Abstract
This review explores the pharmacological potential of chondroitin sulfate and fucoidan as immunomodulatory agents targeting N-acetylgalactosaminidase (nagalase) to normalize immune responses. Nagalase, an enzyme produced by tumor and virus-infected cells, contributes to immune suppression by deactivating macrophage-activating factor. Both chondroitin sulfate and fucoidan, as representatives of glycosaminoglycans and heteropolysaccharides, exhibit significant potential in inhibiting nagalase activity, thereby restoring immune functionality. Chondroitin sulfate, a key component of the extracellular matrix, demonstrates anti-inflammatory and tissue-regenerative properties by modulating nuclear factor (NF)-κB pathways and cytokine expression. Fucoidan, a sulfated polysaccharide derived from brown seaweed, enhances immune responses through macrophage and natural killer cell activation, while also exhibiting antiviral and anticancer activities. This dual action positions these compounds as promising agents for therapeutic interventions in chronic inflammatory conditions, cancer, and infectious diseases. The synergistic effects of chondroitin sulfate and fucoidan highlight their potential to address the root causes of immune dysregulation. This review aims to elucidate the underlying mechanisms of action and explore the clinical applications of these compounds within the framework of innovative immunotherapeutic strategies. However, current evidence is limited by the predominance of preclinical studies and variability in experimental models. Well-designed clinical trials are needed to validate their efficacy for therapeutic use.
1. Introduction
Tumor cells differ from normal cells in several ways. Normal cells become tumor cells when a series of mutations cause them to continue growing and dividing uncontrollably. In this way, tumor cells achieve a kind of immortality. Normal cells grow during developmental stages, such as childhood, or during the repair of damaged tissue. Tumor cells continue to grow even when additional cells are not needed. Tumor cells cannot receive signals that activate the cessation of their growth or signals that activate programmed cell death (apoptosis) in the case of damage or aging [1]. Normal cells respond to signals from other cells that determine their growth boundaries. Tumor cells do not respond to these signals and spread into nearby tissues. This is one of the reasons why it can be difficult to surgically remove a cancerous tumor [2]. The metastatic cascade involves the dissemination of cancer cells from the original tumor site and their establishment of secondary tumors in distant organs. This process requires tumor cells to detach from the primary mass and develop the ability to move and invade other tissues. During epithelial-mesenchymal transition, tumor cells not only alter their adhesive properties but also utilize mechanisms reminiscent of developmental biology to gain motility and invasiveness. These changes involve significant rearrangement of the actin cytoskeleton and the creation of membrane extensions essential for invasion. Normally, cells express adhesion molecules that help them adhere to neighboring cells; however, some cancer cells lose these molecules, enabling them to break away and travel through the bloodstream or lymphatic system to colonize distant sites. For instance, lung cancer cells can spread to locations such as the lymph nodes, brain, liver, or bones [3].
A defining trait of cancer cells is their ability to evade normal homeostatic controls. One early indicator of tumor development is the avoidance of replicative senescence. This is often achieved by reactivating telomerase, which preserves telomere length and contributes to the cells’ capacity for unlimited division in many types of cancer. The shelterin protein complex is crucial for protecting telomeres and facilitating cancer progression. Certain shelterin components are found at elevated levels in tumors and may play a role in promoting tumor development. Additionally, the shelterin complex can influence tumor growth, size, and resistance to various therapies [4].
Human cancer cells are known to produce alpha-N-acetylgalactosaminidase (nagalase), which builds up in the bloodstream of affected individuals. Nagalase removes sugar groups, blocking the formation of Gc-protein-derived macrophage activating factor (GcMAF), a molecule essential for activating macrophages. By inhibiting macrophage function, nagalase serves as an immunosuppressive agent, helping tumor cells escape immune detection and proliferate unchecked within the body [5].
In patients with systemic lupus erythematosus, the precursor function of plasma Gc-protein was diminished or completely lost. This reduction was caused by the deglycosylation of plasma Gc-protein due to the presence of alpha-N-acetylgalactosaminidase enzyme activity in the patients’ plasma. There was an inverse relationship between the levels of this enzyme activity and the macrophage activating factor (MAF) precursor activity of the plasma Gc-protein in these individuals. Once deglycosylated, the Gc-protein is unable to be converted into macrophage activating factor, resulting in impaired macrophage activation. This defect may contribute to a failure in clearing harmful immune complexes from the body [6].
The level of nagalase activity present in the serum of patients with prostate cancer reflects the overall burden of the primary tumor, if prostatectomy has not been done, as well as the extent of metastatic tumor cells. Measuring nagalase activity in each patient can serve as a valuable tool for tracking disease progression and assessing the effectiveness of treatments [7]. In both mouse and human tumor models, the activity of nagalase in the serum correlates directly with the total tumor load. As the precursor function of serum Gc-protein declines, the level of serum nagalase activity rises. Therefore, there is an inverse relationship between the precursor activity of serum Gc-protein and serum nagalase activity.
Patients with advanced cancer had extremely high serum nagalase activity and were severely immunosuppressed. Lack of macrophage activation and immunosuppression may explain why cancer patients often die from severe infections. During curative GcMAF therapy, as tumor burden decreases, GcMAF precursor protein activity increases, while serum nagalase activity decreases. In breast cancer patients, serum nagalase activity showed a similar pattern to that of two other tumor markers. Several studies report that the half-life of nagalase is less than 24 h [8,9,10]. A more precise time specification is not given, apparently because it would require more frequent time sampling. Consequently, measuring serum nagalase activity can be an effective tool for diagnostics and determining the prognosis [11]. Saburi et al. report that a range of nagalase in the serum of healthy people is between 0.32 and 0.95 nM/min/mg [12].
2. Nagalase
Nagalase (synonyms: alpha-N-acetyl-galactosaminamidase; alpha-Na-Galase, α-acetyl-galactosaminidase, N-acetyl-α-d-galactosaminidase; N-acetyl-α-galactosaminidase; α- NAGAL; α-NAGA) is a lysosomal exoglycosidase, an enzyme that hydrolyzes the O-glycosidic bond between the terminal alpha-N-acetyl-galactosamine portions and serine or threonine in a mucin-type glycoprotein [13].
Nagalase is an enzyme belonging to the glycoside hydrolase family, characterized by its ability to cleave terminal alpha-N-acetylgalactosamine residues from glycoproteins and glycolipids. It is a dimeric or multimeric protein with a molecular weight typically ranging between 70 to 90 kDa, depending on the organism and isoform. The enzyme’s structure includes a catalytic domain responsible for its hydrolase activity and additional domains involved in substrate binding and stability [14].
Human nagalase is encoded by a gene located on chromosome 22q13™qter [15]. Molecular cloning of human nagalase revealed a molecular weight of 46 kDa [16]. Endo-nagalase obtained from Diplococcus pneumoniae exhibited a molecular weight around 160 kDa as measured by gel filtration. Its optimal activity occurs at a pH of 7.6, with an isoelectric point falling between pH 8 and 9 [17]. Proteoglycans linked through oxygen bridges typically connect to serine or threonine amino acids via a nagalase sugar residue at their reducing end. Endo-nagalase facilitates the cleavage of the O-glycosidic alpha linkage between galactosyl β1,3 N-acetyl-d-galactosamine and serine or threonine found in mucins and mucin-type glycoproteins derived from various animal species. The physiological substrates for nagalase are glycolipids, glycopeptides and glycoproteins, antigens of blood group A erythrocytes, cell wall lipopolysaccharides and capsules of bacteria [18]. Nagalase is involved in the catabolism of complex oligosaccharides. Its inadequate activity can cause serious clinical disorders in humans, such as Schindler’s disease [19,20].
Nagalase is the enzymatic basis for the process of fusion of viruses into cells, it plays a dual role in the viral infectivity of influenza virus and HIV-I as well as in immunosuppression [7,21].
Figure 1 depicts the N-acetylgalactosaminidase enzyme as a homodimeric protein, with two subunits shown in distinct colors (green and orange). Each subunit displays typical secondary structures, including α-helices and β-sheets characteristic of the enzyme’s fold [14,22]. Several ligand molecules are visible around the structure, represented in stick format, which correspond to oligosaccharide units bound through N-acetyl-galactosamine.
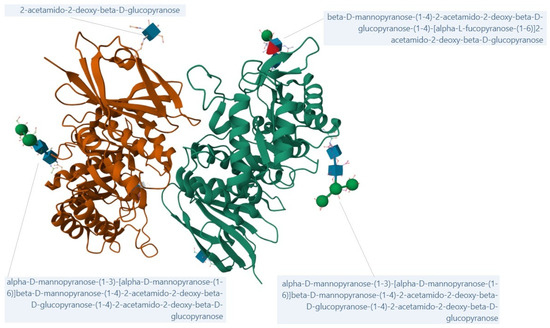
Figure 1.
Crystal structure of human alpha-N-acetylgalactosaminidase; created from 3H53 [23] with Mol* Viewer (https://www.rcsb.org/, accessed on 21 September 2025) [24,25].
2.1. Nagalase as Biomarker
Nagalase is an enzyme degrading the extracellular matrix, it is secreted to an increased extent by tumor cells in the process of tumor invasion. It is also an intrinsic component of the envelope protein of various virions, such as HIV, Epstein-Barr virus (EBV), herpes zoster virus, and influenza virus. Thus, it is also secreted from virus-infected cells [24]. Nagalase deglycosylates the vitamin D3-binding protein (VDTP), also known as the Gc-protein in humans. Gc-protein is a precursor for the main macrophage-activating factor Gc-MAF. The Gc- protein carries a single trisaccharide consisting of N-acetylgalactosamine, galactose, and sialic acid. By deglycosylation, the trisaccharide radical is removed from the Gc-protein. The Gc-protein thus deglycosylated can no longer be converted to Gc-MAF. Gc-MAF is formed from the Gc-protein by the gradual removal of galactose and sialic acid by beta-galactosidase and sialidase, selectively, leaving N-acetylgalactosamine as the remaining monosaccharide radical. Activation of macrophages for phagocytosis and antigen presentation is the first step in the immune cascade of reactions. Loss of precursor activity of the Gc-protein leads to immunosuppression [6].
Elevated levels of nagalase activity have been detected in the blood of individuals afflicted with a different types of cancers, including breast, lung, colon, liver, pancreatic, kidney, bladder, testicular, uterine, ovarian, esophageal, stomach, and prostate cancers, as well as mesothelioma, melanoma, fibrosarcoma, glioblastoma, neuroblastoma, and several forms of leukemia [11,26]. Different levels of nagalase activity have been detected for different types of tumors. The secretory capacity of individual tumor tissues seems to vary depending on the type of tumor, according to the size of the tumor, the stage and degree of malignancy or invasiveness. Increased nagalase activity was not detected in the blood of healthy people.
Nagalase activity correlates with the overall tumor load. Research papers comparing nagalase levels and tumor size indicate that assessing this enzyme may enable the early detection of cancerous lesions, even before they become identifiable by other diagnostic methods [24].
The sera of cancer patients contain nagalase, which deglycosylates the VDTP protein. Deglycosylated VDTP protein is unable to convert into macrophage activating factor, causing immunosuppression. Among 46 oral squamous cell carcinoma patients, about 22% showed a significant reduction in VDTP precursor activity, 61% had a mild decrease, and 17% maintained normal levels. Low VDTP activity was associated with high serum nagalase, while high VDTP activity correlated with low nagalase levels, indicating an inverse relationship. After tumor removal, serum nagalase slightly decreased, with a corresponding increase in VDTP precursor activity. Studies in nude mice with transplanted human oral cancer cells confirmed that serum nagalase activity correlates directly with tumor size [6]. Within one day following surgical removal of the primary tumor, nagalase activity dropped to levels near those of tumor-free controls, indicating a half-life of less than 24 h. This brief biological half-life makes nagalase a useful marker for monitoring disease prognosis during treatment. Additionally, elevated nagalase activity has been observed in the blood of patients suffering from systemic lupus erythematosus [27].
Determination of nagalase activity in the blood is a sensitive test for monitoring the effectiveness of treatments for cancer and some viral infections, including HIV. The high sensitivity of the test can help doctors and oncologists to better monitor and set up treatment [28].
2.2. Nagalase Detection Methods
Bakunina et al. (2018) [5] measured nagalase activity using an enzymatic assay that detects the cleavage of a synthetic substrate, leading to a color change measurable by spectrophotometry. This assay allows sensitive quantification of nagalase enzyme activity in various biological samples including tissue extracts, serum, plasma, and urine. The test is typically performed using microplate formats to facilitate multiple sample analysis simultaneously. Proper sample handling and storage conditions are important to ensure the stability and accuracy of nagalase activity measurements. Serum nagalase activity is generally stable when samples are refrigerated and can provide reproducible results. The enzymatic activity is then expressed relative to substrate concentration and reaction time, allowing comparisons among samples and study conditions [5].
