Pumpkin Seeds Harbor Hidden Agonists: Adenosine-Mediated A1 Receptor Activation and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nucleosides Analysis
2.2.1. Nucleosides Identification and Quantification
2.2.2. Adenosine and Its Derivative Isolation
2.2.3. Chemical Characterization of Isolated Fraction
2.3. Bioactivity Assays
2.3.1. A1AR Direct Activation
2.3.2. A1AR Positive Allosteric Modulation
2.4. Total Phenolic Content and Antioxidant Activity
3. Results
3.1. Nucleosides Analysis
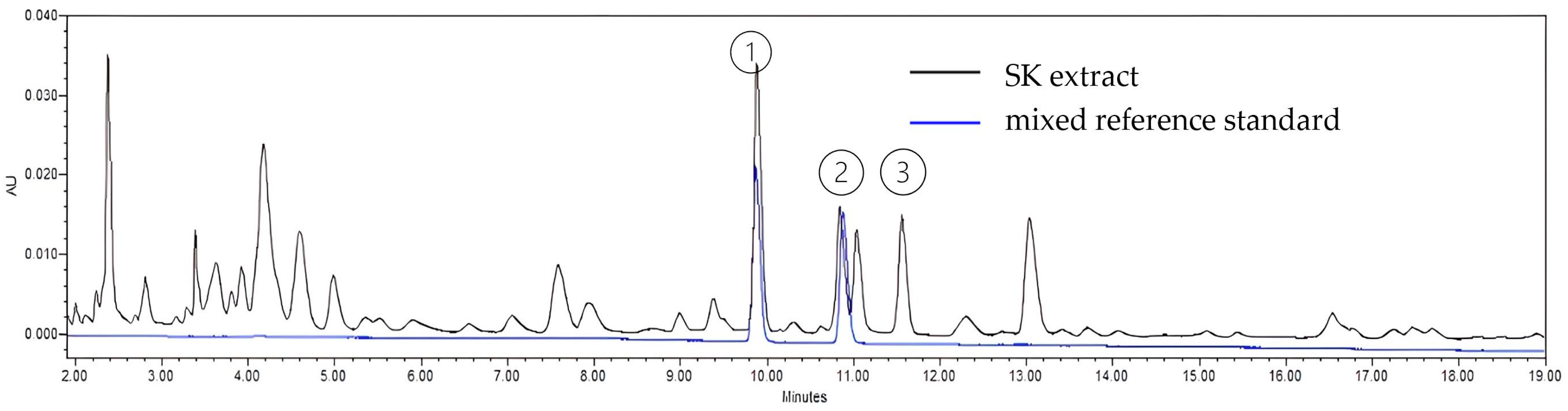
3.1.1. Nucleosides Identification and Quantification
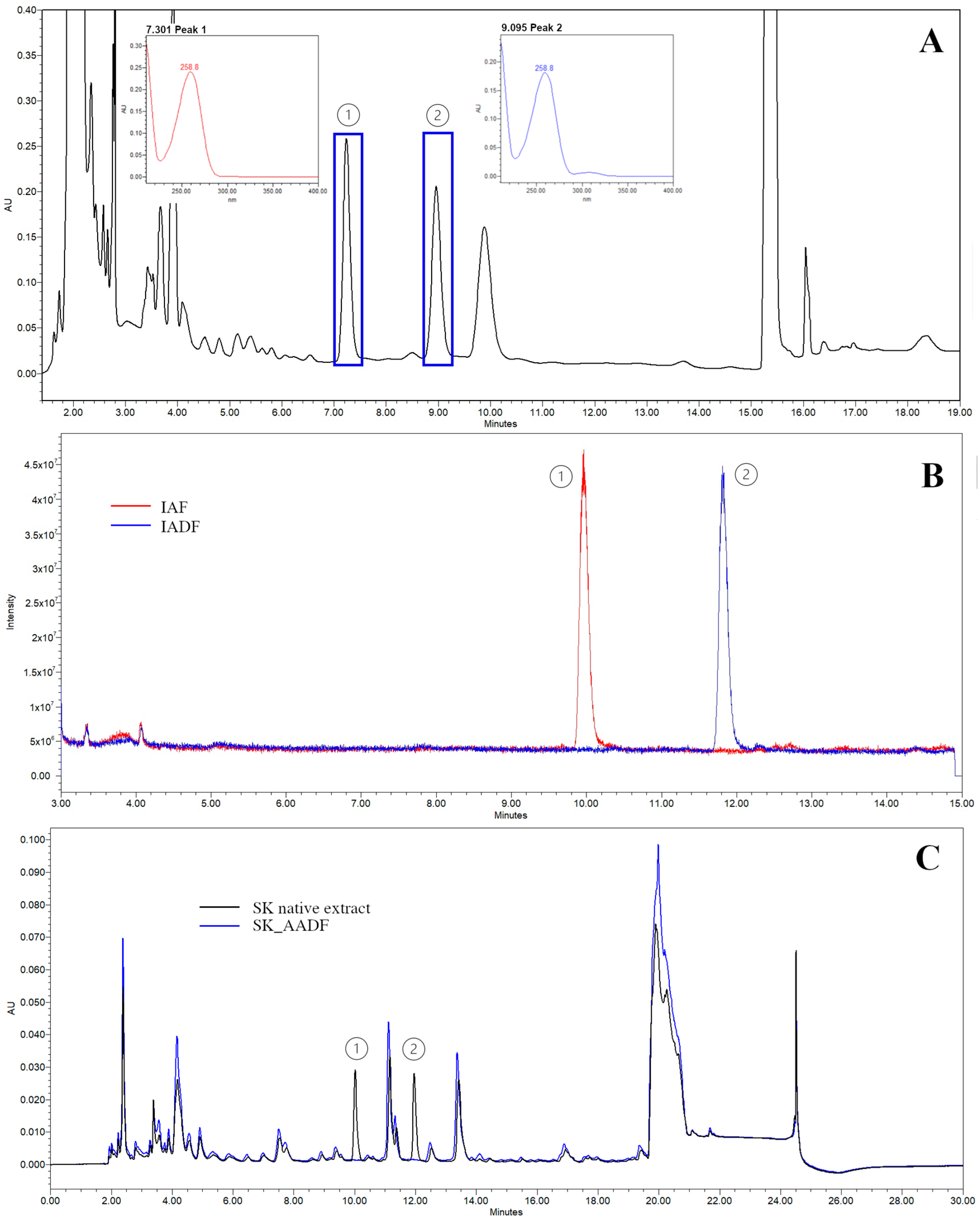
3.1.2. Isolation of Adenosine and Its Derivative Fractions
3.1.3. Chemical Characterization of the Isolated Fraction
3.2. Bioactivity Assays
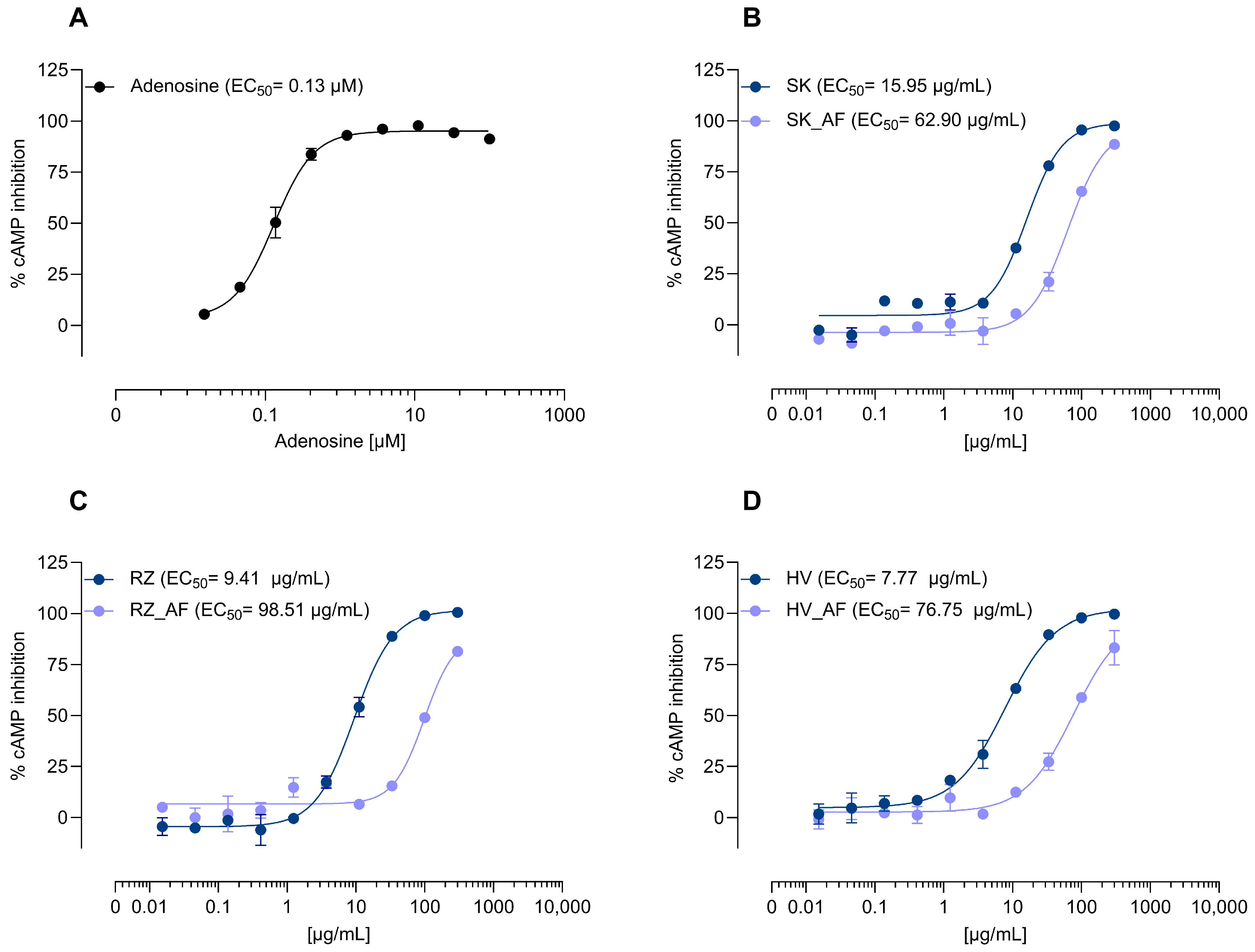
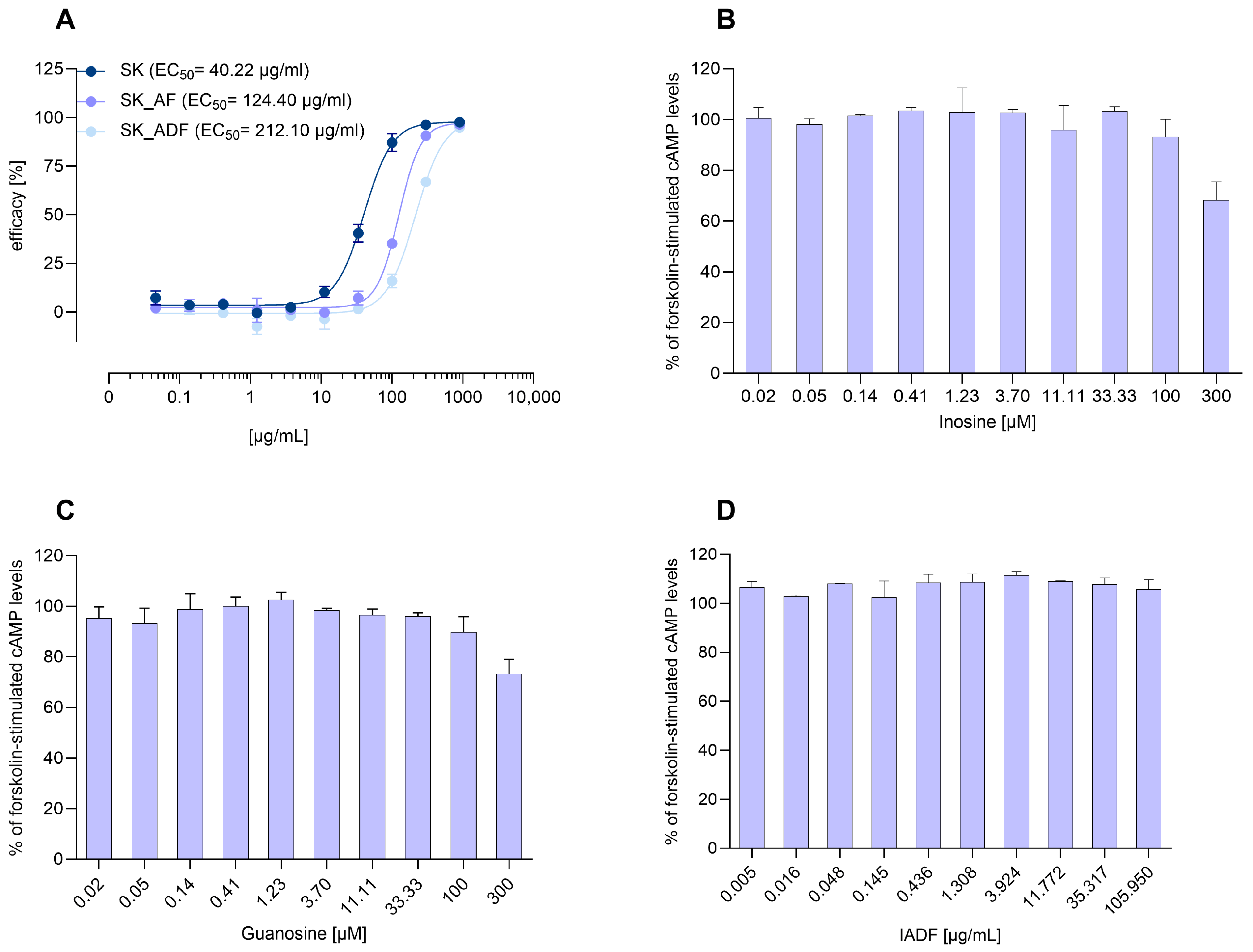
3.2.1. Direct Agonist Activity and Adenosine Role
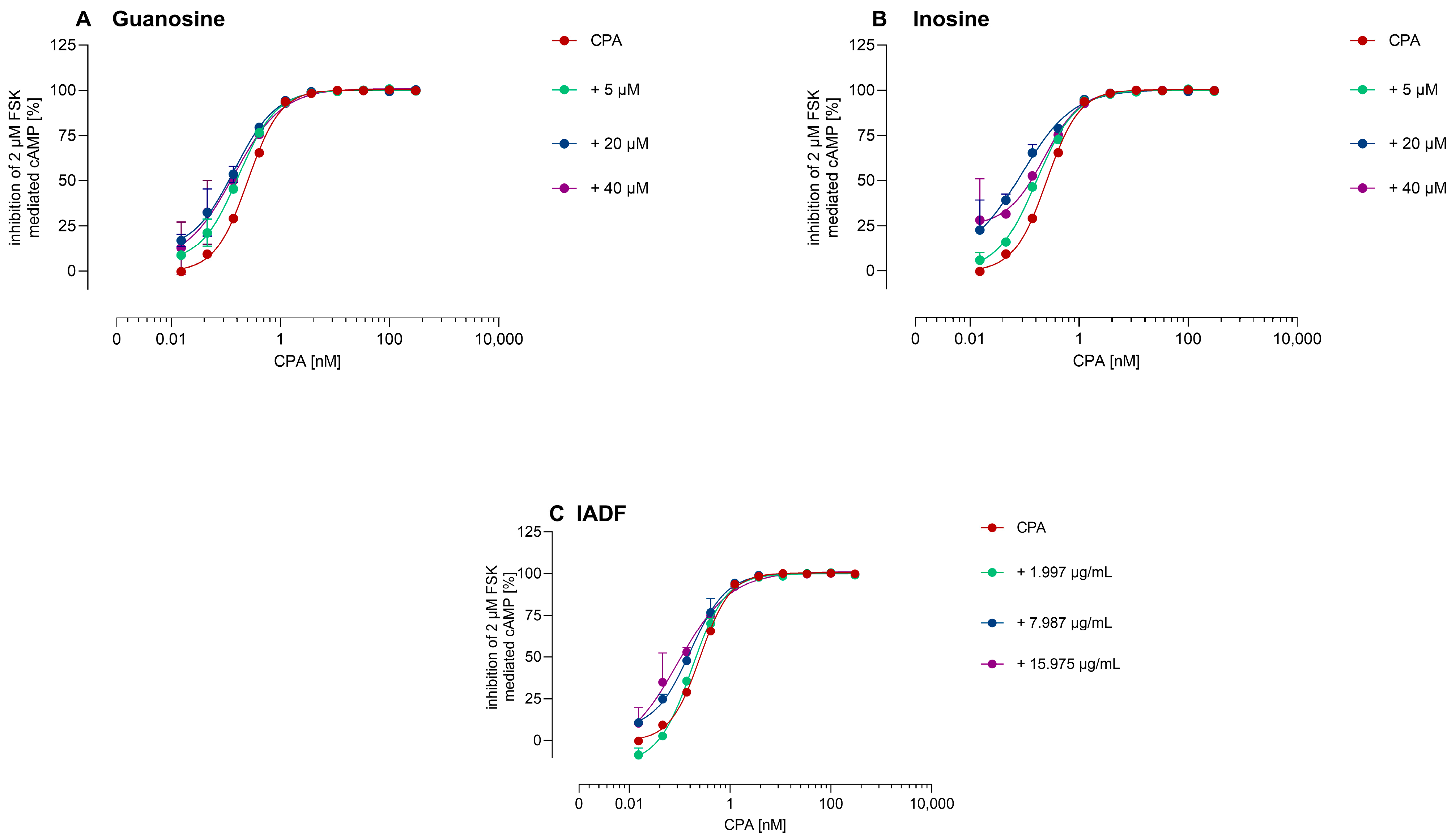
3.2.2. Allosteric Modulation of A1AR
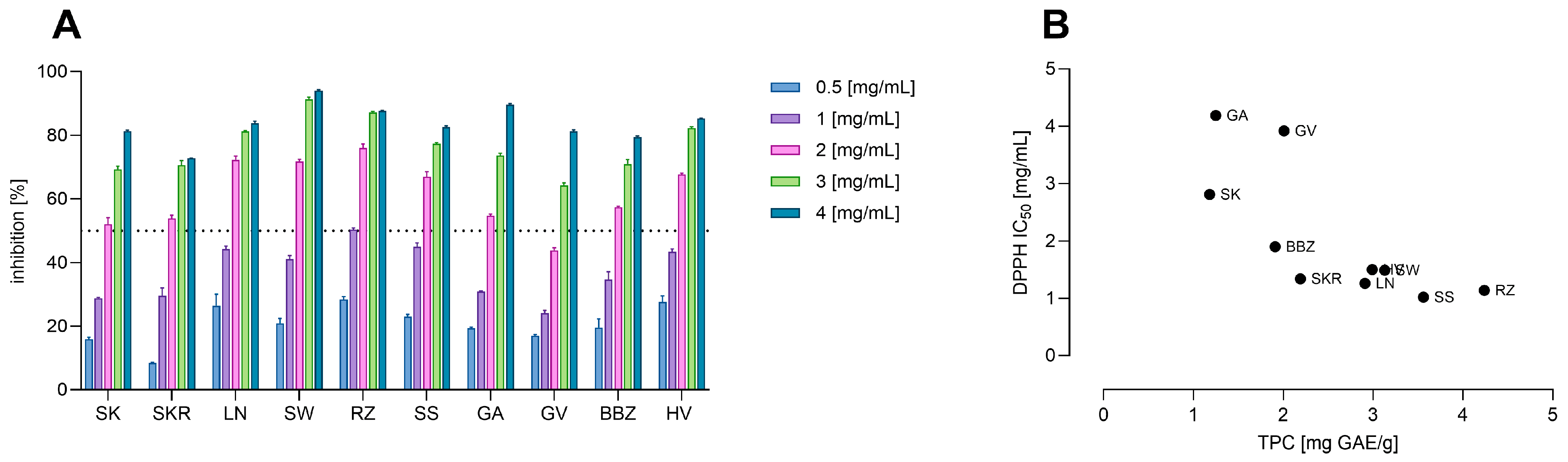
3.3. Total Phenolic Content and Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, N.; Iglesia, C.B. Overactive bladder. Obstet. Gynecol. Clin. 2016, 43, 59–68. [Google Scholar] [CrossRef]
- Singam, P.; Hong, G.E.; Ho, C.; Hee, T.G.; Jasman, H.; Inn, F.X.; Bahadzor, B.; Tamil, A.; Zainuddin, Z. Nocturia in patients with benign prostatic hyperplasia: Evaluating the significance of ageing, co-morbid illnesses, lifestyle and medical therapy in treatment outcome in real life practice. Aging Male 2015, 18, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, N.; Mo, L.; Tian, X.; Liu, H.; Yu, B. Global Prevalence of Overactive Bladder: A Systematic Review and Meta-analysis. Int. Urogynecol. J. 2025; ahead of print. [Google Scholar] [CrossRef]
- Roehrborn, C.G. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med. Clin. 2011, 95, 87–100. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Bixler, B.R.; Dahm, P.; Goueli, R.; Kirkby, E.; Stoffel, J.T.; Wilt, T.J. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA guideline amendment 2023. J. Urol. 2024, 211, 11–19. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Gao, Y. Overactive bladder symptoms within nervous system: A focus on etiology. Front. Physiol. 2021, 12, 747144. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Melcangi, R.C.; Bortolato, M.; Garcia-Segura, L.M.; Zitzmann, M. Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Rev. Endocr. Metab. Disord. 2015, 16, 177–198. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, S.; Li, F.; Li, E.; Kang, R.; Luo, L.; Luo, J.; Wan, S.; Zhao, Z. Effect of 5α-reductase inhibitors on sexual function: A meta-analysis and systematic review of randomized controlled trials. J. Sex. Med. 2016, 13, 1297–1310. [Google Scholar] [CrossRef]
