Abstract
Chronic obstructive pulmonary disease (COPD) is a common disorder with an increasing prevalence, characterised by persistent respiratory symptoms and airflow limitation. Systemic inflammation is involved in the pathogenesis of COPD and can also predispose to metabolic disorders (e.g., metabolic syndrome (MetS) and non-alcoholic fatty liver disease (NAFLD)). Such comorbidities can negatively affect COPD outcomes, cardiovascular risk, and quality of life. Apart from NAFLD, abnormal peri-organ or intra-organ fat (APIFat) could be considered as markers for cardiometabolic diseases and even for COPD. The present narrative review considers the associations of COPD with MetS, NAFLD, and other APIFat, including epicardial, perirenal, peripancreatic, and intramuscular adipose tissue. Further research is needed to define these relationships and identify any potential clinical implications.
1. Introduction
Chronic obstructive pulmonary disease (COPD), mainly caused by tobacco smoking, is characterised by persistent respiratory symptoms and airflow limitation [1]. However, increasing evidence indicates that COPD has important extra-pulmonary manifestations, resulting in significant systemic abnormalities [2,3]. Whether these abnormalities represent direct consequences of the pulmonary disorder, or whether COPD is, in fact, a systemic disease, is still a matter of debate [4].
A central feature in the pathogenesis of COPD includes an impaired inflammatory response of the lungs to noxious particles or gases, caused by an imbalance between oxidant and antioxidant factors; this results in a local increase in oxidative stress and inflammation [5]. The hypothesis that systemic inflammation originates by “spill over” from the pulmonary compartment to the systemic circulation is still to be proven [6]. Genetic and constitutional factors may also predispose individuals with COPD towards the development of systemic and pulmonary inflammation [7]. Systemic inflammation is present in patients with stable COPD, as demonstrated by the increase in serum levels of acute phase proteins, i.e., C-reactive protein (CRP), fibrinogen, interleukins (IL-6 and IL-8), and tumour necrosis factor α (TNF-α), as well as the increased numbers of circulating leucocytes [8]. Additionally, inflammatory biomarkers correlate with an increased risk of COPD exacerbations [9]. There is also increasing evidence supporting that oxidative stress is involved in the development and progression of COPD [10].
Systemic inflammation plays a key role in the pathogenesis of COPD systemic effects, including weight loss, skeletal muscle dysfunction, and cardiovascular (CV) complications [2]. On the other hand, inflammation and oxidative stress have also been implicated in the development of metabolic disorders such as the metabolic syndrome (MetS) and non-alcoholic fatty liver disease (NAFLD) [11,12,13,14]. Both diseases are associated with an increased CV risk [15]. Apart from NAFLD, research has been also extended to the links between other abnormal peri-organ or intra-organ fat (APIFat) deposits and cardiometabolic disorders [16,17].
The aim of the present narrative review was to discuss the associations of COPD with MetS, NAFLD, and APIFat at different locations (namely epicardial, perirenal, peripancreatic, and intramuscular adipose tissue).
2. MetS and COPD
An association between MetS and COPD has been reported [18,19]. Briefly, MetS was present in 62% of 76 COPD patients, whereas COPD was diagnosed in 22% of 59 MetS patients [18]. In another study [19], MetS prevalence was 37.8% among 98 COPD patients. Increased rates and duration of acute COPD exacerbations have been related to the presence of MetS [20]. In addition to increasing the levels of inflammatory markers, MetS has been associated with limited physical activity in patients with COPD, independently of their lung dysfunction [21]. Furthermore, the circulating levels of leptin, endothelin, CRP, and IL-6 were significantly higher, whereas levels of adiponectin were significantly lower, in patients with both COPD and MetS compared with COPD patients without MetS [22,23]. Higher serum leptin levels were associated with greater systemic and airway inflammation in patients with stable COPD [24]. Of note, increased circulating leptin levels have also been observed in obese patients [25] and patients with type 2 diabetes mellitus (T2DM) [26,27]. Furthermore, adiponectin concentrations have been positively related with COPD severity and progression [28] and increased respiratory mortality in COPD patients [29]. Leptin and adiponectin levels may vary according to COPD phenotypes (e.g., in relation to emphysema development and progression) [30] and their circulating levels may be affected by statins and antidiabetic drugs (metformin, pioglitazone, empagliflozin and liraglutide) [31,32,33]. Overall, inflammation, adipokine dysregulation, smoking and physical inactivity may contribute to the link between MetS and COPD [34]. Smoking was also shown to increase the expression of pro-inflammatory mediators by perivascular fat tissue [35].
Among the MetS diagnostic components, dyslipidaemia (i.e., low high-density lipoprotein cholesterol and high triglyceride levels), fasting hyperglycaemia, hypertension, and abdominal obesity (expressed as increased waist circumference) were inversely related with lung function; waist circumference was the strongest predictor of lung dysfunction [36]. Abdominal obesity, hyperglycaemia, and hypertension may represent the most prevalent MetS components found in COPD patients [37,38].
The presence of MetS increases the risk of CV morbidity and mortality in several conditions, including COPD [39,40,41,42]. Therefore, it has been suggested that COPD patients should be screened for MetS [43,44] not only due to the possible co-existence of these disorders, but also because MetS treatment may improve COPD prognosis [45]. In this context, the presence of MetS was associated with increased dyspnoea and co-morbidities (such as DM, heart failure, coronary artery disease, and osteoporosis) in COPD patients hospitalised for an exacerbation [46].
In the context of MetS and COPD, there is a need to consider the “obesity paradox” (i.e., evidence that obesity in patients with COPD is associated with a reduced all-cause mortality) [47]. This pattern is also seen in some other diseases [47]. Several factors are probably involved in mediating this unexpected relationship. These have been reviewed elsewhere [47] and are beyond the scope of the present review. Furthermore, we need to consider the role of “reverse causality”. In other words, that pathological weight loss rather than weight gain contributes to the “obesity paradox” [47].
Overall, it is still not clear whether MetS could be a direct consequence of the COPD-related progressive lung dysfunction, in the absence of cigarette smoking or exposure to air pollution [48]. However, chronic systemic inflammation, adipose tissue dysfunction, oxidative stress, inhaled and oral glucocorticoids therapy, physical inactivity, hyperglycaemia, and aging could be involved in the development of MetS and other metabolic disorders in COPD patients [48].
Figure 1 summarizes the characteristics of MetS presence in COPD patients. Since the presence of MetS has been linked to COPD severity as well as increased CV risk in COPD patients, further research is needed in this field to elucidate the importance of preventing/treating MetS in improving the prognosis of COPD patients.