Increased nagalase activity is generally not detected in the blood of healthy individuals within the established normal reference range. However, elevated nagalase levels have been observed in patients with certain pathological conditions, including systemic lupus erythematosus (SLE) and various cancers, reflecting increased enzymatic activity associated with disease states [27].
2.3. Nagalase as a Therapeutic Target
Macrophages are of great importance in the constant fight against infectious diseases and tumor cells. Their activation occurs by various mechanisms, one of the activation mechanisms is mediated, for example, by the vitamin D-binding protein or VDTP. After conversion through enzymes secreted by inflammation-stimulated lymphocytes, VDTP is modified to Gc-MAF. Several studies, especially those focusing on cancer, report that an enzyme known as nagalase deglycosylates Gc-MAF, which in turn inhibits macrophage activation [25]. Altered glycosylation mechanisms are thought to play an important role in the occurrence of a cascade of cancerous metastases. According to several studies, nagalase acts as an enzyme degrading the extracellular matrix. The degradation of proteoglycans containing GalNAc side chains has an important role in this process for their occurrence in the extracellular matrix and participation in the control of immune mechanisms [22].
In an experimental study on human breast cancer and human ovarian cancer cell lines, a reduction in the expression of the nagalase gene through transfection of the Naga-shRNA gene into cells was demonstrated. Transfection of the Naga-shRNA gene reduced the migration and invasiveness of tumor cell lines [25].
The ability of nagalase to inhibit macrophage activity in patients with developing tumors, where it acts as an immunosuppressant, is the subject of further research to create the basis for new immunomodulatory drugs. A hypothetical therapeutic goal is a solution neutralizing the immunosuppressive effect of nagalase by acting on VDTP and Gc-MAF.
One of the promising pharmacological solutions appears to be in vivo substrate inhibition of nagalase activity through the administration of heteropolysaccharides (HPS) and glycosaminoglycans (GAGs).
3. Heteropolysaccharides
Heteropolysaccharides (HPS), or heteroglycans, are complex carbohydrates composed of two or more different monosaccharides, often linked to proteins or lipids. They include connective tissue polysaccharides, glycoproteins, and glycolipids, which play important physiological roles such as ion binding, calcium accumulation for bone formation, and anticoagulant activity (e.g., heparin). HPS are widely distributed in animal tissues, plants, and fungi [29,30].
Bioactive HPS and HPS-protein complexes from medicinal mushrooms and plants act as biological response modifiers, enhancing immune function and providing therapeutic benefits. In cancer models, HPS reduce tumor growth and improve survival by stimulating immune cells—including macrophages, natural killer cells, dendritic cells—and inducing apoptosis and cell cycle arrest. These effects involve receptors such as toll-like receptors (TLR), dectin-1, and complement receptor 3 (CR3), which trigger cytokine release (interferons, TNFs, interleukins) [31].
Pharmaceutical-grade fungal HPS like lentinan, schizophyllan, and krestin are used as adjuncts to chemotherapy and radiotherapy in East Asia due to their low toxicity and potent antitumor activity. Their immunomodulatory and antitumor properties have been extensively documented in scientific literature [29,31].
The synergistic effect of fucoidan with existing anticancer drugs has prompted researchers to explore its therapeutic potential further. The following subchapters provides an overview of the biological effects, sources, structure, and pharmacodynamic properties of fucoidan.
3.1. Fucoidan
Fucoidan, a sulfated heteropolysaccharide, has attracted increasing scientific interest due to its diverse biological effects and wide availability from natural sources. Its known biological activities include antiviral, anticoagulant, antitumor, antioxidant, anti-inflammatory, and immunomodulatory effects [32], as well as neuroprotective properties [33]. Therapeutic use of fucoidans is intensively directed toward cancer treatment. Many chemotherapy drugs are designed to weaken tumor cell defenses but often cause cytotoxic effects on healthy tissues. Fucoidan, a sulfated polysaccharide primarily composed of fucose and derived from brown seaweed [34], has gained considerable attention as an anticancer agent due to its efficacy against several types of cancer. The known anticancer mechanisms of fucoidan include interruption of the cell cycle, induction of apoptosis, and stimulation of cytotoxic NK cells and macrophages. Additionally, fucoidan protects against toxicity associated with chemotherapy drugs and radiation-induced damage [35].
3.1.1. General Information About Fucoidan
Fucoidan was first isolated from brown algae species, namely Laminaria digitata, Ascophyllum nodosum, and Fucus vesiculosus, in 1913 [36]. It is a well-soluble, hygroscopic, and negatively charged polysaccharide. The leaves of Laminaria digitata, Ascophyllum nodosum, Macrocystis pyrifera, and Fucus vesiculosus are particularly rich in fucoidan [37]. Fucoidan is also highly soluble in acidic environments [38,39]. Most macroalgae contain polysaccharides as their most abundant components. Functionally, polysaccharides can be classified into two main groups: storage polysaccharides (such as starch, glycogen, or laminaran) and structural polysaccharides, which form cell walls, intercellular tissues, and the mucilage matrix [40]. The richest natural sources of fucoidan are brown seaweed (Phaeophyceae) and sea cucumbers (Holothuroidea). Brown algae species with relatively high and variable fucoidan content include Analipus japonicus, Ascophyllum nodosum, Caulerpa racemosa, Dictyota menstrualis, Fucus evanescens, F. serratus, F. distichus, F. vesiculosus, Hizikia fusiforme, Chorda filum, Kjellmaniella crassifolia, Laminaria hyperborea, L. japonica, Macrocystis pyrifera, Padina gymnospora, and Sargassum stenophyllum [41]. Some invertebrates and echinoderms are also rich sources of fucoidan [41]. The most commonly cultivated seaweeds include Saccharina japonica, Undaria pinnatifida, Sargassum fusiforme, Porphyra sp., Eucheuma sp., Kappaphycus alvarezii, Gracilaria sp., Enteromorpha clathrata, and Monostroma sp., depending on their intended use [42].
Fucoidans are a group of heteropolysaccharides consisting of variable polymeric molecules made up of heterogeneous monosaccharide units. In addition to the main carbohydrate fucose (Fuc), fucoidans contain varying proportions of galactose (Gal), mannose (Man), xylose (Xyl), rhamnose (Rha), and glucuronic acid (GlcA) [40]. Later studies also identified acetate esters in their structure. Early purification experiments aimed at isolating “fucan,” a polysaccharide containing only fucose residues, were based on the assumption that other monosaccharides originated from contaminating polysaccharides. However, even in supposedly pure samples, small proportions of galactose, xylose, and uronic acids persisted [43]. Consequently, most fucoidan samples isolated to date are heterofucans, containing a mixture of monosaccharides rather than pure fucose polymers.
Fucoidan acetate esters are notably prevalent, having been detected in nearly every sample where their presence has been investigated. However, the determination of acetyl groups remains uncommon, as they are typically identified only through NMR spectra or specific colorimetric techniques [40]. Due to their high instability in mildly alkaline or acidic environments, these groups may be lost during certain extraction procedures and thus remain undetected [44,45]. Interestingly, fucoidan extracted from Fucus vesiculosus was found to contain the amino sugar galactosamine as one of its subunits [36]. Comprehensive analysis revealed the presence of fucose, galactose, glucose, mannose, xylose, uronic acid, glucosamine, and sulfate in a molar ratio of 1.00:0.04:0.01:0.48:0.24:0.18:0.56:1.90, respectively [46].
When comparing fucoidan composition data across various seaweeds, several factors must be considered. While taxonomy is important, other influences—such as geographical origin, year and season of harvest, extraction and purification protocols, analytical methods, and even the specific part or reproductive stage of the seaweed—play crucial roles in determining the final characteristics of fucoidans [47].
Absorption and Bioavailability: Fucoidans, as high molecular weight polysaccharides (HPS), have traditionally been regarded as dietary fiber following oral administration, since the human gastrointestinal tract lacks the enzymes necessary for their digestion. Accordingly, it was long assumed that fucoidan molecules could not enter the bloodstream [48]. The biological effects of fucoidan differ based on factors such as the source, chemical composition, molecular weight, structural features, and application route. Although numerous studies have documented its effects after oral administration, there is limited research focusing on how fucoidan is absorbed and metabolized in the body [49].
Despite their high molecular weight, fucoidans have been associated with systemic effects. Michel et al. [50] reported that fucoidan is not fermented by human gut flora and is completely eliminated after oral administration. Nevertheless, fucoidan can induce a range of biological responses within the gastrointestinal tract [51]. Using a competitive ELISA assay, Irhimeh et al. [49] measured serum fucoidan levels and found an intestinal absorption rate of 0.6%, which is considerably higher than previously observed by Tokita et al. (2010) [52]. Such discrepancies may be attributed to differences in natural sources or the antibodies used for detection.
Nakazato et al. [53] reported that nitrosamine may enhance intestinal absorption of fucoidan in animal models, though the underlying mechanisms remain unclear. A specific antibody for fucoidan from Cladosiphon okamuranus (Okinawa Mozuku) was developed, and a sandwich ELISA method was introduced for its measurement. Using this method, human serum fucoidan concentrations were detected in seven out of ten men approximately six hours after oral administration, with higher concentrations observed in urine than in serum. However, the precise mechanisms of fucoidan uptake and its fate within the intestinal tract are not yet fully understood. Overall, clinical evidence confirms that fucoidan is absorbed into the bloodstream [52].
Fucoidan can traverse the intestinal barrier via two parallel pathways: transcellular and paracellular. Transport studies using Caco-2 cells showed peak fucoidan transport after one hour, followed by a rapid decline. As this process is not consistent with simple diffusion, it is likely that fucoidan absorption involves specific transporters or pinocytosis [54]. Nagamine et al. [55] further demonstrated that, following oral administration, fucoidan preferentially accumulates in liver macrophages, with only low systemic levels detected in the blood.
Although no studies have directly assessed the specific effects of fucoidan supplementation, several placebo-controlled clinical trials have explored the impact of brown seaweed extract supplementation on cognitive function. For example, Reid et al. [56] investigated the effects of a fermented extract of Laminaria japonica in healthy elderly Japanese adults and found that six weeks of supplementation (1.5 g/day) led to improvements in cognitive and memory tests compared to placebo.
3.1.2. Anticancer Effects of Fucoidan
Fucoidan is widely used in Asia as a medicinal food supplement due to its anti-tumor properties, which have been extensively studied since the 1980s [34,57]. Multiple studies have demonstrated that fucoidan can inhibit tumor growth by arresting the cell cycle, suppressing angiogenesis, inducing apoptosis, and activating natural killer (NK) cells or macrophages [58].
At a concentration of 1.0 mg/mL, fucoidan derived from Cladosiphon okamuranus increased the G0/G1-phase population of Huh7 hepatocarcinoma cells, indicating cell cycle arrest at this phase [59]. Comparative studies have shown that type II fucoidan from F. vesiculosus induces apoptosis in MCF-7 and HeLa cells via activation of caspases-8 and -9, similar to low molecular weight derivatives of type I fucoidan [47,60,61].
The antitumor mechanisms of fucoidan are thought to involve four principal pathways: (i) inhibition of mitosis and cell cycle regulation; (ii) activation of tumor cell apoptosis signals; (iii) inhibition of vascular endothelial growth factor (VEGF) production; and (iv) stimulation of NK cells and T lymphocytes.
Effects Against Colon Cancer: Colon cancer is among the most prevalent cancers worldwide. In a human colon cancer DLD-1 cell model, fucoidan extracted from Saccharina cichorioides suppressed tumor cell proliferation by inhibiting epidermal growth factor activity [62]. Thinh et al. [63] found that three fucoidan fractions from Sargassum mcclurei were not cytotoxic but inhibited colony formation of DLD-1 cells.
In vivo, mice bearing colon tumors were administered fucoidan (5 g/kg daily) extracted from Cladosiphon okamuranus in low, medium, and high molecular weight fractions. All fractions significantly inhibited tumor growth, with the medium molecular weight fraction being the most effective. Mice treated with fucoidan also exhibited increased survival times and a rapid rise in NK cell numbers, particularly with the high molecular weight fraction [64].
Effects on Hepatoma Cells: Fucoidan treatment upregulated NDRG-1/CAP43 and inhibited hepatocarcinoma cells, potentially reducing metastasis via upregulation of p42/44 MAPK-mediated vascular membrane protein 1 (VMP-1), inhibition of caspases-7 and -8, and activation of the Fas-related death domain [65]. In SMMC-7721 human hepatoma cells, fucoidan induced apoptosis through a ROS-mediated mitochondrial pathway, as evidenced by increased ROS, mitochondrial damage, and depolarization of mitochondrial membrane potential [66].