- Chapple, C.; Khullar, V.; Gabriel, Z.; Dooley, J.A. The effects of antimuscarinic treatments in overactive bladder: A systematic review and meta-analysis. Eur. Urol. 2005, 48, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; LEE, K.S.; Jung, R.; Na, S.; KIM, J.c.; Kim, H.G.; Choo, M.S. Health related quality of life in patients with side-effects after antimuscarinic treatment for overactive bladder. Low. Urin. Tract. Symptoms 2017, 9, 171–175. [Google Scholar] [CrossRef]
- Kelleher, C.; Hakimi, Z.; Zur, R.; Siddiqui, E.; Maman, K.; Aballéa, S.; Nazir, J.; Chapple, C. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: A systematic review and network meta-analysis. Eur. Urol. 2018, 74, 324–333. [Google Scholar] [CrossRef]
- Farag, F.; Sakalis, V.I.; Arteaga, S.M.; Sihra, N.; Karavitakis, M.; Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; de Heide, M. What are the short-term benefits and potential harms of therapeutic modalities for the management of overactive bladder syndrome in women? A review of evidence under the auspices of the European Association of Urology, female non-neurogenic lower urinary tract symptoms guidelines panel. Eur. Urol. 2023, 84, 302–312. [Google Scholar]
- Yoshida, M.; Kakizaki, H.; Takahashi, S.; Nagai, S.; Kurose, T. Long-term safety and efficacy of the novel β3-adrenoreceptor agonist vibegron in Japanese patients with overactive bladder: A phase III prospective study. Int. J. Urol. 2018, 25, 668–675. [Google Scholar] [CrossRef]
- Cindolo, L.; Pirozzi, L.; Fanizza, C.; Romero, M.; Tubaro, A.; Autorino, R.; De Nunzio, C.; Schips, L. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: Population-based cohort study. Eur. Urol. 2015, 68, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Carmel, M.E. Quality, Value, and Efficacy of Alternative Medicine in the Treatment of Overactive Bladder. Curr. Bladder Dysfunct. Rep. 2023, 18, 139–146. [Google Scholar] [CrossRef]
- Damiano, R.; Cai, T.; Fornara, P.; Franzese, C.A.; Leonardi, R.; Mirone, V. The role of Cucurbita pepo in the management of patients affected by lower urinary tract symptoms due to benign prostatic hyperplasia: A narrative review. Arch. Ital. Urol. Androl. 2016, 88, 136–143. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D. Cucurbits plants: A key emphasis to its pharmacological potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Wal, A.; Singh, M.R.; Gupta, A.; Rathore, S.; Rout, R.R.; Wal, P. Pumpkin seeds (Cucurbita spp.) as a nutraceutical used in various lifestyle disorders. Nat. Prod. J. 2024, 14, 118–137. [Google Scholar] [CrossRef]