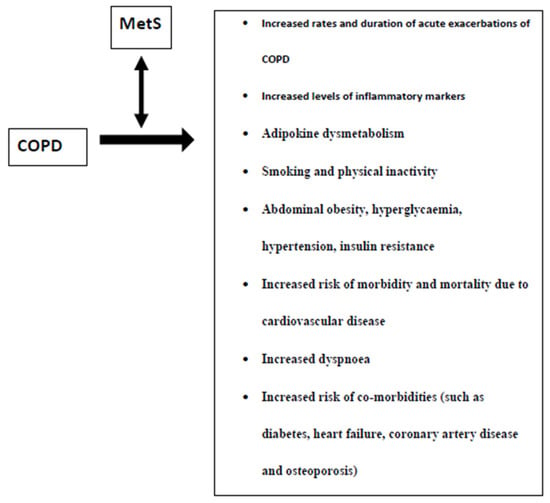
Figure 1.
Characteristics of the presence of the metabolic syndrome (MetS) in patients with chronic obstructive pulmonary disease (COPD).
3. NAFLD and COPD
NAFLD, a hepatic manifestation of MetS, is characterised by fat accumulation in the liver in the absence of alcohol abuse or other causes of chronic liver disease [49]. NAFLD can progress from benign steatosis to steatohepatitis, cirrhosis, and, potentially to hepatocellular carcinoma [50]. Inflammation, oxidative stress, and insulin resistance play a key role in NAFLD pathophysiology [51,52]. NAFLD is associated with increased liver and CV morbidity and mortality [40,53,54,55]. Furthermore, NAFLD patients have a 2-fold increased risk of developing T2DM [56,57]. The prevalence of NAFLD is very high in T2DM patients reaching almost 60% as reported in recent meta- analyses [58,59].
NAFLD was also highly prevalent in COPD patients [60]. Briefly, among 111 COPD patients, the prevalence of liver steatosis, non-alcoholic steatohepatitis and liver fibrosis was 41.4, 36.9, and 61.3%, respectively [60]. Furthermore, NAFLD presence and severity were associated with pulmonary dysfunction [61,62,63]. Vice versa, reduced lung function [defined by forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC)] was a risk factor for NAFLD incidence in a large (n = 96,104) middle-aged (mean age 35.7 years) healthy Korean cohort [64]. This bilateral relationship between NAFLD and pulmonary function has also been confirmed in a recent meta-analysis [65].
Statins and antidiabetic drugs may beneficially affect NAFLD [66,67,68,69,70,71]. An expert panel considered the use of pioglitazone and statins for NAFLD treatment [72]. Statins can also improve lung function, mortality, and exacerbation rates in COPD patients possibly due to their anti- inflammatory properties [73,74]. In this context, a previous meta-analysis found that statins significantly decreased all-cause mortality (HR: 0.65, 95% confidence intervals (CI), 0.57–0.74, p < 0.001) and COPD exacerbation rates with or without hospitalisation (HR: 0.58, 95% CI, 0.48–0.72, p < 0.001) [75]. In other meta-analyses, statins improved pulmonary function and exercise tolerance in COPD patients [76] and decreased COPD mortality [77]. Statins may also protect against pulmonary arterial hypertension (PAH) secondary to lung diseases [78,79]. A recent meta-analysis found that statins significantly reduced all-cause and coronary heart disease mortality, acute exacerbation of COPD, CRP, and PAH in COPD patients [80].
It has been suggested that statin-induced benefits in COPD patients could be more pronounced in those patients with cardiometabolic co-morbidities compared with those without a CV indication for statin use [81]. However, data from randomised controlled studies are missing [82,83]. Further research is needed to elucidate the role of statins in the treatment of COPD.
We also need to consider the role of subcutaneous adipose tissue (SAT) and abdominal visceral adipose tissue (VAT), as well as liver fat. In this context, a study found that, COPD patients (n = 1,267) with prior myocardial infarction (MI) had a significantly greater VAT area than those with no history of MI [84]. All fat deposits were evaluated using computed tomography. However, there was no such relationship between a history of MI and SAT or liver attenuation [84]. Furthermore, after adjusting for several factors (including obesity and diabetes), COPD patients in the upper tertile VAT area had an increased odds ratio (1.86; 95% CI 1.02–3.41) of MI compared with patients in the lower tertiles [84].
Overall, the role of NAFLD on COPD development and progression should be further evaluated in order to improve patients’ prognosis.
4. Epicardial Fat and COPD
Epicardial fat thickness (EFT) has been associated with NAFLD, MetS, and CV disease [85,86,87]. In this context, a recent meta-analysis reported that EFT was significantly increased in NAFLD patients compared with controls (standardized mean difference 0.73, 95% CI 0.51–0.94; p < 0.001); elevated EFT was also related to NAFLD severity and CV disease in NAFLD patients [88]. Similarly, a previous meta-analysis found a significant correlation between EFT and MetS features (i.e., systolic blood pressure, waist circumference, triglycerides, high-density lipoprotein cholesterol, and fasting blood glucose); p < 0.0001 for all comparisons [89]. Increased EFT was also shown to predispose coronary heart disease (CHD) patients to sudden cardiac death [90]. Furthermore, a prospective population-based study (n = 4093; mean follow-up: 8 years) reported that increased EFT was associated with the incidence of both fatal and non-fatal coronary events [91]. Briefly, EFT doubling was related to a 1.5-fold risk of coronary events (HR 1.54, 95% CI 1.09–2.19; p < 0.001) [91]. From a pathophysiological point of view, EFT can exert both local and systemic harmful effects via the secretion of reactive oxygen species and inflammatory and proatherogenic cytokines [92]. Therefore, both inflammation and oxidative stress are involved in the increased CV risk linked to EFT [93,94].
In patients with CV disease (COPD patients were excluded), visceral obesity was associated with an adverse adipokine–cytokine profile [95]. In the same study, epicardial adipose tissue thickness was associated with perivascular adipose tissue [95].
The association between EFT and COPD has been investigated in a small number of studies. Sova et al. [96] found that COPD patients (n = 157) had a significantly higher EFT than controls (n = 45) (5.4 ± 1.6 vs. 4.1 ± 0.9 mm; p < 0.001); multivariate analysis showed that EFT was independently correlated with high-sensitivity CRP (β = 0.300; p < 0.001) and BODE index, an important prognostic predictor of COPD (β = 0.405; p < 0.001) [97]. The BODE index includes body mass index (B), degree of airflow obstruction (O), dyspnoea (D), and exercise capacity (E) [98]. Similarly, in an older study, EFT of COPD patients (n = 171) was significantly higher than controls (n = 70) (143.7 vs. 129.1 cm3; p = 0.02) and was correlated with CRP (r = 0.32; p < 0.001) and coronary calcium score (r = 0.38; p < 0.001) [99].
Likewise, COPD patients (n = 82) also had a significantly increased EFT than controls (n = 84) (6.1 ± 0.9 vs. 4.8 ± 1.1 mm; p < 0.001), as well as COPD patients with MetS compared with those without MetS (7.7 ± 1.8 vs. 6.1 ± 0.9; p < 0.001) [100]. In the same study, each 1 mm increase in EFT resulted in a 2-fold increase for MetS [100]. Furthermore, a positive relationship was found between EFT and the apnoea–hypopnea index (AHI) in 162 COPD patients (β = 0.10; p < 0.001); while continuous positive airway pressure (CPAP) therapy for 24 weeks significantly decreased EFT (from 7.29 ± 1.27 to 6.92 ± 1.26 mm; p = 0.005) [101]. Interestingly, EFT was also reported to be significantly associated with FEV1 (% predicted), FEV1/FVC and airway wall thickness [102].