Effects Against Bladder Cancer: Experimental studies have shown that fucoidan induces apoptosis in bladder tumor cells, accompanied by mitochondrial dysfunction and sequential activation of caspases-8, -9, and -3. Fucoidan also inhibits tumor growth by increasing the expression of cyclin-dependent kinase inhibitors and inducing G1 phase cell cycle arrest [67,68]. Additional studies report that fucoidan reduces the expression of telomerase and key signaling proteins, promoting apoptosis via ROS-dependent inactivation of the PI3K/Akt pathway [69].
Effects Against Lung Cancer: In C57BL/6 mice with transplanted Lewis lung carcinoma, prophylactic fucoidan administration inhibited lung metastases by suppressing VEGF and matrix metalloproteinases [70]. In vitro, fucoidan from Bifurcaria bifurcata irreversibly blocked the growth of non-small cell lung carcinoma cells [71]. Qiu et al. [72] demonstrated that combining fucoidan with gefitinib significantly inhibited the viability of lung cancer cells resistant to tyrosine kinase inhibitors by inducing apoptosis.
Fucoidan and other cancer types: Oral administration of fucoidan (5 mg/kg) effectively inhibited tumor growth in mice with melanoma B16 cells. Over-sulfated fucoidan was particularly potent, suppressing VEGF expression and neoplastic angiogenesis more effectively than standard fucoidan [73]. In prostate cancer models, fucoidan inhibited key signaling pathways, promoted apoptosis, and protected against uncontrolled cell division [74].
3.1.3. Anti-Inflammatory Effects of Fucoidan
Inflammation represents the body’s primary immune reaction to harmful triggers such as injury, stress, or infection. This response activates macrophages and neutrophils, leading to the release of various substances like nitric oxide (NO), both pro-inflammatory and anti-inflammatory cytokines, and prostaglandin E2 (PGE2) [75]. Among the main pro-inflammatory cytokines, interleukin-1β (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are commonly used as markers of inflammation [76]. Although inflammation serves as an immune defense mechanism, it also contributes to the development of numerous diseases.
Fucoidans derived from algae have recently attracted considerable attention for their diverse biological activities and therapeutic potential. Experimental studies have shown that fucoidan can act at multiple stages of the inflammatory process, including blocking lymphocyte adhesion and invasion, inhibiting various enzymes, and inducing apoptosis [77,78].
Menshova et al. [79] examined how complement system activation is related to the sulfate content in water-extractable fucoidans from Fucus evanescens, Laminaria japonica, and Laminaria cichorioides. Their findings indicated that sulfate groups are not crucial for triggering complement activation, whereas the presence of fucose residues within the polysaccharide structure enhances the activation of the alternative complement pathway.
The most widely discussed mechanism underlying fucoidan’s anti-inflammatory effects is the downregulation of MAPK and NF-κB signaling pathways, leading to reduced production of pro-inflammatory cytokines [80]. Cumashi et al. [81] studied fucoidans from nine brown algae species and found that all inhibited leukocyte recruitment in a rat inflammation model, regardless of their fucose or sulfate content or other structural features.
Ni et al. [82] further elucidated the anti-inflammatory mechanism of purified fucoidan from Saccharina japonica, reporting reduced production of NO, iNOS, and COX-2, as well as lower TNF-α, IL-1, and IL-6 levels in LPS-stimulated RAW 264.7 macrophage cells. This activity was associated with attenuation of MAPK and NF-κB phosphorylation.
Keratinocytes, abundant in the epidermis, produce pro-inflammatory cytokines and chemokines in response to pathogen invasion, leading to skin inflammation and potentially chronic conditions such as atopic dermatitis [83]. Fucoidan treatment of human keratinocyte (HaCaT) cell lines resulted in reduced IL-1 and IL-6 levels and suppressed the synthesis of inflammatory chemokines [84].
4. Glycosaminoglycans
Glycosaminoglycans (GAGs) are linear polysaccharides with negative charges found both on human cell surfaces and within the extracellular matrix. They bind to a diverse array of proteins, such as proteases, growth factors, cytokines, chemokines, and adhesion molecules, enabling them to regulate numerous physiological functions including protein activity, cell adhesion, and signaling pathways. These interactions between GAGs and proteins play significant roles in the development and progression of various human diseases like cardiovascular disorders, infectious diseases, neurodegenerative conditions, and cancers [85]. GAGs typically possess a polymeric structure with a molecular weight ranging from about 10 to 100 kDa [86].
Among natural polysaccharides, GAGs exhibit highly complex structures due to variations in the types of sugar residues, the nature of glycosidic linkages, the degree and sites of sulfation, and the length of their chains (Figure 2). Based on the specific types of hexosamines, hexoses, or hexuronic acids present in their repeating disaccharide units, as well as the glycosidic bonds connecting these units, GAGs are categorized into five main groups, including non-sulfated GAGs like hyaluronic acid [87] and sulfated GAGs, including heparin and heparan sulfate [88], chondroitin sulfate [89], dermatan sulfate [90] and keratan sulfate (KS) [91]. The mammalian GAGs, except for hyaluronic acid, are covalently attached to core proteins, forming proteoglycans. The biological functions of proteoglycans are influenced by the structure of their protein cores, the types and composition of the attached GAG chains, and how the proteoglycans are distributed within tissues. Variations in these factors determine the diverse activities and roles of proteoglycans in physiological and pathological processes [92].
GAGs play a vital role due to their diverse functions as signaling molecules that control protein activity and serve as essential structural elements and regulators of cellular functions. They influence numerous biological processes, including embryonic development, enzyme activity regulation, the formation of the extracellular matrix, receptor-ligand interactions, and cell signaling. These effects are mediated through their regulation of various proteins, such as growth factors, chemokines, and adhesion molecules [93].
Increasing research attention on the mechanisms of GAG-protein interactions and their involvement in human diseases is driving the development of targeted therapies. Because GAG-protein interactions influence numerous physiological functions, understanding their role in particular diseases has sparked considerable interest in exploring novel treatment and prevention strategies.
GAGs play a key role in inflammation, neurological diseases such as Parkinson’s disease, Alzheimer’s disease, and other diseases. Numerous studies have highlighted the significant role of glycosaminoglycans (GAGs) in inflammation. The highly sulfated regions of heparan sulfate contribute to various stages of leukocyte movement across the vascular wall by interacting with proteins such as L-selectin, CXCL8 (CXC-chemokine ligand 8), and histidine-rich glycoprotein. Hyaluronic acid’s binding to CD44 and tumor necrosis factor-stimulated gene-6 (TSG-6) activates inflammatory cells [94], and its interaction with TLR-4 on dendritic cells promotes cytokine release [95]. Additionally, low molecular weight heparins (LMWH) can inhibit leukocyte extravasation by interacting with tumor necrosis factor (TNF) and the nuclear transcription factor NF-kB [96].
Several studies indicate that GAGs may have therapeutic potential for Alzheimer’s disease and other dementias. GAGs interact with several important growth factors in the brain, including basic fibroblast growth factor (FGF-2), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and τ-proteins [97]. Heparin is able to inhibit BACE1, the enzyme responsible for amyloid precursor protein cleavage, thereby reducing β-amyloid levels [98]. Similarly, chondroitin sulfate derived from Sardina pilchardus has also been shown to suppress BACE1 activity [99]. Beyond neurological diseases, GAGs provide therapeutic benefits in conditions such as sinusitis, asthma, chronic obstructive pulmonary disease, cystic fibrosis, and primary ciliary dyskinesia. For example, hyaluronic acid can activate TSG-6, CD44, and TLR-4, resulting in calcium channel activation and heightened immune responses [100].
From a chemical perspective, GAGs are polymers made up of repeating disaccharide units connected by oxygen bonds. These disaccharides typically consist of an amino sugar and a uronic acid. An exception is KS, which contains β-d-galactose (Gal) instead of a uronic acid, linked alternately by β1,4 and β1,3 glycosidic bonds to N-acetyl-α-d-glucosamine (GlcNAc). Generally, the amino sugar is acetylated, except in heparin. The uronic acid component can be either glucuronic acid (GlcA) or its isomer iduronic acid [101].
The GAG chains are sulfated in most cases, except for hyaluronan. Sulfation in KS occurs exclusively in the position of 6 galactose and acetylglucosamine rings, while other sulfated GAGs show greater diversity in the position of sulfate groups. This diversity is further enhanced by the epimerization of uronic acid, resulting in a few different disaccharides, partly responsible for the considerable complexity of the GAG chains. Both chondroitin sulfate and dermatan sulfate contain the same amino sugar N-acetyl α-d-galactosamine, but the glucuronic acid in chondroitin sulfate undergoes epimerization to iduric acid in dermatan sulfate. Sulfation at different positions and to varying degrees produces similar disaccharide-type units in both chondroitin and dermatan sulfate [101].
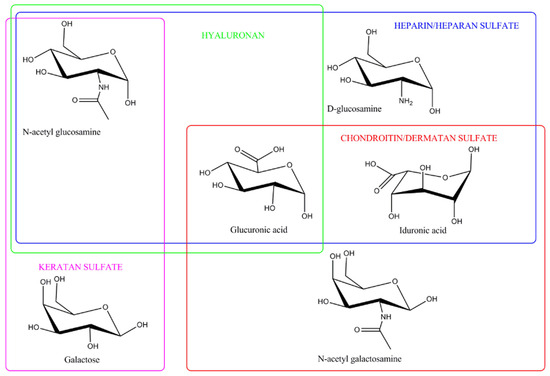
Figure 2.
Structure of glycosaminoglycans. This figure was adapted from Valcarcel et al. [101], with copyright permission from Elsevier.
4.1. Chondroitin Sulfate
Chondroitin sulfate is an essential component of extracellular matrix connective tissues of animals and humans, in which it plays a central role in various physiological and biological processes, such as the strength and elasticity of articular cartilage, hemostasis and inflammation, regulation of cell development, cell adhesion, cell proliferation and differentiation. It is present on all animal cell surfaces and in the extracellular matrix, especially in cartilage, skin, blood vessels, ligaments, tendons and the brain, where it forms a fundamental component of proteoglycans, binds and regulates various proteins, such as growth factors, enzymes, cytokines, participates in re-epithelialization, stimulation of neovascularization, is part of hydrogels [102]. It preserves the integrity and function of cartilage tissue, increases the production of type II collagen and proteoglycans that reduce bone resorption, improves anabolic-catabolic balance in chondrocytes and osteoarthritis [103], therefore it has the potential to become part of a protective strategy to promote health during human aging [104].
Chondroitin sulfate belongs to a class of natural complex heteropolysaccharides, called glycosaminoglycans (GAGs). The molecule has the character of a biopolymer with a complex structure. It is unbranched, polydisperse, has an anionic character. It is one of the most abundant GAGs in mammalian tissues, where it participates in several physiological and biological functions [105].
Chemically, it is (2R,3R,4R,5R,6R)-5-acetamido-4-[(2R,3R,4S,5S,6S)-6-carboxy-4,5-dihydroxy-3-sulfonatooxyoxan-2-yl]oxy-6-hydroxy-2-(hydroxymethyl)oxan-3-yl] sulfate [106]. CS is a heteropolysaccharide formed by repeating disaccharide units composed of glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc). Some of the GlcA residues can be epimerized to L-iduric acid, in which case the resulting glycosaminoglycan is referred to as dermathan sulfate, known also as chondroite sulfate B. Due to the presence of sulfate groups in different amounts and location at different positions, chondroitin sulfate represents a very heterogeneous heteropolysaccharide. Disaccharide subunits are linked β-(1→3) by glycosidic bonds and sulfated at different carbon positions. The classification of chondroitin sulfate (type A to E) depends on the location of the sulfate group at the carbon position of C4 (type A), C4-GalNAc/C2-GlcA (type B), C6 (type C), C6-GalNAc/C2-GlcA (type D), C4 and C6 (type E) [107].
These sulfation steps appear to take place in the Golgi apparatus simultaneously with the formation of the main carbohydrate GAG chain [108]. In addition to chondroitin sulfate types A to E, fucosylated forms of chondroitin sulfate (FCS) have been described. FCS has been discovered exclusively in the body wall of Holothuria (Holothuria tubulosa). It has a structure like mammalian chondroitin sulfate, namely repeating disaccharide units composed of GlcA and GalNAc, but additionally contains fucosylated branches linked to GlcA and/or GalNAc residues via oxygen bonds at position 3 [109,110,111].
4.1.1. General Information About Chondroitin Sulfate
Biosynthesis of chondroitin sulfate: is a multi-step and complex process occurring through enzymatic processes in compartments of the endoplasmic reticulum, the Golgi apparatus [112]. It begins with the covalent attachment of a GAG-protein binding region to specific serine residues that are embedded in various nuclear proteins [113]. Multiple enzymes are involved in this process. Biosynthesis is initiated by glycosyltransferase, xylose is attached to the serine residue by xylosyltransferase, the bond between galactose and serine residues is catalyzed by β1,4-galactosyltransferase I and β1,3-galactosyltransferase II, GlcA residue is attached by β1,3-glucuronyl transferase I, GalNAc transferase attaches GalNAc to the non-reducing terminal GlcA residue and chondroitin skeleton. The final polymerization process is catalyzed by GalNAc-transferase II and GlcA-transferase II. Upon polymerization, epimerases can convert GlcA residues in the chondroitin skeleton to iduric acid, transforming chondroitin domains into dermatanic domains [114].