- Widy-Tyszkiewicz, E.; Matlawska, I.; Bylka, W. Assessment Report on Cucurbita pepo L., Semen; EMA/HMPC/136022/2010; Committee on Herbal Medicinal Products (HMPC): Amsterdam, The Netherlands, 2012; Volume 20. [Google Scholar]
- Gažová, A.; Valášková, S.; Žufková, V.; Castejon, A.M.; Kyselovič, J. Clinical study of effectiveness and safety of CELcomplex® containing Cucurbita pepo seed extract and Flax and Casuarina on stress urinary incontinence in women. J. Trad. Complent. Med. 2019, 9, 138–142. [Google Scholar] [CrossRef]
- Zerafatjou, N.; Amirzargar, M.; Biglarkhani, M.; Shobeirian, F.; Zoghi, G. Pumpkin seed oil (Cucurbita pepo) versus tamsulosin for benign prostatic hyperplasia symptom relief: A single-blind randomized clinical trial. BMC Urol. 2021, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Theil, G.; Richter, M.; Schulze, M.; Köttig, T.; Patz, B.; Heim, S.; Krauß, Y.; Markov, M.; Fornara, P. Extract from Cucurbita pepo improves BPH symptoms without affecting sexual function: A 24-month noninterventional study. World J. Urol. 2022, 40, 1769–1775. [Google Scholar] [CrossRef]
- Yanagisawa, E.; Satoh, I. Clinical study of mixed processed foods containing of pumpkin seed extract and soybean germ extract on stress urinary incontinence (SUI) in women. Jpn. J. Med. Pharm. Sci. 2003, 50, 313–322. [Google Scholar]
- Shim, B.; Jeong, H.; Lee, S.; Hwang, S.; Moon, B.; Storni, C. A randomized double-blind placebo-controlled clinical trial of a product containing pumpkin seed extract and soy germ extract to improve overactive bladder-related voiding dysfunction and quality of life. J. Funct. Foods 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Leibbrand, M.; Siefer, S.; Schön, C.; Perrinjaquet-Moccetti, T.; Kompek, A.; Csernich, A.; Bucar, F.; Kreuter, M.H. Effects of an oil-free hydroethanolic pumpkin seed extract on symptom frequency and severity in men with benign prostatic hyperplasia: A pilot study in humans. J. Med. Food 2019, 22, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Perez Gutierrez, R.M. Review of Cucurbita pepo (pumpkin) its phytochemistry and pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Adnan, M.; Gul, S.; Batool, S.; Fatima, B.; Rehman, A.; Yaqoob, S.; Shabir, H.; Yousaf, T.; Mussarat, S.; Ali, N. A review on the ethnobotany, phytochemistry, pharmacology and nutritional composition of Cucurbita pepo L. J. Phytopharmacol. 2017, 6, 133–139. [Google Scholar] [CrossRef]
