Bioinformatics 2014, 30, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).