Chondroitin sulfate serves as building blocks of proteoglycans. Its supplementation inhibits NF-ƙB-mediated inflammation and promotes extracellular matrix homeostasis (schematic representation is in Figure 3).
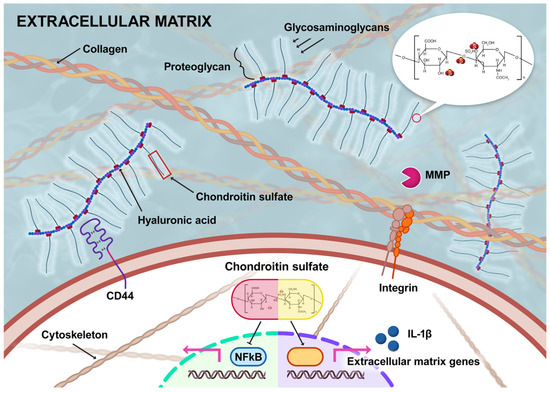
Figure 3.
Structural features of chondroitin sulfate and its role in extracellular matrix homeostasis and anti-inflammatory effects (MMP is matrix metalloproteinase) [104].
Sources: Chondroitin sulfate is commonly obtained from by-products of cattle, pigs, chickens, crustaceans, and fish. Cartilage, a major by-product of slaughterhouses and the fishing industry, serves as the richest and most widely available natural source for chondroitin sulfate extraction. The extraction method of chondroitin sulfate from fish typically involves enzymatic hydrolysis and chemical treatments to break down the proteoglycan structure and isolate chondroitin sulfate. The general process includes delipidation with solvents like acetone and chloroform to remove fats, followed by proteolytic digestion using enzymes such as papain or alcalase to release chondroitin sulfate from tissue. Enzymatic methods are preferred over purely chemical extraction due to higher yield and specificity. Extraction is commonly done on various fish parts like skin, scales, bones, and cartilage. The exact sequence and chemicals may differ depending on the fish species and tissue source, but the principle is enzymatic tissue digestion, protein removal, and purification of glycosaminoglycans including chondroitin sulfate [106].
Notably, chondroitin sulfate is the most abundant glycosaminoglycan found in cartilage, and its presence in the extracellular matrix of connective tissue is crucial for maintaining the elasticity of articular cartilage [115].
For example, Luo et al. [116] extracted proteoglycans from chicken cartilage and precipitated glycosaminoglycans using ethanol. The glycosaminoglycans were then freeze-dried and quantified. Their results indicated that each gram of fresh cartilage yielded 32.9 mg of glycosaminoglycans, of which 75% was chondroitin sulfate. After conversion, the lyophilized chondroitin sulfate content corresponded to 2.47% of the fresh cartilage weight. Similarly, marine sources such as holothurians (sea cucumbers) have been shown to provide relatively high chondroitin sulfate yields, ranging from 6.3 to 9.9% by weight [117]. In another study, chondroitin sulfate extracted from zebrafish (Danio rerio) was found to consist of disaccharide units in the following proportions: 1→3-N-acetylgalactosamine 4-sulfate at 59.4%, 1→3-N-acetylgalactosamine 6-sulfate at 23.1%, and 1→3-N-acetylgalactosamine at 17.5% [118].
However, animal-derived chondroitin sulfate presents certain drawbacks, including high structural variability and the potential mixing of multiple sources during production. This can result in a final product with unpredictable characteristics and poorly defined components, often accompanied by animal-derived contaminants such as proteins.
In addition to animal sources, chondroitin sulfate has also been isolated from the plant Tremella fuciformis. Recent studies suggest that plant-based chondroitin sulfate holds promise as a nutraceutical or pharmaceutical agent, with potential antitumor, immunomodulatory, hypoglycemic, and anti-aging effects [119].
The molecular weight of chondroitin sulfate obtained from animal sources typically ranges from 10 to 100 kD, depending on the specific source. Various depolymerization techniques can reduce this molecular weight to a range of approximately 1.2 to 8 kD. Chondroitin sulfate prepared by these depolymerization methods, or through biofermentation, is classified as low molecular weight chondroitin sulfate, commonly referred to as LMWCS [120]. Valcarcel et al. [121] prepared low molecular weight chondroitin sulfate from fish-derived chondroitin sulfate, which initially had a molecular weight of 51 to 70 kD. This reduction was achieved using enzymatic depolymerization with hyaluronidase and chondroitinases A, B, and C produced by Proteus vulgaris. The resulting low molecular weight chondroitin sulfate exhibited a molecular weight ranging from 1.29 to 4 kD.
Low molecular weight chondroitin sulfate (LMWCS) can be prepared using a variety of methods. The most common approaches include acid or alkaline hydrolysis, oxidative depolymerization, enzymatic depolymerization, ultrasonically assisted acid hydrolysis, and biofermentation-based depolymerization. These techniques enable the production of heterooligosaccharides with different molecular weights and varying degrees of polydispersity, depending on the specific degradation conditions employed. Li et al. prepared low molecular weight chondroitin sulfate using four different depolymerization methods applied to cartilage from sharks, pigs, and cattle [120]. All samples contained mainly chondroitin-6-sulfate and chondroitin-4-sulfate. Depolymerized shark cartilage products also contained chondroitin-2,6-sulfate and -4,6-sulfate. Hydrochloric acid and the microwave-assisted alkaline depolymerization method caused a significant loss of sulfate content. The oxidative degradation method had a less significant effect on the sulphate content and disaccharide composition compared to the use of acidic environments and microwave radiation. The non-reducing and reducing ends of LMWCS from oxidation, HCl and microwave-assisted alkaline methods were the same as the original, intact chondroitin sulfate, which was also confirmed by Li et al. [120]. The enzymatic method was gentle but caused the formation of a residue at the non-reducing end. Table 1 summarizes the key differences in molecular weight, receptor binding efficiency, cytokine modulation, and bioavailability between biofermented low-molecular-weight chondroitin sulfate (LMW CS) and conventional chondroitin sulfate (CS).

Table 1.
Comparison of the immunological profile and properties of biofermented low-molecular-weight chondroitin sulfate (LMW CS) and conventional chondroitin sulfate (CS).
Innovations in Chondroitin Sulfate Manufacturing: The K4 polysaccharide expressed by Escherichia coli (E. coli) K4 closely resembles chondroitin in structure, making it a suitable precursor for chondroitin sulfate production [125].
Biofermentable chondroitin sulfate—also known as non-animal chondroitin sulfate (NACS)—is chemically identical to chondroitin sulfate. It is produced through enzymatic sulfation of an unsulfated chondroitin main chain. This main chain is obtained by acid-thermal hydrolysis of the capsular polysaccharide naturally synthesized by a specific strain of E. coli. The sulfation process employs chondroitin sulfate-sulfotransferases, which are integral transmembrane glycoproteins normally found in the Golgi apparatus. For industrial use, these enzymes are biotechnologically engineered into soluble forms and expressed to serve as efficient catalysts for E. coli-based chondroitin sulfate synthesis [126,127].
NACS is highly purified, standardized, and structurally validated through analytical methods. Its defining features include sulfate groups in well-defined positions, consistent charge density, a defined molecular weight range, and a homogeneous structure that closely matches that of synovial fluid. NACS is primarily monosulfated, with most sulfate groups located at the 6th carbon position (chondroitin-6-sulfate), and only trace or no sulfation at the 4th carbon position (chondroitin-4-sulfate). This product is very similar to natural chondroitin sulfate, but it is distinguished by the absence of trisulfated and polysulfated disaccharides. Additionally, NACS has a lower molecular weight (1–5 kDa) compared to chondroitin sulfate extracted from animal tissues.
The charge density of NACS ranges from 1.0 to 1.25, and its sulfation pattern and charge density most closely resemble those of shark-derived chondroitin sulfate. Notably, NACS exhibits extremely low protein contamination (≤0.5%), in contrast to animal-derived chondroitin sulfate, which typically contains residual protein levels of about 10% after extraction [128].
Degradation: Some bacteria utilize adhesion mechanisms to attach to host cells, enabling them to establish themselves within the host organism. This process often involves interaction with glycosaminoglycans (GAGs), which are important components of the host extracellular matrix.
Until recently, probiotic strains capable of degrading GAGs were not widely recognized. However, a study by Kawai et al. [129] demonstrated that certain probiotic strains from the human gut microbiota are capable of degrading GAGs and can adhere to human gut cells through GAG-mediated interactions. These GAG-degrading bacteria have been isolated from human feces, with Enterococcus faecium identified as one such species. Some well-known probiotics, including Lactobacillus casei, Lactobacillus rhamnosus, and Enterococcus faecalis, have also been shown to degrade heparin, another type of GAG.
Genetic analysis has revealed that GAG-degrading lactobacilli and enterococci, such as the isolated E. faecium, possess a genetic cluster responsible for encoding GAG-degrading and metabolizing enzymes. For instance, in Lactobacillus rhamnosus, the enzymes KduI and KduD—encoded within the GAG cluster—function as 4-deoxy-L-threo-5-hexosulose-uronate ketol isomerase and 2-keto-3-deoxy-d-gluconate dehydrogenase, respectively. These enzymes are essential for GAG metabolism [129].
Additionally, certain species of Bacteroides, considered next-generation probiotics, have demonstrated the ability to degrade chondroitin sulfate C and hyaluronan. In contrast, while Lactobacillus casei and Lactobacillus pantheris showed slight degradation of chondroitin sulfate C, other probiotics—including Lactobacillus acidophilus, Lactobacillus aviarius subsp. aviarius, Lactobacillus brevis, Lactobacillus parabuchneri, Lactobacillus paracasei subsp. tolerans, Lactobacillus reuteri, Lactobacillus saerimneri, and Bifidobacterium bifidum—exhibited little or no detectable degradation of other GAGs [129,130,131].
Moreover, genes encoding GAG-degrading enzymes are frequently detected in Bacteroides within the human gut microbiota. Collectively, these findings confirm that certain probiotic strains in the human gut microbiota are indeed capable of degrading GAGs [129].
4.1.2. Anticancer Effects of Chondroitin Sulfate
He et al. (2014) [132] studied the antimetastatic properties of fucosylated chondroitin sulfate derived from the sea cucumber Isostichopus badionotus. This compound significantly inhibited cell proliferation, adhesion, migration, and invasion in cancer cell models. In the chorioallantoic membrane (CAM) assay, it reduced neovascularization in chicken embryos. Fucosylated chondroitin sulfate also suppressed the expression of matrix metalloproteinases (MMP-2/9), hypoxia-inducible factor (Hif-1α), heparanase, and vascular endothelial growth factor (VEGF), while increasing the levels of tissue inhibitors of metalloproteinases (TIMP-1/2). In vivo, it inhibited tumor growth, reduced metastatic foci, and lowered the expression of key pro-metastatic proteins.
Further research by Borsig et al. (2007) [133] demonstrated that fucosylated chondroitin sulfate is a potent, concentration-dependent inhibitor of P- and L-selectin binding to cancer cells, being 4–8 times more effective than heparin in blocking these interactions. It did not inhibit E-selectin. In mouse models, it reduced lung colonization by cancer cells and neutrophil recruitment during inflammation without affecting blood coagulation times. Removal of the sulfated fucose branches abolished its inhibitory effects, highlighting their importance. These findings suggest that invertebrate-derived fucosylated chondroitin sulfate could be a promising alternative to heparins for preventing metastasis and inflammation, without the side effects typically associated with heparin.
4.1.3. Anti-Inflammatory Effects of Chondroitin Sulfate
Inflammatory responses are triggered by infectious agents, immune reactions, or tissue injury, which activate membrane receptors on cells. This activation initiates phosphorylation of signaling pathways such as mitogen-activated protein kinase (MAPK) and NF-κB. When NF-κB binds to the promoters of target genes, it increases the expression of pro-inflammatory cytokines, inducible nitric oxide synthase, cyclooxygenase-2 (COX-2), phospholipase A2, and matrix metalloproteinases—proteins that contribute to tissue damage and inflammation. NF-κB activation is central to immune homeostasis and the inflammatory process, playing a key role in the development of many diseases.
Chondroitin sulfate has been shown to reduce NF-κB activation and its nuclear translocation in both chondrocytes and synovial membrane cells. These effects may explain the clinical benefits of chondroitin sulfate in osteoarthritis. Systemic administration of chondroitin sulfate also reduces NF-κB nuclear translocation in macrophages and hepatocytes, suggesting potential benefits in other diseases with strong inflammatory components [134,135].