- Ramak, P.; Mahboubi, M. The beneficial effects of pumpkin (Cucurbita pepo L.) seed oil for health condition of men. Food Rev. Int. 2019, 35, 166–176. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; SadeghiMahoonak, A.; Alami, M.; Ghorbani, M. Amino acid composition and antioxidative properties of hydrolysed pumpkin (Cucurbita pepo L.) oil cake protein. Int. J. Food Prop. 2017, 20, 3244–3255. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—In vitro and in vivo studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Grasu, A.-E.; Senn, R.; Halbsguth, C.; Schenk, A.; Butterweck, V.; Miron, A. Profiling Hydrophilic Cucurbita pepo Seed Extracts: A Study of European Cultivar Variability. Plants 2025, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Xu, G.-Y.; Tang, Y. Purinergic signaling in the central nervous system in health and disease. Neurosci. Bull. 2020, 36, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Tanahashi, S.; Wakida, Y.; Tatsuzaki, M.; Koide, A. Effects of pumpkin seed extract on urinary bladder function in anesthesized rats. Med. Sci. Pharm. Sci. 2005, 54, 1–10. [Google Scholar]
- Persson, K.; Igawa, Y.; Mattiasson, A.; Andersson, K.E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br. J. Pharmacol. 1992, 107, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, D.Y.; Cho, K.J.; Hashimoto, M.; Matsuoka, K.; Kamijo, T.; Wang, Z.; Karnup, S.; Robertson, A.M.; Tyagi, P. Pathophysiology of overactive bladder and pharmacologic treatments including β3-adrenoceptor agonists-basic research perspectives. Int. Neurourol. J. 2024, 28, S2. [Google Scholar] [CrossRef]
- Searl, T.J.; Dynda, D.I.; Alanee, S.R.; El-Zawahry, A.M.; McVary, K.T.; Silinsky, E.M. A1 adenosine receptor–mediated inhibition of parasympathetic neuromuscular transmission in human and murine urinary bladder. J. Pharmacol. Exp. Ther. 2016, 356, 116–122. [Google Scholar] [CrossRef]
- Silva, I.; Costa, A.F.; Moreira, S.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Calejo, I.; Silva-Ramos, M.; Correia-de-Sá, P. Inhibition of cholinergic neurotransmission by β3-adrenoceptors depends on adenosine release and A1-receptor activation in human and rat urinary bladders. Am. J. Physiol. Ren. Physiol. 2017, 313, F388–F403. [Google Scholar] [CrossRef]
- Silva-Ramos, M.; Silva, I.; Faria, M.; Magalhães-Cardoso, M.; Correia, J.; Ferreirinha, F.; Correia-de-Sá, P. Impairment of ATP hydrolysis decreases adenosine A 1 receptor tonus favoring cholinergic nerve hyperactivity in the obstructed human urinary bladder. Purinergic Signal. 2015, 11, 595–606. [Google Scholar] [CrossRef]