On the other hand, there are studies with contradictory results. EFT was found to be decreased in COPD patients (n = 98) compared with healthy controls (n = 40) [103]. COPD patients with severe right ventricular systolic dysfunction (RVSD) also had a thinner EFT compared with those with mild RVSD [93]. Another study reported no differences in EFT between COPD patients (n = 81) and controls (n = 81) [104]. This diversity in findings may be explained, at least partly, by differences in patient characteristics, duration, and severity of COPD, as well as in the differences in the applied methodology of EFT measurements.
Currently, there is a knowledge gap in relation to the clinical implications of changes in EFT in COPD patients. This should be addressed in terms of identifying potential benefits for treating these patients.
4.1. Perirenal Fat and COPD
There is evidence of a link between perirenal adiposity and CV disease [105]. Associations between perirenal fat and other fat depots (e.g., NAFLD, EFT) have also been reported [106]. The presence of perirenal adipose tissue in COPD patients has not been investigated yet.
4.2. Peripancreatic Fat and COPD
Non-alcoholic fatty pancreas disease (NAFPD) has been related to CV disease [107]. Links between NAFPD and NAFLD, as well as EFT have also been reported [108,109,110]. It has been hypothesized that COPD may contribute to NAFPD development through systemic and local inflammation, excessive lipolysis and increased lipid synthesis, as well as intermittent hypoxia- induced pancreatic beta cell damage, thus representing potential mechanisms linking COPD with NAFPD [111]. Insulin resistance has also been related to COPD [112,113], as well as to NAFPD [114]. However, no data exist on peripancreatic adipose tissue in relation to COPD.
4.3. Intramuscular Fat and COPD
COPD is characterized by gradual skeletal muscle wasting, contributing to impaired exercise capacity, reduced health-related quality of life, and increased mortality [115].
The aetiology of muscle weakness/atrophy in patients with COPD is complex [116]. Cytokines, oxidative stress, hormones, nutrition, and obviously, decreased physical activity are likely to be involved [116]. This topic has been reviewed elsewhere [116].
Accumulation of adipocytes and intramyocellular lipid species in skeletal muscle can lead to loss of muscle strength and reduced insulin sensitivity [117]. The mechanisms involved in causing “myosteatosis” have not been well documented but are likely to involve aging, glucocorticoid treatment, estrogen deficiency, and disuse atrophy [117]. Inflammation and oxidative stress may also be involved in intramuscular fat accumulation [118,119].
Robles et al. reported significantly higher fat infiltration in calf and thin muscles in 10 COPD patients compared with controls, in addition to the reported muscle atrophy [120]. Of note, intramuscular fat infiltration was more profound than muscle atrophy in COPD patients [120]. Similarly, the percentage of intramuscular adipose tissue was higher in COPD patients (n = 101) than in healthy controls (n = 10) (6.7 ± 3.5 vs. 4.3 ± 1.2%; p = 0.03); this fat depot was associated with exercise capacity and physical activity level, thus potentially affecting COPD-related muscle dysfunction [121]. Furthermore, muscle fat infiltration was linked to oxidative stress and endothelial dysfunction in both obese and lean COPD patients (n = 78) [122]. In another study, intramuscular adipose tissue of knee extensors and flexors was 2-fold higher in COPD patients (n = 21) than healthy individuals (n = 21) [123]. It was recently reported that in patients with advanced emphysema, bronchoscopic lung volume reduction treatment was associated with increases in intramuscular fat [124]. The associations between intramuscular adiposity and COPD severity/prognosis should be further elucidated.
In the context of treatment, we should consider the potential adverse effects of statins on muscles [125,126,127] in COPD where there may already be impaired muscle function, as mentioned above [115,120].
5. Current Knowledge and Future Perspectives
COPD may represent a “systemic” disease with inflammation, oxidative stress and insulin resistance playing key roles as the underlying mechanisms linking COPD with cardiometabolic disorders. Among the latter, MetS and NAFLD have been related to the presence and severity of COPD, as well as with increased CV morbidity and mortality [18,20,40,54,60,63]. In this context, dyslipidaemia, hyperglycaemia, hypertension, and abdominal obesity (i.e., features of both MetS and NAFLD) are predictors of lung dysfunction [36]. In other words, the pathogenesis of COPD and MetS has been described as “intertwined” [48]. Figure 2 summarizes the pathophysiological links between MetS, NAFLD, and COPD. On the other hand, treatment of MetS and NAFLD may improve survival and COPD prognosis [45,72]. Therefore, screening COPD patients for MetS and NAFLD may result in early diagnosis and treatment, leading to better outcomes.
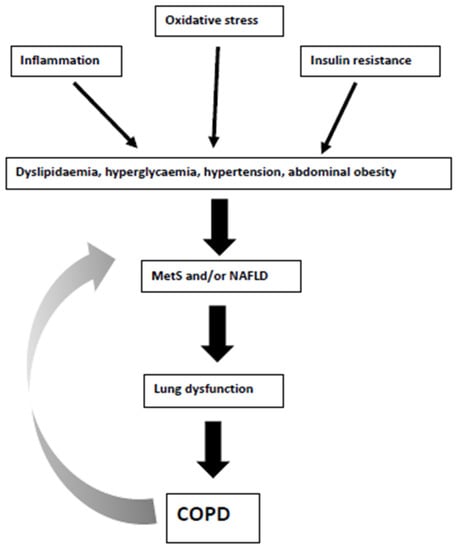
Figure 2.
Pathophysiological links between metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD) and chronic obstructive pulmonary disease (COPD). The grey arrow shows the potential bilateral interactions between MetS/NAFLD and COPD.
Apart from NAFLD, other APIFat deposits (i.e., epicardial, perirenal, peripancreatic, and intramuscular) have been related to cardiometabolic disorders [16,17,128]. Furthermore, we should consider that excessive “orthotopic” APIFat fat accumulation may be a “systemic” rather than a “local” phenomenon. Therefore, the reported associations of these fat depots with COPD may also be relevant. In this context, EFT has been found increased in COPD patients, being also linked to inflammation (i.e., CRP levels), COPD prognosis and severity (i.e., the BODE index, FEV1, FEV1/FVC, and airway wall thickness) [102]. However, as mentioned previously, there are also conflicting results [103,104]. Perirenal and NAFPD have been associated with CV risk, NAFLD, and EFT [106,108,109,110], but there are no data on their association with COPD, despite the common pathogenetic mechanisms (e.g., oxidative stress, inflammation, and insulin resistance). Future research on this field is certainly needed.
Finally, intramuscular fat infiltration may be more profound than muscle atrophy in COPD patients [120], thus potentially affecting their exercise capacity and physical activity level [121]. A link between intramuscular adiposity and lung function has also been reported in patients with advanced emphysema [124]. The associations of intramuscular fat with COPD should be further investigated.