Imada et al. (2010) [136] investigated the anti-arthritic effects of chondroitin sulfate on human articular chondrocytes and synovial fibroblasts. Chondroitin sulfate (1–100 μg/mL) effectively suppressed interleukin-1β (IL-1β)-induced gene expression of aggrecanase-1, metalloproteinase, and aggrecanase-2 in chondrocytes and synovial fibroblasts. It also reduced expression of the collagenase-3 gene (MMP-13) in chondrocytes and modulated tissue metalloproteinase inhibitor (TIMP) expression, enhancing TIMP-1 production in synovial fibroblasts. These findings support the multifunctional, chondroprotective role of chondroitin sulfate in degenerative arthritis.
In a randomized, double-blind, 6-months-lasting study, Reginster et al. (2017) [137] evaluated the efficacy and safety of chondroitin sulfate (800 mg/day) in 604 patients from five European countries diagnosed with symptomatic knee osteoarthritis. The study assessed changes in pain measured by a Visual Analogue Scale and function by the Lequesne Index. Chondroitin sulfate, administered as an oral gel, demonstrated efficacy comparable to celecoxib, with benefits apparent after just 30 days and persisting for at least three months post-treatment. The treatment was well tolerated and associated with minimal side effects.
5. Discussion
Immunotherapy represents one of the most progressive therapeutic approaches, particularly applicable in the treatment of chronic diseases. This therapeutic strategy has achieved significant clinical successes, particularly in oncology and the treatment of infectious diseases. Its foundation lies in modulating the function of immune cells to restore their physiological function and eliminate pathological cells, including cancerous ones, through various mechanisms [138,139]. Chronic infectious diseases and cancer are often characterized by dysregulated immune responses, manifesting predominantly as immunosuppression [140].
Targeted modulation of immune components toward physiological levels can significantly contribute to the treatment of these conditions. In cases where some immune components exhibit hyperfunction while others show hypofunction, disease progression ensues [141]. A therapeutic approach aimed at restoring physiological immune responses is referred to as immunonormalization. Unlike traditional approaches involving nonspecific immune stimulation or overall immune suppression, immunonormalization seeks to restore the functional integrity of the immune system. This concept is particularly applicable in the treatment of chronic diseases, including immune dysregulations, immunodeficiency states, chronic bacterial and viral infections, and oncological conditions [142].
Immune normalization is particularly relevant in conditions where immune dysregulation plays a critical role, such as cancer, autoimmune diseases, and inflammatory disorders. This concept differs from traditional strategies, such as immunostimulation or immunosuppression, by focusing on correcting dysregulation without inducing excessive activation or suppression of immune processes [143]. Three principal mechanisms of immune normalization are distinguished. First, targeted modulation focuses on correcting dysfunctional components of the immune system to prevent pathological conditions, such as autoimmune diseases or immunosuppressive states [144]. Second, controlled enhancement of immune responses involves temporarily increasing immune activity, for example, to support antitumor reactions. This process is feedback-regulated to minimize the risk of chronic inflammation or tissue damage [145] (Schreiber, 2011). Third, unblocking immune responses aims to restore the activity of suppressed immune mechanisms, thereby enabling effective management of pathological conditions such as tumors or chronic infections [144,146].
The immunomodulatory effects of chondroitin sulfate are the subject of research in the field of innovative immunotherapeutic strategies that focus on specific aspects of immune normalization. Their mechanism of action is based on inhibiting the enzyme N-acetylgalactosaminidase, which functions as an immunosuppressor. Excessive activity of this enzyme leads to states of immunosuppression and may contribute to the development of chronic diseases [134].
Chondroitin sulfate and fucoidan have both attracted considerable attention for their potential adjunctive roles in cancer therapy. Clinical and epidemiological studies have indicated a supportive therapeutic role for chondroitin sulfate. For example, population-based studies have suggested an association between the use of glucosamine and/or chondroitin supplements and a reduced risk of colorectal cancer, highlighting their potential as safe, adjunctive agents in cancer management [147]. Several clinical trials are currently investigating the effects of chondroitin supplementation in inflammation and cancer-related endpoints [148,149].
Fucoidan has been also studied preclinically for its anticancer properties, including inhibition of tumor growth, metastasis, and enhancement of immune responses [150,151,152]. Clinical trials are ongoing to evaluate oligo-fucoidan as an adjunctive therapy for cancer cachexia and sarcopenia, conditions commonly associated with advanced cancer and chemotherapy [153].
Regarding nagalase, fucoidan has been found not to inhibit free alpha-nagalase enzyme activity directly but to reduce its expression in colon cancer cell lines, suggesting an immunomodulatory effect that may contribute to its anticancer activity [5].
So far, only a few authors have focused on the synergistic effects of the combination of chondroitin sulfate and fucoidan, not only because of their immunomodulatory properties. For example, Carvalho et al. (2023) [154] in their study concentrate on their regenerative effects. They developed cryo-processed biomaterials combining marine collagen, chitosan, fucoidan, and chondroitin sulfate, aiming to support cartilage regeneration by loading primary human cells. In laboratory tests using human adipose-derived stem cells (hASCs), the cryogels exhibited a favorable microenvironment that promoted both cell survival and growth.
Anisimova et al. (2017) [155] investigated two sulfated polysaccharides derived from marine organisms for their potential to stimulate hematopoiesis in a mouse model of chemotherapy-induced myelosuppression. Chemotherapy, while targeting cancer cells, causes collateral damage to healthy cells, notably impairing hematopoiesis. This hematopoietic suppression leads to decreased levels of critical blood components, including white blood cells, which compromises the immune response.
The investigated compouds were fucoidan extracted from the seaweed Chordaria flagelliformis (PS-Fuc) and fucosylated chondroitin sulfate from the sea cucumber Massinium magnum (PS-FCS). Their efficacy was compared to that of recombinant granulocyte colony-stimulating factor (r G-CSF), a clinically established agent used to enhance white blood cell recovery.
Both PS-Fuc and PS-FCS significantly promoted neutrophil recovery, demonstrating comparable stimulatory effects to r G-CSF. Moreover, these polysaccharides also enhanced thrombopoiesis and erythropoiesis, indicating a broad stimulatory effect on hematopoietic lineages. Notably, PS-FCS exhibited greater activity than r G-CSF in this model.
These findings suggest that PS-Fuc and PS-FCS are promising candidates as supportive agents to mitigate hematological toxicity in cancer patients undergoing chemotherapy or radiotherapy, warranting further investigation [155].
Similarly, another study by Ustyuzhanina et al. (2021) [156] reported that the polysaccharides under investigation promoted the release of white blood cells, red blood cells, and platelets from bone marrow in immunosuppressed mice, while r G-CSF primarily led to a significant increase only in leukocyte levels [156]. These findings motivated us to explore their combined effects, and we successfully confirmed their synergistic action [157].
We investigated the effects of chondroitin sulfate, fucoidan, and their combination on the growth and viability of 3D spheroids derived from the non-tumor NIH3T3 and tumor Hepa1c1c7 cell lines. Using the liquid overlay method and flow cytometry, it was found out that chondroitin sulfate and fucoidan promoted proliferation in non-tumor NIH3T3 spheroids, with chondroitin sulfate showing the most significant increase in viable cells. Conversely, in tumor-derived Hepa1c1c7 spheroids, fucoidan and the chondroitin sulfate-fucoidan combination exerted a synergistic antiproliferative effect, significantly increasing cell death [157].
As part of our research, we plan to further investigate the antiproliferative, antitumor, and immunomodulatory effects of the substances, chondroitin sulfate and fucoidan. We have conducted pilot studies evaluating the biological activity of pharmaceutical substances based on glycosaminoglycans and sulfated heteropolysaccharides in vitro using stabilized cell lines. Specifically, we will test these substances on selected HeLa, A549, and MRC-5 cell lines, assessing their impact on cell proliferation and viability (cell metabolic activity) through impedance analysis with the xCELLigence system, alongside MTT and XTT assays.
Subsequently, we examined the effects of fucoidan, chondroitin sulfate, and their combination on cell growth and the morphology of three-dimensional (3D) spheroids. We monitored spheroid viability and clonogenicity, followed by protein isolation to analyze the expression of apoptotic initiator caspases and related markers within the spheroids.
For in vivo experiments, following targeted subcutaneous administration of tumor cells to laboratory mice, we aim to evaluate the influence of an oral medicine containing chondroitin sulfate and fucoidan on xenograft progression and inhibition. We will monitor granulocyte (neutrophil) presence in mouse blood to assess phagocytic activity by flow cytometry. Central to the immunomodulatory effects of chondroitin sulfate and fucoidan is their capacity to enhance in vivo production of GcMAF protein via inhibition of nagalase activity. Nagalase, an enzyme secreted by viruses and tumor cells, disrupts GcMAF production, leading to immunosuppression.
GcMAF, derived from plasma Gc protein, is vital for optimal immune system function, particularly through macrophage activation and facilitating the phagocytic arm of immunity. Impaired GcMAF production compromises immune communication pathways, manifesting clinically as varying degrees of immunodeficiency and even potential complete immune failure. Chronic immunodeficiency heightens the risk of uncontrolled tumor cell growth, malignancy development, and oncological disease progression. GcMAF mediates immune responses by interacting with the vitamin D receptor (VDR) and activating several immune components, including macrophages [158].
Multiple strategies exist to stimulate GcMAF production in vivo. Preclinical data highlight the promising clinical potential of GcMAF, with one of the most clinically significant effects being the antitumor activity of GcMAF-activated macrophages. Cancer patients, especially those with advanced disease, often have impaired macrophage activation due to tumor-released endoglycosidase enzymes that deglycosylate VDTP (vitamin D-binding protein-derived GcMAF precursor), effectively inactivating it. Since macrophage activation is the first critical step in the immune cascade triggered by inflammation, its disruption results in immune system suppression, permitting cancer cell survival and metastasis.
Intramuscular administration of GcMAF has been shown to activate systemic macrophages, leading to the development of receptors that recognize tumor cell surface abnormalities and transform macrophages into tumoricidal cells. Additionally, GcMAF exhibits mitogenic activity on myeloid progenitor cells, causing a substantial systemic increase in activated macrophages within days [10,159,160].
Given these findings, our future studies will focus on monitoring macrophage phagocytic activity by enhancing endogenous GcMAF production through oral administration of a suspension designed to modulate the enzymatic activity of key enzymes, including nagalase. This innovative approach aims to harness the immunomodulatory potential of chondroitin sulfate and fucoidan in vivo.
Safety Profile of Chondroitin Sulfate and Fucoidan: Clinical studies show no toxicity of fucoidan at oral doses of 1–3 g per day over periods up to 3 months [161]. Some coagulation-modifying effects have been observed (e.g., prolongation of clotting times), but these remain within normal clinical ranges and are not typically associated with bleeding risk [162]. Occasional mild gastrointestinal symptoms such as diarrhea have been reported at higher doses in a few patients [161]. Chondroitin sulfate is widely used as a dietary supplement for osteoarthritis with an excellent safety record. Adverse effects are rare and usually mild, such as gastrointestinal discomfort [163].
In subchronic toxicity, genotoxicity, and bioavailability studies, Sprague Dawley rats were orally administered non-animal chondroitin sulfate (NACS) at doses of 0, 250, 500, and 1000 mg per kilogram of body weight per day for 90 days. No mortality or significant changes in clinical signs, body weight, body weight gain, or feed consumption were observed during the study. Similarly, no treatment-related toxicologically relevant alterations were detected in hematology, clinical chemistry, urinalysis, or organ weights. Both macroscopic and microscopic examinations revealed no abnormalities attributable to NACS administration [164].
In vitro genotoxicity assessments—including the Ames test, chromosomal aberration assay, and micronucleus assay—confirmed that NACS did not exhibit mutagenic or clastogenic potential. Study results demonstrated that even at the highest tested dose of 1000 mg/kg body weight per day, NACS did not cause any adverse effects in subchronic toxicity studies [164].
The pharmacokinetic profile of NACS was evaluated in healthy volunteers using 800 mg oral tablets, with comparisons made to chondroitin sulfate of animal origin. Blood samples were collected over a 48-h period following a single oral dose of 2400 mg. Total chondroitin sulfate, specific disaccharide composition, and total charge density were quantified in plasma [165].
The safety and tolerability profile after a single dose of NACS was excellent. After baseline correction, plasma concentrations of chondroitin sulfate were found to be overall higher at 24 h and remained 45% higher at 48 h following NACS administration compared to animal-derived chondroitin sulfate. NACS administration led to an increase in specific 6-sulfation of N-acetyl-galactosamine within the chondroitin sulfate present in human plasma, resulting in a total charge density that was twice as high as that observed with cattle-derived chondroitin sulfate after 48 h. NACS, which has a lower molecular weight than animal-derived chondroitin sulfate, achieved sustained higher plasma concentrations over a longer period and increased both the charge density and the specific 6-sulfation of endogenous plasma chondroitin sulfate [165].