- Prakasam, H.S.; Herrington, H.; Roppolo, J.R.; Jackson, E.K.; Apodaca, G. Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am. J. Physiol. Ren. Physiol. 2012, 303, F279–F292. [Google Scholar] [CrossRef]
- Kitta, T.; Chancellor, M.B.; de Groat, W.C.; Kuno, S.; Nonomura, K.; Yoshimura, N. Roles of adenosine A1 and A2A receptors in the control of micturition in rats. Neurourol. Urodyn. 2014, 33, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Loizzo, M.R.; Gortzi, O.; Çankaya, İ.T.; Tundis, R.; Suntar, İ.; Shirooie, S.; Zengin, G.; Devkota, H.P.; Reboredo-Rodríguez, P. The potential of pumpkin seed oil as a functional food—A comprehensive review of chemical composition, health benefits, and safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4422–4446. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, A.; Jamil, M.A.; Noreen, S.; Iqbal, M.A. Antioxidant and antimicrobial properties of pumpkin (Cucurbita maxima) peel, flesh and seeds powders. J. Biol. Agric. Healthc. 2021, 11, 42–51. [Google Scholar]
- Kaltsas, A.; Giannakas, T.; Stavropoulos, M.; Kratiras, Z.; Chrisofos, M. Oxidative stress in benign prostatic hyperplasia: Mechanisms, clinical relevance and therapeutic perspectives. Diseases 2025, 13, 53. [Google Scholar] [CrossRef]
- Khosla, L.; Gong, S.; Weiss, J.P.; Birder, L.A. Oxidative stress biomarkers in age-related lower urinary tract disorders: A systematic review. Int. Neuroul. J. 2022, 26, 3–19. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Inferrera, A.; Navarra, M.; Calapai, G.; Magno, C.; Gangemi, S. Oxidative stress in benign prostatic hyperplasia: A systematic review. Urol. Int. 2015, 94, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chueh, K.-S.; Chuang, S.-M.; Long, C.-Y.; Lu, J.-H.; Juan, Y.-S. Bladder hyperactivity induced by oxidative stress and bladder ischemia: A review of treatment strategies with antioxidants. Int. J. Mol. Sci. 2021, 22, 6014. [Google Scholar] [CrossRef] [PubMed]
- Haslauer, K.E.; Hemmler, D.; Schmitt-Kopplin, P.; Heinzmann, S.S. Guidelines for the use of deuterium oxide (D2O) in 1H NMR metabolomics. Anal. Chem. 2019, 91, 11063–11069. [Google Scholar] [CrossRef]
- Senn, R.; Schertler, L.; Bussmann, H.; Drewe, J.; Boonen, G.; Butterweck, V. Valerenic Acid and Pinoresinol as Positive Allosteric Modulators: Unlocking the Sleep-Promoting Potential of Valerian Extract Ze 911. Molecules 2025, 30, 2344. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Esslinger, N.; Grubelnik, A.; Wolfram, E.; Skalicka-Woźniak, K.; Minceva, M.; Luca, S.V. Influence of the post-harvest storage time on the multi-biological potential, phenolic and pyrrolizidine alkaloid content of comfrey (Symphytum officinale L.) roots collected from different European regions. Plants 2021, 10, 1825. [Google Scholar] [CrossRef]