Statins may improve both biochemical and histological features of NAFLD, as well as pulmonary function, exercise tolerance, (all-cause and CV) mortality, and exacerbation rates in COPD patients [72,73,75,80]. However, strong evidence from randomised controlled studies is lacking and further research should focus on elucidating the role of statins in COPD treatment.
The Overlap syndrome (OS) is characterised by the coexistence of COPD and obstructive sleep apnoea syndrome (OSAS) in the same patient [129]. Increasing evidence now acknowledges the frequent association between OSAS and MetS [130,131] as well as between OSAS and NAFLD [132,133]. Increased EFT has also been reported in OSAS patients compared with controls, even in the absence of obesity [134,135]. Interestingly, EFT has been linked to OSAS severity [136]. In this context, EFT was the strongest predictor of AHI among other parameters (i.e., age, gender, BMI, carotid intima-media thickness, and left ventricular mass index) in patients with both MetS and OSAS [137].
Overall, it has been suggested that OSAS may predispose to APIFat depositions via intermittent hypoxia, hormonal derangements, systemic and local inflammation, excessive lipolysis, and enhanced lipid synthesis [111]. OSAS severity has also been positively associated with adipose accumulation in skeletal muscles, a link that may be largely mediated by obesity [138].
Continuous positive airway pressure (CPAP) therapy was shown to reduce EFT [101]. No data exist on perirenal and peripancreatic fat deposition in OSAS patients.
Patients with OS exhibit worse parameters of nocturnal hypoxia, as well as frequent daytime hypoxaemia and hypercapnia, associated with pulmonary hypertension. In this context, hypoxia-inducible factor 1-alpha levels may be associated with tissue hypoxia in patients with COPD [139]. In turn, this factor may contribute to cardiac fibrosis and impaired cardiac function [139].
Furthermore, COPD and OSAS may share the same risk factors including smoking, obesity, inflammation (both systemic and local) as well as increased airway resistance [140]. Of note, OS patients may also have an increased CV morbidity compared with patients with OSAS alone [141,142,143]. These additive effects of concurrent COPD and OSAS point to an increased prevalence of comorbidities, particularly cardio-metabolic [144,145]. In this context, the presence of abnormal APIFat deposits in patients with OSAS and OS merits further research.
6. Conclusions
COPD patients may have an increased risk of developing MetS, NAFLD, increased EFT, and intramuscular fat. One should also consider that excessive “orthotopic” APIFat accumulation may be a “systemic” rather than a “local” phenomenon. These comorbidities may affect COPD progression as well as individual CV risk and also should be considered as a marker for developing cardiovascular disease and evolution for COPD. The role of peripancreatic and perirenal adipose tissue in COPD patients has not been investigated yet. There will also be a need to consider that every APIFat may not share the same characteristics (e.g., adipokine–cytokine secretion) and the effect on different organs may vary [16,17,86,87,95,106].
Further research is needed in this field to elucidate these associations as well as their potential clinical implications.
Author Contributions
Conceptualization, N.K., A.P.S. and D.P.M.; literature search, N.K. and A.P.S.; writing—original draft preparation, N.K., A.P.S., N.P., P.S. and D.P.M.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
N.K. has given talks, attended conferences, and participated in trials sponsored by Angelini, Astra Zeneca, Bausch Health, Boehringer Ingelheim, Elpen, Mylan, Novo Nordisk, Sanofi, and Servier. P.S. has given talks, attended conferences and participated in advisory boards and trials sponsored by AstraZeneca, Boehringer Ingelheim GlaxoSmithKline, Chiesi, Elpen, Menarini, Novartis, and Pfizer. A.P.S. has given talks, attended conferences, and participated in advisory boards, and clinical trials sponsored by various pharmaceutical companies and she is currently Vice-President, National Diabetes Commission, Ministry of Health, Romania. N.P. has been an advisory board member of TrigoCare International, Abbott, AstraZeneca, Elpen, MSD, Novartis, Novo Nordisk, Sanofi-Aventis, and Takeda; has participated in sponsored studies by Eli Lilly, MSD, Novo Nordisk, Novartis, and Sanofi-Aventis; received honoraria as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elpen, Galenica, MSD, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis, Takeda, and Vianex; and attended conferences sponsored by TrigoCare International, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Pfizer, and Sanofi-Aventis. D.P.M has given talks and attended conferences sponsored by Amgen, Novo Nordisk, and Libytec.
Abbreviations
| APIFat | abnormal peri-organ or intra-organ fat |
| BODE index | body mass index (B), degree of airflow obstruction (O), dyspnoea (D) and exercise capacity (E) |
| COPD | chronic obstructive pulmonary disease |
| CPAP | continuous positive airway pressure |
| CRP | C-reactive protein |
| CV | cardiovascular |
| EFT | epicardial fat thickness |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| IL | interleukins |
| MetS | metabolic syndrome |
| MI | myocardial infarction |
| NAFLD | non-alcoholic fatty liver disease |
| NAFPD | non-alcoholic fatty pancreas disease |
| OS | overlap syndrome |
| OSAS | obstructive sleep apnoea syndrome |
| PAH | pulmonary arterial hypertension |
| RVSD | right ventricular systolic dysfunction |
| SAT | subcutaneous adipose tissue |
| T2DM | type 2 diabetes mellitus |
| TNF-α | tumour necrosis factor α |
| VAT | visceral adipose tissue |
References
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Huertas, A.; Palange, P. COPD: A multifactorial systemic disease. Ther. Adv. Respir. Dis. 2011, 5, 217–224. [Google Scholar] [CrossRef]
- Agusti, A.; Soriano, J.B. COPD as a systemic disease. COPD 2008, 5, 133–138. [Google Scholar] [CrossRef]