Together, these data support the safety of chondroitin sulfate and fucoidan, even at doses common in supplementation. A randomized observational study of a combined formulation of chondroitin sulfate and fucoidan in osteoarthritis patients confirmed good safety and tolerability over 12 weeks [163]. Nevertheless, caution is advised when using fucoidan in individuals with bleeding disorders or on anticoagulant therapy, due to its clotting-modifying effects.
Summarizing, cancer remains one of the leading causes of death globally. Extensive research efforts are ongoing to develop effective cancer therapies. Potential drug candidates must meet critical criteria including efficacy, safety, good bioavailability, and minimal adverse side effects.
The preclinical phase of drug development involves assessing drug candidates in vitro using 2D and 3D cell culture models, as well as in vivo studies on animal models. These living systems provide valuable insights due to their complex interactions among cells, tissues, and organs, allowing researchers to better predict human responses. Data obtained from in vivo studies often translate more readily to clinical settings compared to in vitro or purely theoretical results, thereby facilitating the progression of new therapeutic approaches.
Our further research is focused on glycosaminoglycans and sulfated heteropolysaccharides, which have demonstrated various beneficial biological effects including anticancer, anti-inflammatory, antiviral, antiangiogenic, and immunomodulatory properties. Specifically, we aim to investigate the inhibitory mechanism of nagalase activity through the administration of glycosaminoglycans containing N-acetylgalactosamine, using an in vivo mouse model.
We hypothesize that monitoring the immune response alterations induced by oral administration of these substances can provide valuable biomarkers and inform strategies for GcMAF immunotherapy.
6. Conclusions
This review highlights the promising pharmacological potential of chondroitin sulfate and fucoidan as immunomodulatory agents, particularly through their inhibition of N-acetylgalactosaminidase (nagalase), an enzyme implicated in immune suppression and cancer progression. Both compounds not only exhibit anti-inflammatory, tissue-regenerative, and immunostimulatory effects, but also demonstrate the ability to restore macrophage function and normalize immune responses in the context of chronic inflammation, cancer, and infectious diseases. The synergistic action of chondroitin sulfate and fucoidan further enhances their therapeutic value, offering a multifaceted approach to targeting the underlying mechanisms of immune dysregulation. These findings underscore the need for further preclinical and clinical studies to fully elucidate the mechanisms of action and to validate the clinical efficacy of these marine-derived polysaccharides as innovative agents in immunotherapy and supportive cancer care.
Author Contributions
Conceptualization, J.Z. and M.Š. (Miroslava Šupolíková); investigation, J.Z., E.N. and M.Š. (Miroslava Šupolíková); resources, M.Š. (Miroslava Šupolíková); writing—original draft preparation, J.Z., M.Š. (Miroslava Šupolíková) and M.Š. (Miroslava Špaglová); writing review and editing, J.Z., M.Š. (Miroslava Šupolíková) and M.Š. (Miroslava Špaglová); visualization, J.Z., M.Š. (Miroslava Šupolíková) and M.Š. (Miroslava Špaglová); supervision, M.Š. (Miroslava Šupolíková); project administration, M.Š. (Miroslava Šupolíková); funding acquisition, M.Š. (Miroslava Šupolíková). All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support to the Scientific Grant Agency of the Ministry of Education of Slovak Republic and Slovak Academy of Sciences VEGA, Project No.: 1/0146/23.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used Perplexity AI, version 2.48.2, for the purposes of language enhancement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BDNF | brain neurotrophic factor |
| CAM | chorioallantoic membrane |
| CNS | central nervous system |
| CS | chondroitin sulfate |
| EBV | Epstein-Barr virus |
| FCS | fucosylated forms of chondroitin sulfate |
| HPS | heteropolysaccharides |
| KS | keratan sulfate |
| LMWCS | low molecular weight chondroitin sulfate |
| LMWH | low molecular weight heparins |
| MAPK | mitogen-activated protein kinase |
| NACS | non-animal chondroitin sulfate |
| NF | nuclear factor |
| NK | natural killer |
| NO | nitric oxide |
| TIMP | tissue metalloproteinase inhibitor |
| TLR | toll-like receptors |
| VDTP | vitamin D-binding protein |
| VEGF | vascular endothelial growth factor |
References
- Kaczanowski, S. Apoptosis: Its Origin, History, Maintenance and the Medical Implications for Cancer and Aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the Cytoskeleton, and Cancer Cell Invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Benhamou, Y.; Picco, V.; Pagès, G. The Telomere Proteins in Tumorigenesis and Clinical Outcomes of Oral Squamous Cell Carcinoma. Oral Oncol. 2016, 57, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, I.; Chadova, O.; Malyarenko, O.; Ermakova, S. The Effect of Fucoidan from the Brown Alga Fucus Evanescence on the Activity of α-N-Acetylgalactosaminidase of Human Colon Carcinoma Cells. Mar. Drugs 2018, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Naraparaju, V.R.; Urade, M. Prognostic Utility of Serum Alpha-N-Acetylgalactosaminidase and Immunosuppression Resulted from Deglycosylation of Serum Gc Protein in Oral Cancer Patients. Cancer Res. 1997, 57, 295–299. [Google Scholar]
- Yamamoto, N.; Urade, M. Pathogenic Significance of Alpha-N-Acetylgalactosaminidase Activity Found in the Hemagglutinin of Influenza Virus. Microbes Infect. 2005, 7, 674–681. [Google Scholar] [CrossRef]
- Korbelik, M.; Naraparaju, V.R.; Yamamoto, N. The Value of Serum Alpha-N-Acetylgalactosaminidase Measurement for the Assessment of Tumour Response to Radio- and Photodynamic Therapy. Br. J. Cancer 1998, 77, 1009–1014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoga, M.; Nikou, T.; Ioannidis, A.; Tzavellas, E.; Paparrigopoulos, T.; Lambrokostopoulos, K.T.; Vasdekis, V.G.; Magana, M.; Chatzipanagiotou, S. Alteration of α-N-Acetylgalactosaminidase (Nagalase) Concentration in Alcohol-Dependent Individuals without Liver Disease, during the Detoxification Therapy. Drug Alcohol Depend. 2017, 170, 147–151. [Google Scholar] [CrossRef]
- Yamamoto, N.; Suyama, H.; Yamamoto, N. Immunotherapy for Prostate Cancer with Gc Protein-Derived Macrophage-Activating Factor, GcMAF. Transl. Oncol. 2008, 1, 65–72. [Google Scholar] [CrossRef]
- Thyer, L.; Ward, E.; Smith, R.; Branca, J.J.; Morucci, G.; Gulisano, M.; Noakes, D.; Eslinger, R.; Pacini, S. GC Protein-Derived Macrophage-Activating Factor Decreases α-N-Acetylgalactosaminidase Levels in Advanced Cancer Patients. Oncoimmunology 2013, 2, e25769. [Google Scholar] [CrossRef]
- Saburi, E.; Tavakol-Afshari, J.; Biglari, S.; Mortazavi, Y. Is α-N-Acetylgalactosaminidase the Key to Curing Cancer? A Mini-Review and Hypothesis. J. BUON 2017, 22, 1372–1377. [Google Scholar]
- Zhu, A.; Wang, Z.-K.; Beavis, R. Structural Studies of α-N-Acetylgalactosaminidase: Effect of Glycosylation on the Level of Expression, Secretion Efficiency, and Enzyme Activity. Arch. Biochem. Biophys. 1998, 352, 1–8. [Google Scholar] [CrossRef]
- Garman, S.C.; Hannick, L.; Zhu, A.; Garboczi, D.N. The 1.9 Å Structure of α-N-Acetylgalactosaminidase: Molecular Basis of Glycosidase Deficiency Diseases. Structure 2002, 10, 425–434. [Google Scholar] [CrossRef]
- Wang, A.M.; Desnick, R.J. Structural Organization and Complete Sequence of the Human Alpha-N-Acetylgalactosaminidase Gene: Homology with the Alpha-Galactosidase A Gene Provides Evidence for Evolution from a Common Ancestral Gene. Genomics 1991, 10, 133–142. [Google Scholar] [CrossRef]
- Tsuji, S.; Yamauchi, T.; Hiraiwa, M.; Isobe, T.; Okuyama, T.; Sakimura, K.; Takahashi, Y.; Nishizawa, M.; Uda, Y.; Miyatake, T. Molecular Cloning of a Full-Length cDNA for Human α-N-Acetylgalactosaminidase (α-Galactosidase B). Biochem. Biophys. Res. Commun. 1989, 163, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, J.; Bhavanandan, V.P.; Davidson, E.A. Purification and Properties of an Endo-Alpha-N-Acetyl-D-Galactosaminidase from Diplococcus pneumoniae. J. Biol. Chem. 1977, 252, 8609–8614. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Schachter, H.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; ISBN 978-0-87969-770-9. [Google Scholar]
- Schindler, D.; Bishop, D.F.; Wolfe, D.E.; Wang, A.M.; Egge, H.; Lemieux, R.U.; Desnick, R.J. Neuroaxonal Dystrophy Due to Lysosomal Alpha-N-Acetylgalactosaminidase Deficiency. N. Engl. J. Med. 1989, 320, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Schindler, D.; Desnick, R. Schindler Disease: The Molecular Lesion in the Alpha-N-Acetylgalactosaminidase Gene That Causes an Infantile Neuroaxonal Dystrophy. J. Clin. Investig. 1990, 86, 1752–1756. [Google Scholar] [CrossRef]
- Yamamoto, N. Pathogenic Significance of Alpha-N-Acetylgalactosaminidase Activity Found in the Envelope Glycoprotein Gp160 of Human Immunodeficiency Virus Type 1. AIDS Res. Hum. Retroviruses 2006, 22, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.E.; Metcalf, M.C.; Best, D.; Fleet, G.W.J.; Garman, S.C. Pharmacological Chaperones for Human α-N-Acetylgalactosaminidase. Proc. Natl. Acad. Sci. USA 2012, 109, 17400–17405. [Google Scholar] [CrossRef]
- Clark, N.E.; Garman, S.C. The 1.9 Å Structure of Human α-N-Acetylgalactosaminidase: The Molecular Basis of Schindler and Kanzaki Diseases. J. Mol. Biol. 2009, 393, 435–447. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.B.; Nagasawa, H.; Uto, Y.; Hori, H. Tumor Cell Alpha-N-Acetylgalactosaminidase Activity and Its Involvement in GcMAF-Related Macrophage Activation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Naraparaju, V.R.; Moore, M.; Brent, L.H. Deglycosylation of Serum Vitamin D3-Binding Protein by α-N-Acetylgalactosaminidase Detected in the Plasma of Patients with Systemic Lupus Erythematosus. Clin. Immunol. Immunopathol. 1997, 82, 290–298. [Google Scholar] [CrossRef]
- Reddi, A.L.; Sankaranarayanan, K.; Arulraj, H.S.; Devaraj, N.; Devaraj, H. Serum Alpha-N-Acetylgalactosaminidase Is Associated with Diagnosis/Prognosis of Patients with Squamous Cell Carcinoma of the Uterine Cervix. Cancer Lett. 2000, 158, 61–64. [Google Scholar] [CrossRef]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from Lactic Acid Bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Chen, D.; Wang, A.; Lv, J.; Tang, C.; Jin, C.-H.; Liu, J.; Zeng, X.; Wang, L. Structural and Digestive Characters of a Heteropolysaccharide Fraction from Tea (Camellia sinensis L.) Flower. Food Chem. X 2024, 21, 101058. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom Heteropolysaccharides: A Review on Their Sources, Structure and Biological Effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Ponce, N.M.A.; Stortz, C.A. Seaweed Polysaccharides: Structure and Applications. In Industrial Applications of Renewable Biomass Products: Past, Present and Future; Goyanes, S.N., D’Accorso, N.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 75–116. ISBN 978-3-319-61288-1. [Google Scholar]
- Wang, T.; Zhu, M.; He, Z.-Z. Low-Molecular-Weight Fucoidan Attenuates Mitochondrial Dysfunction and Improves Neurological Outcome After Traumatic Brain Injury in Aged Mice: Involvement of Sirt3. Cell. Mol. Neurobiol. 2016, 36, 1257–1268. [Google Scholar] [CrossRef]