- Fuso, A.; Dejonghe, W.; Cauwenberghs, L.; Rosso, G.; Rosso, F.; Manera, I.; Caligiani, A. DPPH radical scavenging activity of xylo-oligosaccharides mixtures of controlled composition: A step forward in understanding structure–activity relationship. J. Funct. Foods 2023, 101, 105417. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.K.; Raghvendra, D.K. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu. Rev. Physiol. 2004, 66, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Sichardt, K.; Nieber, K. Adenosine A 1 receptor: Functional receptor-receptor interactions in the brain. Purinergic Signal. 2007, 3, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A receptors in the brain: Current research and their role in neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Li, M.; Lv, R.; Gu, B.; Chen, Z. Activation of the adenosine A1 receptor in the lumbosacral spinal cord improves bladder overactivity in rats with cystitis induced by cyclophosphamide. Int. Urol. Nephrol. 2023, 55, 2183–2191. [Google Scholar] [CrossRef]
- Yu, W.; Zacharia, L.C.; Jackson, E.K.; Apodaca, G. Adenosine receptor expression and function in bladder uroepithelium. Am. J. Physiol. Cell Physiol. 2006, 291, C254–C265. [Google Scholar] [CrossRef]
- Pakzad, M.; Ikeda, Y.; McCarthy, C.; Kitney, D.G.; Jabr, R.I.; Fry, C.H. Contractile effects and receptor analysis of adenosine-receptors in human detrusor muscle from stable and neuropathic bladders. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Nahrstedt, A. What is the best strategy for preclinical testing of botanicals? A critical perspective. Planta Med. 2012, 78, 747–754. [Google Scholar] [CrossRef]
- Verzijl, D.; IJzerman, A.P. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011, 7, 171–192. [Google Scholar] [CrossRef]
- Haskó, G.; Sitkovsky, M.V.; Szabó, C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef]
- Domínguez Moré, G.P.; Cardona, M.I.; Sepúlveda, P.M.; Echeverry, S.M.; Oliveira Simões, C.M.; Aragón, D.M. Matrix effects of the hydroethanolic extract of Calyces of Physalis peruviana L. on rutin pharmacokinetics in Wistar rats using population modeling. Pharmaceutics 2021, 13, 535. [Google Scholar] [CrossRef]
- Huang, N.-K.; Chern, Y.; Fang, J.-M.; Lin, C.-I.; Chen, W.-P.; Lin, Y.-L. Neuroprotective principles from Gastrodia elata. J. Nat. Prod. 2007, 70, 571–574. [Google Scholar] [CrossRef]