- Sinden, N.J.; Stockley, R.A. Systemic inflammation and comorbidity in COPD: A result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010, 65, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Kirkham, P.; Barczyk, A.; Di Stefano, A.; Adcock, I. Molecular pathogenesis of cigarette smoking-induced stable COPD. Ann. N. Y. Acad. Sci. 2015, 1340, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A. Systemic effects of chronic obstructive pulmonary disease: What we know and what we don’t know (but should). Proc. Am. Thorac. Soc. 2007, 4, 522–525. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic obstructive pulmonary disease. N. Engl. J. Med. 2000, 343, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Ingebrigtsen, T.S.; Marott, J.L.; Dahl, M.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013, 309, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, S.F.; Sin, D.D. Oxidative stress in chronic obstructive pulmonary disease: A lung and systemic process. Can. Respir. J. 2013, 20, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011, 15, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Katsiki, N.; Perez-Martinez, P.; Anagnostis, P.; Mikhailidis, D.P.; Karagiannis, A. Is Nonalcoholic Fatty Liver Disease Indeed the Hepatic Manifestation of Metabolic Syndrome? Curr. Vasc. Pharmacol. 2018, 16, 219–227. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Mikhailidis, D.P. Abnormal Peri-Organ or Intra-organ Fat (APIFat) Deposition: An Underestimated Predictor of Vascular Risk? Curr. Vasc. Pharmacol. 2016, 14, 432–441. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P. Abnormal Peri-Organ or Intra-Organ Fat Deposition and Vascular Risk. Angiology 2018, 69, 841–842. [Google Scholar] [CrossRef]
- Piazzolla, G.; Castrovilli, A.; Liotino, V.; Vulpi, M.R.; Fanelli, M.; Mazzocca, A.; Candigliota, M.; Berardi, E.; Resta, O.; Sabbà, C.; et al. Metabolic syndrome and Chronic Obstructive Pulmonary Disease (COPD): The interplay among smoking, insulin resistance and vitamin D. PLoS ONE 2017, 12, e0186708. [Google Scholar] [CrossRef]
- Vujic, T.; Nagorni, O.; Maric, G.; Popovic, L.; Jankovic, J. Metabolic syndrome in patients with chronic obstructive pulmonary disease: Frequency and relationship with systemic inflammation. Hippokratia 2016, 20, 110–114. [Google Scholar]
- Küpeli, E.; Ulubay, G.; Ulasli, S.S.; Sahin, T.; Erayman, Z.; Gürsoy, A. Metabolic syndrome is associated with increased risk of acute exacerbation of COPD: A preliminary study. Endocrine 2010, 38, 76–82. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztajn, R.; Przybyłowski, T.; Maskey-Warzęchowska, M.; Paplińska-Goryca, M.; Nejman-Gryz, P.; Karwat, K.; Chazan, R. Metabolic Syndrome as a Factor Affecting Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Adv. Exp. Med. Biol. 2017, 1021, 55–62. [Google Scholar] [PubMed]
- Minas, M.; Kostikas, K.; Papaioannou, A.I.; Mystridou, P.; Karetsi, E.; Georgoulias, P.; Liakos, N.; Pournaras, S.; Gourgoulianis, K.I. The association of metabolic syndrome with adipose tissue hormones and insulin resistance in patients with COPD without co-morbidities. COPD 2011, 8, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sood, A. Obesity, adipokines, and lung disease. J. Appl. Physiol. 2010, 108, 744–753. [Google Scholar] [CrossRef]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Gotzamani-Psarrakou, A.; Yovos, J.G.; Karamitsos, D. Effect of various treatments on leptin, adiponectin, ghrelin and neuropeptide Y in patients with type 2 diabetes mellitus. Expert Opin. Ther. Targets 2011, 15, 401–420. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Gotzamani-Psarrakou, A.; Didangelos, T.P.; Yovos, J.G.; Karamitsos, D.T. Effects of improving glycemic control with insulin on leptin, adiponectin, ghrelin and neuropeptide levels in patients with type 2 diabetes mellitus: A pilot study. Open Cardiovasc. Med. J. 2011, 5, 136–147. [Google Scholar] [CrossRef]
- Jaswal, S.; Saini, V.; Kaur, J.; Gupta, S.; Kaur, H.; Garg, K. Association of Adiponectin with Lung Function Impairment and Disease Severity in Chronic Obstructive Pulmonary Disease. Int. J. Appl. Basic Med. Res. 2018, 8, 14–18. [Google Scholar] [CrossRef]
- Yoon, H.I.; Li, Y.; Man, S.F.P.; Tashkin, D.; Wise, R.A.; Connett, J.E.; Anthonisen, N.A.; Churg, A.; Wright, J.L.; Sin, D.D. The complex relationship of serum adiponectin to COPD outcomes COPD and adiponectin. Chest 2012, 142, 893–899. [Google Scholar] [CrossRef]
- Oh, Y.M.; Jeong, B.H.; Woo, S.Y.; Kim, S.Y.; Kim, H.; Lee, J.H.; Lim, S.Y.; Rhee, C.K.; Yoo, K.H.; Lee, J.H.; et al. Association ofplasma adipokines with chronic obstructive pulmonary disease severity and progression. Ann. Am. Thorac. Soc. 2015, 12, 1005–1012. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.S. Statins in relation to adiponectin: A significant association with clinical implications. Atherosclerosis 2016, 253, 270–272. [Google Scholar] [CrossRef][Green Version]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mantzoros, C.; Mikhailidis, D.P. Adiponectin, lipids and atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Clini, E.; Crisafulli, E.; Radaeli, A.; Malerba, M. COPD and the metabolic syndrome: An intriguing association. Intern. Emerg. Med. 2013, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Santini, E.; Chiarugi, M.; Salvati, A.; Comassi, M.; Vitolo, E.; Madec, S.; Solini, A. The complex P2X7 receptor/inflammasome in perivascular fat tissue of heavy smokers. Eur. J. Clin. Investig. 2014, 44, 295–302. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jégo, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef]
- Wouters, E.F.M. Obesity and Metabolic Abnormalities in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2017, 14, S389–S394. [Google Scholar] [CrossRef]
- Cebron Lipovec, N.; Beijers, R.J.; van den Borst, B.; Doehner, W.; Lainscak, M.; Schols, A.M. The Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD 2016, 13, 399–406. [Google Scholar] [CrossRef]
- Naik, D.; Joshi, A.; Paul, T.V.; Thomas, N. Chronic obstructive pulmonary disease and the metabolic syndrome: Consequences of a dual threat. Indian J. Endocrinol. Metab. 2014, 18, 608–616. [Google Scholar]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Wierzbicki, A.S.; Mikhailidis, D.P. Should we expand the concept of coronary heart disease equivalents? Curr. Opin. Cardiol. 2014, 29, 389–395. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Metabolic syndrome and non-cardiac vascular diseases: An update from human studies. Curr. Pharm. Des. 2014, 20, 4944–4952. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Characteristics other than the diagnostic criteria associated with metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2014, 12, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.; Maltais, F.; Duguay, V.; Bezeau, A.M.; LeBlanc, P.; Jobin, J.; Poirier, P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2005, 25, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Larson, J.L. Metabolic syndrome and associated factors in people with chronic obstructive pulmonary disease. West. J. Nurs. Res. 2014, 36, 620–642. [Google Scholar] [CrossRef]
- Nussbaumer-Ochsner, Y.; Rabe, K.F. Systemic manifestations of COPD. Chest 2011, 139, 165–173. [Google Scholar] [CrossRef]