- Jin, J.-O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown Seaweed Fucoidan: Biological Activity and Apoptosis, Growth Signaling Mechanism in Cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wu, J. Fucoidan: Biological Activity in Liver Diseases. Am. J. Chin. Med. 2020, 48, 1617–1632. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential Beneficial Actions of Fucoidan in Brain and Liver Injury, Disease, and Intoxication—Potential Implication of Sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Percival, E. The Polysaccharides of Green, Red and Brown Seaweeds: Their Basic Structure, Biosynthesis and Function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Bernhard, S.A.; Hammett, L.P. Specific Effects in Acid Catalysis by Ion Exchange Resins. II. Hydrolysis of Esters in Water Solution1. J. Am. Chem. Soc. 1953, 75, 5834–5835. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-58932-8. [Google Scholar]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and Partial Characterization of a Noval Amino Sugar-Containing Fucan Sulfate from Commercial Fucus Vesiculosus Fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a Natural Biopolymer in Cancer Combating: From Edible Algae to Nanocarrier Tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A Quantitative Method to Detect Fucoidan in Human Plasma Using a Novel Antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef]
- Michel, C.; Lahaye, M.; Bonnet, C.; Mabeau, S.; Barry, J.L. In vitro Fermentation by Human Faecal Bacteria of Total and Purified Dietary Fibres from Brown Seaweeds. Br. J. Nutr. 1996, 75, 263–280. [Google Scholar] [CrossRef]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The Effect of Dietary Laminaria-Derived Laminarin and Fucoidan on Nutrient Digestibility, Nitrogen Utilisation, Intestinal Microflora and Volatile Fatty Acid Concentration in Pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a Fucoidan-Specific Antibody and Measurement of Fucoidan in Serum and Urine by Sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K.; Takada, H.; Iha, M.; Nagamine, T. Attenuation of N-Nitrosodiethylamine-Induced Liver Fibrosis by High-Molecular-Weight Fucoidan Derived from Cladosiphon Okamuranus. J. Gastroenterol. Hepatol. 2010, 25, 1692–1701. [Google Scholar] [CrossRef]
- Bakhru, S.H.; Furtado, S.; Morello, A.P.; Mathiowitz, E. Oral Delivery of Proteins by Biodegradable Nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 811–821. [Google Scholar] [CrossRef]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal Absorption of Fucoidan Extracted from the Brown Seaweed, Cladosiphon okamuranus. Mar. Drugs 2014, 13, 48–64. [Google Scholar] [CrossRef]
- Reid, S.N.S.; Ryu, J.; Kim, Y.; Jeon, B.H. The Effects of Fermented Laminaria Japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evid.-Based Complement. Altern. Med. 2018, 2018, 8109621. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Zeng, L.; Jin, J.-O. Rehmannia Glutinosa Polysaccharide Induced an Anti-Cancer Effect by Activating Natural Killer Cells. Int. J. Biol. Macromol. 2017, 105, 680–685. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Peng, X.; Li, J.; Zhu, C. The Natural Product Fucoidan Inhibits Proliferation and Induces Apoptosis of Human Ovarian Cancer Cells: Focus on the PI3K/Akt Signaling Pathway. Cancer Manag. Res. 2020, 12, 6195–6207. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Yang, C.; Lee, H.; Lee, B.-Y. Molecular Characteristics of Partially Hydrolyzed Fucoidans from Sporophyll of Undaria Pinnatifida and Their in Vitro Anticancer Activity. Food Chem. 2010, 119, 554–559. [Google Scholar] [CrossRef]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of Structure and in vitro Anticancer Activity of Native and Modified Fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum Mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S.; et al. Effects of Oral Administration of Fucoidan Extracted from Cladosiphon okamuranus on Tumor Growth and Survival Time in a Tumor-Bearing Mouse Model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, J.-H.; Yoo, J.; Lee, M.; Lee, D.H.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y. Fucoidan Protects Hepatocytes from Apoptosis and Inhibits Invasion of Hepatocellular Carcinoma by Up-Regulating P42/44 MAPK-Dependent NDRG-1/CAP43. Acta Pharm. Sin. B 2015, 5, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan Derived from Undaria Pinnatifida Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells via the ROS-Mediated Mitochondrial Pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.-Y.; Moon, S.-K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan Inhibits the Proliferation of Human Urinary Bladder Cancer T24 Cells by Blocking Cell Cycle Progression and Inducing Apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef]
- Park, H.Y.; Choi, I.-W.; Kim, G.-Y.; Kim, B.W.; Kim, W.-J.; Choi, Y.H. Fucoidan Induces G1 Arrest of the Cell Cycle in EJ Human Bladder Cancer Cells through Down-Regulation of pRB Phosphorylation. Rev. Bras. Farmacogn. 2015, 25, 246–251. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, D.-S.; Jeong, J.-W.; Hong, S.-H.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Park, C.; Kim, G.-Y.; et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Moreau, D.; Thomas-Guyon, H.; Jacquot, C.; Jugé, M.; Culioli, G.; Ortalo-Magné, A.; Piovetti, L.; Roussakis, C. An Extract from the Brown Alga Bifurcaria bifurcata Induces Irreversible Arrest of Cell Proliferation in a Non-Small-Cell Bronchopulmonary Carcinoma Line. J. Appl. Phycol. 2006, 18, 87–93. [Google Scholar] [CrossRef]
- Qiu, W.-L.; Tseng, A.-J.; Hsu, H.-Y.; Hsu, W.-H.; Lin, Z.-H.; Hua, W.-J.; Lin, T.-Y. Fucoidan Increased the Sensitivity to Gefitinib in Lung Cancer Cells Correlates with Reduction of TGFβ-Mediated Slug Expression. Int. J. Biol. Macromol. 2020, 153, 796–805. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of Fucoidan Enhances Its Anti-Angiogenic and Antitumor Activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.-J.; Hong, J.-Y.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Kim, E.-J.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S.; Kwon, J.-M.; et al. The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells. Mar. Drugs 2013, 11, 2982–2999. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan Inhibits Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Macrophages and Zebrafish Larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Aleissa, M.S.; Alkahtani, S.; Abd Eldaim, M.A.; Ahmed, A.M.; Bungău, S.G.; Almutairi, B.; Bin-Jumah, M.; AlKahtane, A.A.; Alyousif, M.S.; Abdel-Daim, M.M. Fucoidan Ameliorates Oxidative Stress, Inflammation, DNA Damage, and Hepatorenal Injuries in Diabetic Rats Intoxicated with Aflatoxin B1. Oxid. Med. Cell. Longev. 2020, 2020, 9316751. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from Brown Alga Fucus Evanescens: Structure and Biological Activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, Q.; Thorlacius, H. Inhibition of Selectin Function and Leukocyte Rolling Protects against Dextran Sodium Sulfate-Induced Murine Colitis. Scand. J. Gastroenterol. 2001, 36, 270–275. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A Comparative Study of the Anti-Inflammatory, Anticoagulant, Antiangiogenic, and Antiadhesive Activities of Nine Different Fucoidans from Brown Seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In vitro and in vivo Anti-Inflammatory Activities of a Fucose-Rich Fucoidan Isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Cho, N.; Cho, S.H.; Yoo, H.M.; Fernando, I.P.S.; Ahn, G. Fine-Dust-Induced Skin Inflammation: Low-Molecular-Weight Fucoidan Protects Keratinocytes and Underlying Fibroblasts in an Integrated Culture Model. Mar. Drugs 2022, 21, 12. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical Application of Glycosaminoglycans: A Review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Dymarska, M. Hyaluronic Acid. Structure, Properties and Uses Kwas Hialuronowy. Budowa, Właściwości i Zastosowanie. Chem. Rev. 2016, 1, 136–138. [Google Scholar] [CrossRef]
- Shriver, Z.; Capila, I.; Venkataraman, G.; Sasisekharan, R. Heparin and Heparan Sulfate: Analyzing Structure and Microheterogeneity. In Heparin-A Century of Progress; Lever, R., Mulloy, B., Page, C.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 159–176. ISBN 978-3-642-23056-1. [Google Scholar]
- Purushothaman, A.; Sugahara, K.; Faissner, A. Chondroitin Sulfate “Wobble Motifs” Modulate Maintenance and Differentiation of Neural Stem Cells and Their Progeny. J. Biol. Chem. 2012, 287, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K. Potential Therapeutic Application of Chondroitin Sulfate/Dermatan Sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Paradigms in the Structural Biology of the Mitogenic Ternary Complex FGF:FGFR:Heparin. Biochimie 2016, 127, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Vallet, S.D.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2021, 69, 93–104. [Google Scholar] [CrossRef]
- Baranova, N.S.; Nilebäck, E.; Haller, F.M.; Briggs, D.C.; Svedhem, S.; Day, A.J.; Richter, R.P. The Inflammation-Associated Protein TSG-6 Cross-Links Hyaluronan via Hyaluronan-Induced TSG-6 Oligomers. J. Biol. Chem. 2011, 286, 25675–25686. [Google Scholar] [CrossRef]
- Taylor, K.R.; Gallo, R.L. Glycosaminoglycans and Their Proteoglycans: Host-Associated Molecular Patterns for Initiation and Modulation of Inflammation. FASEB J. 2006, 20, 9–22. [Google Scholar] [CrossRef]
- Luan, Z.-G.; Naranpurev, M.; Ma, X.-C. Treatment of Low Molecular Weight Heparin Inhibits Systemic Inflammation and Prevents Endotoxin-Induced Acute Lung Injury in Rats. Inflammation 2014, 37, 924–932. [Google Scholar] [CrossRef]
- Huynh, M.B.; Ouidja, M.O.; Chantepie, S.; Carpentier, G.; Maïza, A.; Zhang, G.; Vilares, J.; Raisman-Vozari, R.; Papy-Garcia, D. Glycosaminoglycans from Alzheimer’s Disease Hippocampus Have Altered Capacities to Bind and Regulate Growth Factors Activities and to Bind Tau. PLoS ONE 2019, 14, e0209573. [Google Scholar] [CrossRef]
- Cui, H.; Hung, A.C.; Klaver, D.W.; Suzuki, T.; Freeman, C.; Narkowicz, C.; Jacobson, G.A.; Small, D.H. Effects of Heparin and Enoxaparin on APP Processing and Aβ Production in Primary Cortical Neurons from Tg2576 Mice. PLoS ONE 2011, 6, e23007. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Procter, P.; Miller, G.J.; Fernig, D.G.; Guerrini, M.; Guimond, S.E.; Lima, M.A.; Yates, E.A.; et al. Inhibition of BACE1, the β-Secretase Implicated in Alzheimer’s Disease, by a Chondroitin Sulfate Extract from Sardina Pilchardus. Neural Regen. Res. 2020, 15, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Brezina, M.; Castelnuovo, P.; Drago, L. The Role of Hyaluronan in the Pathobiology and Treatment of Respiratory Disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L785–L795. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Lauder, R.M. Chondroitin Sulphate: A Complex Molecule with Potential Impacts on a Wide Range of Biological Systems. Complement. Ther. Med. 2009, 17, 56–62. [Google Scholar] [CrossRef]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular Interactions between Chondroitin–Dermatan Sulfate and Growth Factors/Receptors/Matrix Proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef]
- Ewald, C.Y. Drug Screening Implicates Chondroitin Sulfate as a Potential Longevity Pill. Front. Aging 2021, 2, 741843. [Google Scholar] [CrossRef]
- Volpi, N. Analytical Aspects of Pharmaceutical Grade Chondroitin Sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Urbi, Z.; Azmi, N.S.; Ming, L.C.; Hossain, M.S. A Concise Review of Extraction and Characterization of Chondroitin Sulphate from Fish and Fish Wastes for Pharmacological Application. Curr. Issues Mol. Biol. 2022, 44, 3905–3922. [Google Scholar] [CrossRef]
- Malavaki, C.; Mizumoto, S.; Karamanos, N.; Sugahara, K. Recent Advances in the Structural Study of Functional Chondroitin Sulfate and Dermatan Sulfate in Health and Disease. Connect. Tissue Res. 2008, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Stick, R.V.; Williams, S. Carbohydrates: The Essential Molecules of Life; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-092702-2. [Google Scholar]