- Nakamukai, S.; Ise, Y.; Ohtsuka, S.; Okada, S.; Matsunaga, S. Isolation and identification of N6-isopentenyladenosine as the cytotoxic constituent of a marine sponge Oceanapia sp. Biosci. Biotechnol. Biochem. 2019, 83, 1985–1988. [Google Scholar] [CrossRef]
- Takaoka, E.i.; Kurobe, M.; Matsuoka, K.; Kamijo, T.; Kimura, S.; Watton, P.N.; Robertson, A.M.; Yoshimura, N. Adenosine receptor mechanisms underlying bladder dysfunction in male rats with bladder outlet obstruction. Neurourol. Urodyn. 2025, 44, 1370–1377. [Google Scholar] [CrossRef]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs targeting adenosine signaling pathways: A current view. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.; Aires, A.; Dias, C.; Almeida, J.; De Vasconcelos, M.; Santos, P.; Rosa, E. Evaluation of the potential of squash pumpkin by-products (seeds and shell) as sources of antioxidant and bioactive compounds. J. Food Sci. Technol. 2015, 52, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Lu, D.; Liu, J.; Jiang, B.; Chen, J. Effect of Roasting on the Antioxidant Activity, Phenolic Composition, and Nutritional Quality of Pumpkin (Cucurbita pepo L.) Seeds. Front. Nutr. 2021, 8, 647354. [Google Scholar] [CrossRef] [PubMed]
- Akomolafe, S.F.; Olasehinde, T.A.; Aluko, B.T. Diets supplemented with raw and roasted pumpkin (Cucurbita pepo L.) seeds improved some biochemical parameters associated with erectile function in rats. J. Food Biochem. 2021, 45, e13629. [Google Scholar] [CrossRef] [PubMed]
- Monica, S.J.; John, S.; Madhanagopal, R.; Sivaraj, C.; Khusro, A.; Arumugam, P.; Gajdacs, M.; Lydia, D.E.; Sahibzada, M.U.K.; Alghamdi, S. Chemical composition of pumpkin (Cucurbita maxima) seeds and its supplemental effect on Indian women with metabolic syndrome. Arab. J. Chem. 2022, 15, 103985. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Nascimento da Silva, L.C.; Bezerra Filho, C.M.; Paula, R.A.d.; Silva e Silva, C.S.; Oliveira de Souza, L.I.; Silva, M.V.d.; Correia, M.T.d.S.; Figueiredo, R.C.B.Q.d. In vitro cell-based assays for evaluation of antioxidant potential of plant-derived products. Free Radic. Res. 2016, 50, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hou, X.; Lee, J.H.; Nayak, A.; Alexander, V.; Sharma, P.K.; Chang, H.; Phan, K.; Gao, Z.-G.; Jacobson, K.A. Subtle chemical changes cross the boundary between agonist and antagonist: New A3 adenosine receptor homology models and structural network analysis can predict this boundary. J. Med. Chem. 2021, 64, 12525–12536. [Google Scholar] [CrossRef] [PubMed]