- Díez-Manglano, J.; Barquero-Romero, J.; Almagro, P.; Cabrera, F.J.; López García, F.; Montero, L.; Soriano, J.B.; Working Group on COPD; Spanish Society of Internal Medicine. COPD patients with and without metabolic syndrome: Clinical and functional differences. Intern. Emerg. Med. 2014, 9, 419–425. [Google Scholar] [CrossRef]
- Spelta, F.; Fratta Pasini, A.M.; Cazzoletti, L.; Ferrari, M. Body weight and mortality in COPD: Focus on the obesity paradox. Eat. Weight Disord. 2018, 23, 15–22. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): Clinical significance and therapeutic strategies. Pharmacol. Ther. 2019, 198, 160–188. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef]
- Alkhouri, N.; Poordad, F.; Lawitz, E. Management of nonalcoholic fatty liver disease: Lessons learned from type 2 diabetes. Hepatol. Commun. 2018, 2, 778–785. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Lee, Y.H. Non-Alcoholic Fatty Liver Disease: The Emerging Burden in Cardiometabolic and Renal Diseases. Diabetes Metab. J. 2017, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Tziomalos, K.; Katsiki, N.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J. Gastroenterol. 2015, 21, 6820–6834. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Dai, W.; Ye, L.; Liu, A.; Wen, S.W.; Deng, J.; Wu, X.; Lai, Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine 2017, 96, e8179. [Google Scholar] [CrossRef]
- Amiri Dash Atan, N.; Koushki, M.; Motedayen, M.; Dousti, M.; Sayehmiri, F.; Vafaee, R.; Norouzinia, M.; Gholami, R. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed Bench 2017, 10, S1–S7. [Google Scholar]
- Viglino, D.; Jullian-Desayes, I.; Minoves, M.; Aron-Wisnewsky, J.; Leroy, V.; Zarski, J.P.; Tamisier, R.; Joyeux-Faure, M.; Pépin, J.L. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur. Respir. J. 2017, 49, 1601923. [Google Scholar] [CrossRef]
- Jung, D.H.; Shim, J.Y.; Lee, H.R.; Moon, B.S.; Park, B.J.; Lee, Y.J. Relationship between non-alcoholic fatty liver disease and pulmonary function. Intern. Med. J. 2012, 42, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Choi, S.H.; Chung, G.E.; Park, B.; Kwak, M.S. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018, 38, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Kim, E.; Jang, E.J.; Lee, C.H. The association of non-alcoholic fatty liver disease with lung function: A survey design analysis using propensity score. Respirology 2018, 23, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Song, J.U.; Jang, Y.; Lim, S.Y.; Ryu, S.; Song, W.J.; Byrne, C.D.; Sung, K.C. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: A longitudinal cohort study. PLoS ONE 2019, 14, e0208736. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Lonardo, A.; Vinco, G.; Zoppini, G.; Lippi, G.; Bonora, E.; Loomba, R.; Tilg, H.; Byrne, C.D.; Fabbri, L.; et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: A systematic review and meta-analysis. Diabetes Metab. 2019, 45, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Vonghia, L. Pharmacological Treatment for Non-alcoholic Fatty Liver Disease. Adv. Ther. 2019, 36, 1052–1074. [Google Scholar] [CrossRef]
- Snyder, H.S.; Sakaan, S.A.; March, K.L.; Siddique, O.; Cholankeril, R.; Cummings, C.D.; Gadiparthi, C.; Satapathy, S.K.; Ahmed, A.; Cholankeril, G. Non-alcoholic Fatty Liver Disease: A Review of Anti-diabetic Pharmacologic Therapies. J. Clin. Transl. Hepatol. 2018, 6, 168–174. [Google Scholar] [CrossRef]
- Athyros, V.G.; Katsiki, N.; Mikhailidis, D.P. Editorial: Resolution of Non-Alcoholic-Steatohepatitis. More than One Drug Needed? Curr. Vasc. Pharmacol. 2016, 14, 313–315. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Mikhailidis, D.P. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: Effects of statins and antidiabetic drugs. J. Diabetes Complicat. 2017, 31, 521–522. [Google Scholar] [CrossRef]
- Amor, A.J.; Perea, V. Dyslipidemia in nonalcoholic fatty liver disease. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 103–108. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. The role of statins in the treatment of type 2 diabetes mellitus: An update. Curr. Pharm. Des. 2014, 20, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Alexandrides, T.K.; Bilianou, H.; Cholongitas, E.; Doumas, M.; Ganotakis, E.S.; Goudevenos, J.; Elisaf, M.S.; Germanidis, G.; Giouleme, O.; et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism 2017, 71, 17–32. [Google Scholar] [CrossRef] [PubMed]
- So, J.Y.; Dhungana, S.; Beros, J.J.; Criner, G.J. Statins in the treatment of COPD and asthma-where do we stand? Curr. Opin. Pharmacol. 2018, 40, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.L.; Vincent, A.H. Statin Effects on Exacerbation Rates, Mortality, and Inflammatory Markers in Patients with Chronic Obstructive Pulmonary Disease: A Review of Prospective Studies. Pharmacotherapy 2016, 36, 536–547. [Google Scholar] [CrossRef]
- Li, W.F.; Huang, Y.Q.; Huang, C.; Feng, Y.Q. Statins reduce all-cause mortality in chronic obstructive pulmonary disease: An updated systematic review and meta-analysis of observational studies. Oncotarget 2017, 8, 73000–73008. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Li, C.W.; Jones, P.; Wang, C.; Fan, Y. Effect of Statins on COPD: A Meta-Analysis of Randomized Controlled Trials. Chest 2017, 152, 1159–1168. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.; Xu, Z.; Lv, D.; Zhang, C.; Lai, T.; Li, W.; Shen, H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: A systematic review and meta-analysis of observational research. Sci. Rep. 2015, 5, 16461. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Qian, D.H.; Xu, J.C.; Yao, W.; Fan, Y.; Wang, C.Z. Statins may be beneficial for patients with pulmonary hypertension secondary to lung diseases. J. Thorac. Dis. 2017, 9, 2437–2446. [Google Scholar] [CrossRef]
- Katsiki, N.; Wierzbicki, A.S.; Mikhailidis, D.P. Pulmonary arterial hypertension and statins: An update. Curr. Opin. Cardiol. 2011, 26, 322–326. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, R.; Yao, J.; Xu, X.; Teng, Y.; Cheng, N. Effectiveness of long-term using statins in COPD—A network meta-analysis. Respir. Res. 2019, 20, 17. [Google Scholar] [CrossRef]
- Thomson, N.C. Clinical Studies of Statins in Asthma and COPD. Curr. Mol. Pharmacol. 2017, 10, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.A.; Smith, E.A.; Reid, D.J. The stats are in: An update on statin use in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2277–2284. [Google Scholar] [PubMed]