- Xu, H.; Zhou, Q.; Liu, B.; Chen, F.; Wang, M. Holothurian Fucosylated Chondroitin Sulfates and Their Potential Benefits for Human Health: Structures and Biological Activities. Carbohydr. Polym. 2022, 275, 118691. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nikogosova, S.P.; Hang, C.T.T.; Thinh, P.D.; Trung, D.T.; Van, T.T.T.; Shashkov, A.S.; et al. Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and Anticoagulant Activity. Mar. Drugs 2022, 20, 380. [Google Scholar] [CrossRef]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef]
- Wang, W.; Shi, L.; Qin, Y.; Li, F. Research and Application of Chondroitin Sulfate/Dermatan Sulfate-Degrading Enzymes. Front. Cell Dev. Biol. 2020, 8, 560442. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Recent Advances in the Study of the Biosynthesis and Functions of Sulfated Glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Maccarana, M.; Olander, B.; Malmström, J.; Tiedemann, K.; Aebersold, R.; Lindahl, U.; Li, J.-P.; Malmström, A. Biosynthesis of Dermatan Sulfate: Chondroitin-Glucuronate C5-Epimerase Is Identical to SART2. J. Biol. Chem. 2006, 281, 11560–11568. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Gatta, A.L.; Rosa, M.D.; Schiraldi, C.; Gatta, A.L.; Rosa, M.D. Biotechnological Production and Application of Hyaluronan. In Biopolymers; IntechOpen: London, UK, 2010; ISBN 978-953-307-109-1. [Google Scholar]
- Luo, X.; Fosmire, G.; Leach, R. Chicken Keel Cartilage as a Source of Chondroitin Sulfate. Poult. Sci. 2002, 81, 1086–1089. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of Structures and Anticoagulant Activities of Fucosylated Chondroitin Sulfates from Different Sea Cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Souza, A.R.C.; Kozlowski, E.O.; Cerqueira, V.R.; Castelo-Branco, M.T.L.; Costa, M.L.; Pavão, M.S.G. Chondroitin Sulfate and Keratan Sulfate Are the Major Glycosaminoglycans Present in the Adult Zebrafish Danio rerio (Chordata-Cyprinidae). Glycoconj. J. 2007, 24, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Galla, R.; Ruga, S.; Ferrari, S.; Saccone, S.; Saccuman, L.; Invernizzi, M.; Uberti, F. In Vitro Analysis of the Effects of Plant-Derived Chondroitin Sulfate from Intestinal Barrier to Chondrocytes. J. Funct. Foods 2022, 98, 105285. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; García, M.R.; Sampayo, L.F.; Vázquez, J.A. Marine Chondroitin Sulfate of Defined Molecular Weight by Enzymatic Depolymerization. Carbohydr. Polym. 2020, 229, 115450. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Q.; Song, J.; Guo, R.; Ma, T.; Liu, Z.; Liu, Q. Intervention Effects of Low-Molecular-Weight Chondroitin Sulfate from the Nasal Cartilage of Yellow Cattle on Lipopolysaccharide-Induced Behavioral Disorders: Regulation of the Microbiome-Gut-Brain Axis. Front. Nutr. 2024, 11, 1371691. [Google Scholar] [CrossRef]
- Yang, S.-R.; Peng, S.; Ko, C.-Y.; Chu, I.-M. The Effects of Different Molecular Weight Chondroitin-4-Sulfates in Chondrocyte Pellet Culture. Cytotechnology 2016, 68, 371–379. [Google Scholar] [CrossRef][Green Version]
- Volpi, N. Quality of Different Chondroitin Sulfate Preparations in Relation to Their Therapeutic Activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef]
- Xu, S.; Qiu, M.; Zhang, Q.; Wu, J.; Huimin, X.; Chen, J. Chain Structure and Immunomodulatory Activity of a Fructosylated Chondroitin from an Engineered Escherichia coli K4. Int. J. Biol. Macromol. 2019, 133, 702–711. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Awofiranye, A.; Datta, P.; Xia, K.; He, W.; Fraser, K.; Dordick, J.S.; Linhardt, R.J.; Koffas, M.A.G. Complete Biosynthesis of a Sulfated Chondroitin in Escherichia coli. Nat. Commun. 2021, 12, 1389. [Google Scholar] [CrossRef]
- Goldenzweig, A.; Goldsmith, M.; Hill, S.E.; Gertman, O.; Laurino, P.; Ashani, Y.; Dym, O.; Unger, T.; Albeck, S.; Prilusky, J.; et al. Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability. Mol. Cell 2016, 63, 337–346. [Google Scholar] [CrossRef]
- Rondanelli, M.; Braschi, V.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; Peroni, G.; Naso, M.; Iannello, G.; Spadaccini, D.; Miraglia, N.; et al. Effectiveness of Non-Animal Chondroitin Sulfate Supplementation in the Treatment of Moderate Knee Osteoarthritis in a Group of Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 2027. [Google Scholar] [CrossRef]
- Kawai, K.; Kamochi, R.; Oiki, S.; Murata, K.; Hashimoto, W. Probiotics in Human Gut Microbiota Can Degrade Host Glycosaminoglycans. Sci. Rep. 2018, 8, 10674. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Dai, W.; Shang, Q.; Yu, G. Bacteroides Salyersiae Is a Potent Chondroitin Sulfate-Degrading Species in the Human Gut Microbiota. Microbiome 2024, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Yin, Y.; Zhu, L.; Li, G.; Yu, G.; Wang, X. Degradation of Chondroitin Sulfate by the Gut Microbiota of Chinese Individuals. Int. J. Biol. Macromol. 2016, 86, 112–118. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Hu, S.; Wang, Y.; Xue, C.; Li, H. The Effects of Fucosylated Chondroitin Sulfate Isolated from Isostichopus badionotus on Antimetastatic Activity via Down-Regulation of Hif-1α and Hpa. Food Sci. Biotechnol. 2014, 23, 1643–1651. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourąo, P.A.S.; Esko, J.D.; Pavąo, M.S.G. Selectin Blocking Activity of a Fucosylated Chondroitin Sulfate Glycosaminoglycan from Sea Cucumber: Effect on Tumor Metastasis and Neutrophil Recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef]
- du Souich, P.; García, A.G.; Vergés, J.; Montell, E. Immunomodulatory and Anti-Inflammatory Effects of Chondroitin Sulphate. J. Cell. Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Anti-Inflammatory Activity of Chondroitin Sulphate: New Functions from an Old Natural Macromolecule. Inflammopharmacology 2011, 19, 299–306. [Google Scholar] [CrossRef]
- Imada, K.; Oka, H.; Kawasaki, D.; Miura, N.; Sato, T.; Ito, A. Anti-Arthritic Action Mechanisms of Natural Chondroitin Sulfate in Human Articular Chondrocytes and Synovial Fibroblasts. Biol. Pharm. Bull. 2010, 33, 410–414. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Dudler, J.; Blicharski, T.; Pavelka, K. Pharmaceutical-Grade Chondroitin Sulfate Is as Effective as Celecoxib and Superior to Placebo in Symptomatic Knee Osteoarthritis: The ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann. Rheum. Dis. 2017, 76, 1537–1543. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.J.; Moon, S.; Lee, H.; Lee, J.; Kim, B.-S.; Jung, K.; Seo, H.; Chung, Y. Strategies to Overcome Hurdles in Cancer Immunotherapy. Biomater. Res. 2024, 28, 0080. [Google Scholar] [CrossRef]
- Fayyaz, H.; Zaman, A.; Rafiq, W.; Murtaza, M.H.; Ullah, I.; Fayyaz, H.; Zaman, A.; Rafiq, W.; Murtaza, M.H.; Ullah, I. Immunosuppression in Infectious Diseases: Causes and Effects. In Innate Immunity-New Perspectives and Therapeutic Opportunities; IntechOpen: London, UK, 2024; ISBN 978-1-83634-127-7. [Google Scholar]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation—A General Review of the Current State-of-the-Art and New Therapeutic Strategies for Targeting the Immune System. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Cancer Immunosuppression and Autoimmune Disease: Beyond Immunosuppressive Networks for Tumour Immunity. Immunology 2006, 119, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jung, K. Normalization of the Tumor Microenvironment by Harnessing Vascular and Immune Modulation to Achieve Enhanced Cancer Therapy. Exp. Mol. Med. 2023, 55, 2308–2319. [Google Scholar] [CrossRef]
- Khan, A.A.; Mannan, V.; Pervaiz, M.A.; Akram, A.; Momin, E.S.; Sanusi, M.; Kashyap, T.; Elshaikh, A.O. The Role of Glucosamine and Chondroitin Sulfate in the Prevention of Colorectal Cancer: A Systematic Review. Cureus 2022, 14, e25401. [Google Scholar] [CrossRef]
- Kantor, E.; Lampe, J.; Peters, U.; Shen, D.; Vaughan, T.; White, E. Use of Glucosamine and Chondroitin Supplements and Risk of Colorectal Cancer. Cancer Causes Control 2013, 24, 1137–1146. [Google Scholar] [CrossRef]
- Fred Hutchinson Cancer Center GLANCE 2—Glucosamine and Chondroitin (G&C) Effects Study. 2019. Available online: https://clinicaltrials.gov/study/NCT01682694 (accessed on 21 September 2025).
- Cao, L.-M.; Sun, Z.-X.; Makale, E.C.; Du, G.-K.; Long, W.-F.; Huang, H.-R. Antitumor Activity of Fucoidan: A Systematic Review and Meta-Analysis. Transl. Cancer Res. 2021, 10, 5390–5405. [Google Scholar] [CrossRef]
- Yue, Q.; Liu, Y.; Li, F.; Hong, T.; Guo, S.; Cai, M.; Zhao, L.; Su, L.; Zhang, S.; Zhao, C.; et al. Antioxidant and Anticancer Properties of Fucoidan Isolated from Saccharina japonica Brown Algae. Sci. Rep. 2025, 15, 8962. [Google Scholar] [CrossRef]
- Jin, J.-O.; Yadav, D.; Madhwani, K.; Puranik, N.; Chavda, V.; Song, M. Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review. Molecules 2022, 27, 6032. [Google Scholar] [CrossRef]
- Turrini, E.; Maffei, F.; Fimognari, C. Ten Years of Research on Fucoidan and Cancer: Focus on Its Antiangiogenic and Antimetastatic Effects. Mar. Drugs 2023, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.N.; Lobo, F.C.M.; Rodrigues, L.C.; Fernandes, E.M.; Williams, D.S.; Mearns-Spragg, A.; Sotelo, C.G.; Perez-Martín, R.I.; Reis, R.L.; Gelinsky, M.; et al. Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues. Gels 2023, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, N.; Ustyuzhanina, N.; Bilan, M.; Donenko, F.; Usov, A.; Kiselevskiy, M.; Nifantiev, N. Fucoidan and Fucosylated Chondroitin Sulfate Stimulate Hematopoiesis in Cyclophosphamide-Induced Mice. Mar. Drugs 2017, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Anisimova, N.Y.; Bilan, M.I.; Donenko, F.V.; Morozevich, G.E.; Yashunskiy, D.V.; Usov, A.I.; Siminyan, N.G.; Kirgisov, K.I.; Varfolomeeva, S.R.; et al. Chondroitin Sulfate and Fucosylated Chondroitin Sulfate as Stimulators of Hematopoiesis in Cyclophosphamide-Induced Mice. Pharmaceuticals 2021, 14, 1074. [Google Scholar] [CrossRef] [PubMed]
- Nováková, E.; Zima, J.; Špaglová, M.; Labudová, M.; Šupolíková, M. Synergistic Antiproliferative Effects of Chondroitin Sulfate and Fucoidan in Tumor-Derived Spheroids: Insights From a 3D Cell Culture Approach. Eur. Pharm. J. 2025, 71, 25–33. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kumashiro, R. Conversion of Vitamin D3 Binding Protein (Group-Specific Component) to a Macrophage Activating Factor by the Stepwise Action of Beta-Galactosidase of B Cells and Sialidase of T Cells. J. Immunol. 1993, 151, 2794–2802. [Google Scholar] [CrossRef]
- Kisker, O.; Onizuka, S.; Becker, C.M.; Fannon, M.; Flynn, E.; D’Amato, R.; Zetter, B.; Folkman, J.; Ray, R.; Swamy, N.; et al. Vitamin D Binding Protein-Macrophage Activating Factor (DBP-Maf) Inhibits Angiogenesis and Tumor Growth in Mice. Neoplasia 2003, 5, 32–40. [Google Scholar] [CrossRef]
- Koga, Y.; Naraparaju, V.R.; Yamamoto, N. Antitumor Effect of Vitamin D-Binding Protein-Derived Macrophage Activating Factor on Ehrlich Ascites Tumor-Bearing Mice. Proc. Soc. Exp. Biol. Med. 1999, 220, 20–26. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Mourão, P.A.S. Pharmacological Activities of Sulfated Fucose-Rich Polysaccharides after Oral Administration: Perspectives for the Development of New Carbohydrate-Based Drugs. Mar. Drugs 2021, 19, 425. [Google Scholar] [CrossRef]
- Martin-Martin, L.; Pierluigi, B.; La Medica, C.; Melis, G.; Nuvoli, G.; Piccinni, V.; Pietrapertosa, M.; Vincenti, M.; Vinicola, V. Randomized Observational Multicenter Study to Assess the Efficacy and Safety of the Association of Fortigel (10 Gr) and Fucoidan (100 Mg) in Patients with Gonarthrosis. Clin. Res. Trials 2016, 2, 253–257. [Google Scholar] [CrossRef]
- Miraglia, N.; Bianchi, D.; Trentin, A.; Volpi, N.; Soni, M.G. Safety Assessment of Non-Animal Chondroitin Sulfate Sodium: Subchronic Study in Rats, Genotoxicity Tests and Human Bioavailability. Food Chem. Toxicol. 2016, 93, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Mantovani, V.; Galeotti, F.; Bianchi, D.; Straniero, V.; Valoti, E.; Miraglia, N. Oral Bioavailability and Pharmacokinetics of Nonanimal Chondroitin Sulfate and Its Constituents in Healthy Male Volunteers. Clin. Pharmacol. Drug Dev. 2019, 8, 336–345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).