| Nr. | Sample | Adenosine [mg/g] ± SD | Guanosine [mg/g] ± SD | Adenosine Derivative [mg/g Adenosine Equivalents] ± SD |
|---|---|---|---|---|
| 1. | SK | 0.60 ± 0.01 | 0.84 ± 0.00 | 0.58 ± 0.06 |
| 2. | SKR | 0.99 ± 0.00 | 0.52 ± 0.00 | 0.50 ± 0.05 |
| 3. | LN | 0.86 ± 0.01 | 1.03 ± 0.04 | 0.68 ± 0.07 |
| 4. | SW | 1.02 ± 0.00 | 0.33 ± 0.02 | 1.58 ± 0.16 |
| 5. | RZ | 0.83 ± 0.01 | 0.71 ± 0.04 | 0.48 ± 0.05 |
| 6. | SS | 0.92 ± 0.01 | 0.91 ± 0.06 | 0.40 ± 0.04 |
| 7. | GA | 0.67 ± 0.00 | 1.23 ± 0.04 | 0.67 ± 0.07 |
| 8. | GV | 1.05 ± 0.00 | 0.74 ± 0.01 | 0.14 ± 0.01 |
| 9. | BBZ | 0.76 ± 0.00 | 1.05 ± 0.01 | 0.29 ± 0.03 |
| 10. | HV | 1.17 ± 0.01 | 0.90 ± 0.05 | 0.47 ± 0.05 |
| Nr. | Sample | TPC [mg GAE/g Extract] | DPPH IC50 [mg/mL] | 95% CI [mg/mL] |
|---|---|---|---|---|
| 1. | SK | 1.18 ± 0.16 | 2.81 ± 0.26 | 2.27–5.40 |
| 2. | SKR | 2.19 ± 0.26 | 1.34 ± 0.16 | 0.99–1.60 |
| 3. | LN | 2.91 ± 0.04 | 2.26 ± 0.04 | 1.16–1.36 |
| 4. | SW | 3.13 ± 0.16 | 1.49 ± 0.16 | 1.35–1.66 |
| 5. | RZ | 4.24 ± 0.17 | 1.14 ± 0.17 | 1.04–1.23 |
| 6. | SS | 3.56 ± 0.31 | 1.02 ± 0.31 | 0.59–1.19 |
| 7. | GA | 1.25 ± 0.38 | 4.19 ± 0.38 | 3.35–6.45 |
| 8. | GV | 2.01 ± 0.08 | 3.92 ± 0.08 | 3.25–5.58 |
| 9. | BBZ | 1.91 ± 0.39 | 1.90 ± 0.39 | 1.56–2.13 |
| 10. | HV | 2.99 ± 0.22 | 1.50 ±0.22 | 1.37–1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasu, A.-E.; Senn, R.; Halbsguth, C.; Schenk, A.; Butterweck, V.; Zecchin, G.; Mangalagiu, I.I.; Ciobanu, C.-I.; Miron, A. Pumpkin Seeds Harbor Hidden Agonists: Adenosine-Mediated A1 Receptor Activation and Antioxidant Activity. Sci. Pharm. 2025, 93, 48. https://doi.org/10.3390/scipharm93040048
Grasu A-E, Senn R, Halbsguth C, Schenk A, Butterweck V, Zecchin G, Mangalagiu II, Ciobanu C-I, Miron A. Pumpkin Seeds Harbor Hidden Agonists: Adenosine-Mediated A1 Receptor Activation and Antioxidant Activity. Scientia Pharmaceutica. 2025; 93(4):48. https://doi.org/10.3390/scipharm93040048
Chicago/Turabian StyleGrasu, Adina-Elena, Roman Senn, Christiane Halbsguth, Alexander Schenk, Veronika Butterweck, Giulia Zecchin, Ionel I. Mangalagiu, Cătălina-Ionica Ciobanu, and Anca Miron. 2025. "Pumpkin Seeds Harbor Hidden Agonists: Adenosine-Mediated A1 Receptor Activation and Antioxidant Activity" Scientia Pharmaceutica 93, no. 4: 48. https://doi.org/10.3390/scipharm93040048
APA StyleGrasu, A.-E., Senn, R., Halbsguth, C., Schenk, A., Butterweck, V., Zecchin, G., Mangalagiu, I. I., Ciobanu, C.-I., & Miron, A. (2025). Pumpkin Seeds Harbor Hidden Agonists: Adenosine-Mediated A1 Receptor Activation and Antioxidant Activity. Scientia Pharmaceutica, 93(4), 48. https://doi.org/10.3390/scipharm93040048