- Amariei, D.E.; Reed, R.M. The role of statins in chronic obstructive pulmonary disease: Is cardiovascular disease the common denominator? Curr. Opin. Pulm. Med. 2019, 25, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.A.; Young, T.P.; Kurugol, S.; Eckbo, E.; Muralidhar, N.; Chapman, J.K.; Kinney, G.L.; Ross, J.C.; San Jose Estepar, R.; Harmouche, R.; et al. Abdominal Visceral Adipose Tissue is Associated with Myocardial Infarction in Patients with COPD. Chronic Obstr. Pulm. Dis. 2015, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P. Epicardial fat: A novel marker of subclinical atherosclerosis in clinical practice? Anatol. J. Cardiol. 2017, 17, 64–65. [Google Scholar] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Wierzbicki, A.S. Epicardial fat and vascular risk: A narrative review. Curr. Opin. Cardiol. 2013, 28, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Li, Y.; Liu, Y.; Yan, Y.; Luo, A.; Ren, H.; She, Q. Association of epicardial adipose tissue with non-alcoholic fatty liver disease: A meta-analysis. Hepatol. Int. 2019, 13, 757–765. [Google Scholar] [CrossRef]
- Rabkin, S.W. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metab. Syndr. Relat. Disord. 2014, 12, 31–42. [Google Scholar] [CrossRef]
- Fuller, B.; Garland, J.; Anne, S.; Beh, R.; McNevin, D.; Tse, R. Increased Epicardial Fat Thickness in Sudden Death From Stable Coronary Artery Atherosclerosis. Am. J. Forensic Med. Pathol. 2017, 38, 162–166. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kälsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jöckel, K.H.; Erbel, R.; et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, A.; Hamilton, D.J.; Deng, T. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist Debakey Cardiovasc. J. 2017, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Matloch, Z.; Cinkajzlova, A.; Mraz, M.; Haluzik, M. The Role of Inflammation in Epicardial Adipose Tissue in Heart Diseases. Curr. Pharm. Des. 2018, 24, 297–309. [Google Scholar] [CrossRef]
- Demir, B.; Demir, E.; Acıksarı, G.; Uygun, T.; Utku, I.K.; Gedikbasi, A.; Caglar, I.M.; Pirhan, O.; Tureli, H.O.; Oflar, E.; et al. The Association between the Epicardial Adipose Tissue Thickness and Oxidative Stress Parameters in Isolated Metabolic Syndrome Patients: A Multimarker Approach. Int. J. Endocrinol. 2014, 2014, 954045. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Borodkina, D.; Akbasheva, O.; Karetnikova, V.; Brel, N.; Alexander, K.; Barbarash, O. Relationship between epicardial and perivascular fatty tissue and adipokine-cytokine level in coronary artery disease patients. PLoS ONE 2019, 14, e0208156. [Google Scholar] [CrossRef]
- Sova, M.; Genzor, S.; Kolek, V.; Čtvrtlík, F.; Asswad, A.G.; Zela, O.; Tauber, Z. Epicardial fat in patients with chronic obstructive pulmonary disease as a marker of high cardiovascular risk—Review. Adv. Respir. Med. 2018, 86, 314–318. [Google Scholar]
- Kiraz, K.; Gökdeniz, T.; Kalaycıoglu, E.; Börekçi, A.; Akyol, S.; Baykan, A.O.; Acele, A.; Karakoyun, S.; Seker, T.; Gür, M. Epicardial fat thickness is associated with severity of disease in patients with chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4508–4515. [Google Scholar]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Zagaceta, J.; Zulueta, J.J.; Bastarrika, G.; Colina, I.; Alcaide, A.B.; Campo, A.; Celli, B.R.; de Torres, J.P. Epicardial adipose tissue in patients with chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e65593. [Google Scholar] [CrossRef]
- Demir, M.; Acet, H.; Kaya, H.; Taylan, M.; Yüksel, M.; Yılmaz, S.; Sezgi, C.; Karadeniz, G.; Yenibertiz, D. Relationship between metabolic syndrome and epicardial fat tissue thickness in patients with chronic obstructive pulmonary disease. Anatol. J. Cardiol. 2016, 16, 405–411. [Google Scholar]
- Çetin, S.; Vural, M.G.; Gündüz, H.; Akdemir, R.; Fırat, H. Epicardial fat thickness regression with continuous positive airway pressure therapy in patients with obstructive sleep apnea: Assessment by two-dimensional echocardiography. Wien. Klin. Wochenschr. 2016, 128, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Higami, Y.; Ogawa, E.; Ryujin, Y.; Goto, K.; Seto, R.; Wada, H.; Van Tho, N.; Lan, L.T.T.; Paré, P.D.; Nakano, Y. Increased Epicardial Adipose Tissue Is Associated with the Airway Dominant Phenotype of Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0148794. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, O.; Kurtoglu, E.; Gozubuyuk, G.; Dogan, C.; Acar, Z.; EyupKoca, F.; Pekdemir, H. Epicardial adipose tissue thickness in patients with chronic obstructive pulmonary disease having right ventricular systolic dysfunction. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2461–2467. [Google Scholar]
- Gaisl, T.; Schlatzer, C.; Schwarz, E.I.; Possner, M.; Stehli, J.; Sievi, N.A.; Clarenbach, C.F.; Dey, D.; Slomka, P.J.; Kaufmann, P.A.; et al. Coronary artery calcification, epicardial fat burden, and cardiovascular events in chronic obstructive pulmonary disease. PLoS ONE 2015, 10, e0126613. [Google Scholar] [CrossRef]
- Liu, B.X.; Sun, W.; Kong, X.Q. Perirenal Fat: A Unique Fat Pad and Potential Target for Cardiovascular Disease. Angiology 2019, 70, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Dimitriadis, G.; Mikhailidis, D.P. Perirenal Adiposity and Other Excessive Intra- and Peri-Organ Fat Depots: What Is the Connection? Angiology 2019, 70, 581–583. [Google Scholar] [CrossRef]
- Blaho, M.; Dítě, P.; Kunovský, L.; Martínek, A. Fatty pancreas disease: Clinical impact. Vnitr. Lek. 2018, 64, 949–952. [Google Scholar]
- Wang, C.Y.; Ou, H.Y.; Chen, M.F.; Chang, T.C.; Chang, C.J. Enigmatic ectopic fat: Prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J. Am. Heart Assoc. 2014, 3, e000297. [Google Scholar] [CrossRef]
- Ozturk, K.; Dogan, T.; Celikkanat, S.; Ozen, A.; Demirci, H.; Kurt, O.; Turker, T.; Yilmaz, Y.; Uygun, A. The association of fatty pancreas with subclinical atherosclerosis in nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 411–417. [Google Scholar] [CrossRef]
- Kul, S.; Karadeniz, A.; Dursun, İ.; Şahin, S.; Çırakoğlu, O.F.; Sayın, M.R.; Turan, T.; Ateş, A.H. Non-Alcoholic Fatty Pancreas Disease is Associated with Increased Epicardial Adipose Tissue and Aortic Intima-Media Thickness. Acta Cardiol. Sin. 2019, 35, 118–125. [Google Scholar]
- Mirrakhimov, A.E. Nonalcoholic fatty pancreatic disease and cardio-metabolic risk: Is there is a place for obstructive sleep apnea? Cardiovasc. Diabetol. 2014, 13, 29. [Google Scholar] [CrossRef]
- Katsiki, N.; Steiropoulos, P.; Papanas, N.; Mikhailidis, D.P. Diabetes Mellitus and Chronic Obstructive Pulmonary Disease: An Overview. Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.V.C.; Pitta, F.; Hernandes, N.A.; Bertolini, G.L. Physiopathological relationship between chronic obstructive pulmonary disease and insulin resistance. Endocrine 2018, 61, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar]
- Langen, R.C.; Gosker, H.R.; Remels, A.H.; Schols, A.M. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int. J. Biochem. Cell Biol. 2013, 45, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Passey, S.L.; Hansen, M.J.; Bozinovski, S.; McDonald, C.F.; Holland, A.E.; Vlahos, R. Emerging therapies for the treatment of skeletal muscle wasting in chronic obstructive pulmonary disease. Pharmacol. Ther. 2016, 166, 56–70. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Addison, O.; Drummond, M.J.; LaStayo, P.C.; Dibble, L.E.; Wende, A.R.; McClain, D.A.; Marcus, R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging 2014, 18, 532–538. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Robles, P.G.; Sussman, M.S.; Naraghi, A.; Brooks, D.; Goldstein, R.S.; White, L.M.; Mathur, S. Intramuscular Fat Infiltration Contributes to Impaired Muscle Function in COPD. Med. Sci. Sports Exerc. 2015, 47, 1334–1341. [Google Scholar] [CrossRef]
- Maddocks, M.; Shrikrishna, D.; Vitoriano, S.; Natanek, S.A.; Tanner, R.J.; Hart, N.; Kemp, P.R.; Moxham, J.; Polkey, M.I.; Hopkinson, N.S. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur. Respir. J. 2014, 44, 1188–1198. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Moncharmont, L.; Tamisier, R.; Borel, J.C.; Arbib, F.; Wuyam, B.; Lévy, P.; Maltais, F.; Ferretti, G.; Pépin, J.L. Quadriceps muscle fat infiltration is associated with cardiometabolic risk in COPD. Clin. Physiol. Funct. Imaging 2018, 38, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Eng, J.J.; MacIntyre, D.L.; Road, J.D.; Reid, W.D. Deficits in muscle strength, mass, quality, and mobility in people with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. Prev. 2011, 31, 120–124. [Google Scholar] [CrossRef]
- Sanders, K.J.C.; Klooster, K.; Vanfleteren, L.E.G.W.; Slebos, D.J.; Schols, A.M.W.J. CT-derived muscle remodelling after bronchoscopic lung volume reduction in advanced emphysema. Thorax 2019, 74, 206–207. [Google Scholar] [CrossRef]
- Di Stasi, S.L.; MacLeod, T.D.; Winters, J.D.; Binder-Macleod, S.A. Effects of statins on skeletal muscle: A perspective for physical therapists. Phys. Ther. 2010, 90, 1530–1542. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Expert Opin. Drug Saf. 2015, 14, 935–955. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Fabis, J.; Mikhailidis, D.P.; von Haehling, S.; Sahebkar, A.; Rysz, J.; Banach, M. Prosarcopenic Effects of Statins May Limit Their Effectiveness in Patients with Heart Failure. Trends Pharmacol. Sci. 2018, 39, 331–353. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P. Excessive “orthotopic” fat accumulation: Links with cardiometabolic diseases and potential drug treatment. J. Cell. Physiol. 2020, 235, 6321–6322. [Google Scholar] [CrossRef]
- McNicholas, W.T. Chronic obstructive pulmonary disease and obstructive sleep apnea: Overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2009, 180, 692–700. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Voulgaris, A.; Papanas, N.; Nena, E.; Xanthoudaki, M.; Pataka, A.; Schiza, S.; Rizzo, M.; Froudarakis, M.E.; Steiropoulos, P. Metabolic Syndrome in Patients with Coexistent Obstructive Sleep Apnea Syndrome and Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Metab. Syndr. Relat. Disord. 2020, 18, 296–301. [Google Scholar] [CrossRef]
- Xu, S.; Wan, Y.; Xu, M.; Ming, J.; Xing, Y.; An, F.; Ji, Q. The association between obstructive sleep apnea and metabolic syndrome: A systematic review and meta-analysis. BMC Pulm. Med. 2015, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Olivetti, C.; Rosina, F.; Carbone, G.; Gambino, R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 2013, 14, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Jiang, S.; Hu, A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sleep Breath. 2018, 22, 841–851. [Google Scholar] [CrossRef]
- Mariani, S.; Fiore, D.; Barbaro, G.; Basciani, S.; Saponara, M.; D’Arcangelo, E.; Ulisse, S.; Moretti, C.; Fabbri, A.; Gnessi, L. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. Int. J. Cardiol. 2013, 167, 2244–2249. [Google Scholar] [CrossRef]
- Derin, S.; Altun, I.; Koseoglu, S.; Yilmaz, M.; Akin, F.; Sahan, M. Association of epicardial fat thickness with clinical and polysomnographic parameters in non-obese obstructive sleep apnoea patients. J. Laryngol. Otol. 2018, 132, 439–445. [Google Scholar] [CrossRef]
- Akilli, H.; Kayrak, M.; Bekci, T.T.; Erdogan, H.İ.; Aribas, A.; Yildirim, O.; Taner, A.; Erer, M.; Unlu, A. Gender-related changes of the epicardial fat thickness and leptin in obstructive sleep apnea. Echocardiography 2014, 31, 411–419. [Google Scholar] [CrossRef]
- Lubrano, C.; Saponara, M.; Barbaro, G.; Specchia, P.; Addessi, E.; Costantini, D.; Tenuta, M.; Di Lorenzo, G.; Genovesi, G.; Donini, L.M.; et al. Relationships between body fat distribution, epicardial fat and obstructive sleep apnea in obese patients with and without metabolic syndrome. PLoS ONE 2012, 7, e47059. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanizawa, K.; Tachikawa, R.; Murase, K.; Minami, T.; Inouchi, M.; Handa, T.; Oga, T.; Hirai, T.; Chin, K. Associations of obstructive sleep apnea with truncal skeletal muscle mass and density. Sci. Rep. 2018, 8, 6550. [Google Scholar] [CrossRef] [PubMed]
- Warbrick, I.; Rabkin, S.W. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes. Rev. 2019, 20, 701–712. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Teodorescu, M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013, 18, 421–431. [Google Scholar] [CrossRef]
- Voulgaris, A.; Archontogeorgis, K.; Papanas, N.; Pilitsi, E.; Nena, E.; Xanthoudaki, M.; Mikhailidis, D.P.; Froudarakis, M.E.; Steiropoulos, P. Increased risk for cardiovascular disease in patients with obstructive sleep apnoea syndrome-chronic obstructive pulmonary disease (overlap syndrome). Clin. Respir. J. 2019, 13, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Shawon, M.S.; Perret, J.L.; Senaratna, C.V.; Lodge, C.; Hamilton, G.S.; Dharmage, S.C. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med. Rev. 2017, 32, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Leung, R.S.; Aaron, S.D.; Ayas, N.; Sandoz, J.S.; Gershon, A.S. Cardiovascular Outcomes and All-Cause Mortality in Patients with Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease (Overlap Syndrome). Ann. Am. Thorac. Soc. 2019, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lacedonia, D.; Carpagnano, G.E.; Patricelli, G.; Carone, M.; Gallo, C.; Caccavo, I.; Sabato, R.; Depalo, A.; Aliani, M.; Capozzolo, A.; et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin. Respir. J. 2018, 12, 1905–1911. [Google Scholar] [CrossRef]
- McNicholas, W.T. Chronic obstructive pulmonary disease and obstructive sleep apnoea-the overlap syndrome. J. Thorac. Dis. 2016, 8, 236–242. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).