Simultaneous Profiling of Terpenes and Cannabinoids in Hemp Essential Oils Using Static Headspace Gas Chromatography–Mass Spectrometry for Quality Control and Chemotype Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Essential Oil Extraction
2.3. Standard and Sample Preparation for SHS-GC-MS
2.4. SHS-GC-MS Analysis
2.5. Optimization of the SHS Sampling
2.6. SHS-GC-MS Method Validation
2.7. Data Processing and Multivariate Analysis of Essential Oils
3. Results and Discussion
3.1. Optimization of the SHS Sampling
3.2. SHS-GC-MS Method Validation
3.3. Quantitative Analysis of Terpenes and Cannabinoids in Hemp Essential Oil (EO)
3.4. Multivariate Analysis of Hemp Essential Oils (EO)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BQL | below the quantification limit |
| CA | Charlotte’s Angel |
| CBD | cannabidiol |
| CW | Cherry Wine |
| EO | hemp essential oil |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| QC | quality control |
| RSD | relative standard deviation |
| SHS-GC-MS | static headspace gas chromatography–mass spectrometry |
| Δ9-THC | delta-9-tetrahydrocannabinol |
References
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.-H.; Deng, G. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut. Res. 2021, 28, 52832–52843. [Google Scholar] [CrossRef] [PubMed]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2020, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Johnson, L.; Malone, M.; Paulson, E.; Swider, J.; Marelius, D.; Andersen, S.; Black, D. Potency and safety analysis of hemp delta-9 products: The hemp vs. cannabis demarcation problem. J. Cannabis Res. 2023, 5, 29. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Noller, J.S.; Maggi, F.; Dale, R. Terpenes and cannabinoids yields and profile from direct-seeded and transplanted CBD-Cannabis sativa. J. Agric. Food Chem. 2022, 70, 10417–10428. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ascrizzi, R.; Iannone, M.; Cinque, G.; Marianelli, A.; Pistelli, L.; Flamini, G. “Hemping” the drinks: Aromatizing alcoholic beverages with a blend of Cannabis sativa L. flowers. Food Chem. 2020, 325, 126909. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 hemp genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Mead, A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10515–10525. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z. In vitro evaluation of the activity of terpenes and cannabidiol against human coronavirus E229. Life 2021, 11, 290. [Google Scholar] [CrossRef]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef]
- Capetti, F.; Rubiolo, P.; Mastellone, G.; Marengo, A.; Sgorbini, B.; Cagliero, C. A sustainable approach for the reliable and simultaneous determination of terpenoids and cannabinoids in hemp inflorescences by vacuum assisted headspace solid-phase microextraction. Adv. Sample Prep. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Shapira, A.; Berman, P.; Futoran, K.; Guberman, O.; Meiri, D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 11425–11432. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, J.C.; Gokool, V.A.; Holness, H.K.; Furton, K.G.; DeGreeff, L.E. Method development for an untargeted HS-SPME-GC–MS analysis of terpenes and cannabinoids for the geographical sourcing of Marijuana. Talanta Open 2024, 9, 100300. [Google Scholar] [CrossRef]
- Afshar Mogaddam, M.R.; Mohebbi, A.; Pazhohan, A.; Khodadadeian, F.; Farajzadeh, M.A. Headspace mode of liquid phase microextraction: A review. Trends Anal. Chem. 2019, 110, 8–14. [Google Scholar] [CrossRef]
- Deng, H.; He, R.; Huang, R.; Pang, C.; Ma, Y.; Xia, H.; Liang, D.; Liao, L.; Xiong, B.; Wang, X.; et al. Optimization of a static headspace GC-MS method and its application in metabolic fingerprinting of the leaf volatiles of 42 citrus cultivars. Front. Plant Sci. 2022, 13, 1050289. [Google Scholar] [CrossRef]
- Cox, A.O.; Daw, R.C.; Mason, M.D.; Grabenauer, M.; Pande, P.G.; Davis, K.H.; Wiley, J.L.; Stout, P.R.; Thomas, B.F.; Huffman, J.W. Use of SPME-HS-GC–MS for the analysis of herbal products containing synthetic cannabinoids. J. Anal. Toxicol. 2012, 36, 293–302. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. AK-1Guidelines for Dietary Supplements and Botanicals. In Official Methods of Analysis of AOAC International; Latimer, G.W., Jr., Ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Ilias, Y.; Rudaz, S.; Mathieu, P.; Christen, P.; Veuthey, J.L. Extraction and analysis of different Cannabis samples by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. J. Sep. Sci. 2005, 28, 2293–2300. [Google Scholar] [CrossRef]
- Czégény, Z.; Nagy, G.; Babinszki, B.; Bajtel, Á.; Sebestyén, Z.; Kiss, T.; Csupor-Löffler, B.; Tóth, B.; Csupor, D. CBD, a precursor of THC in e-cigarettes. Sci. Rep. 2021, 11, 8951. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.H.; Khan, I.A.; Chandra, S.; Lata, H.; et al. Analysis of terpenes in Cannabis sativa L. using GC/MS: Method development, validation, and application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef]
- Hatton, C.K. Beyond sports-doping headlines: The science of laboratory tests for performance-enhancing drugs. Pediatr. Clin. N. Am. 2007, 54, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High added-value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Maggi, F. Valorization of CBD-hemp through distillation to provide essential oil and improved cannabinoids profile. Sci. Rep. 2021, 11, 19890. [Google Scholar] [CrossRef]
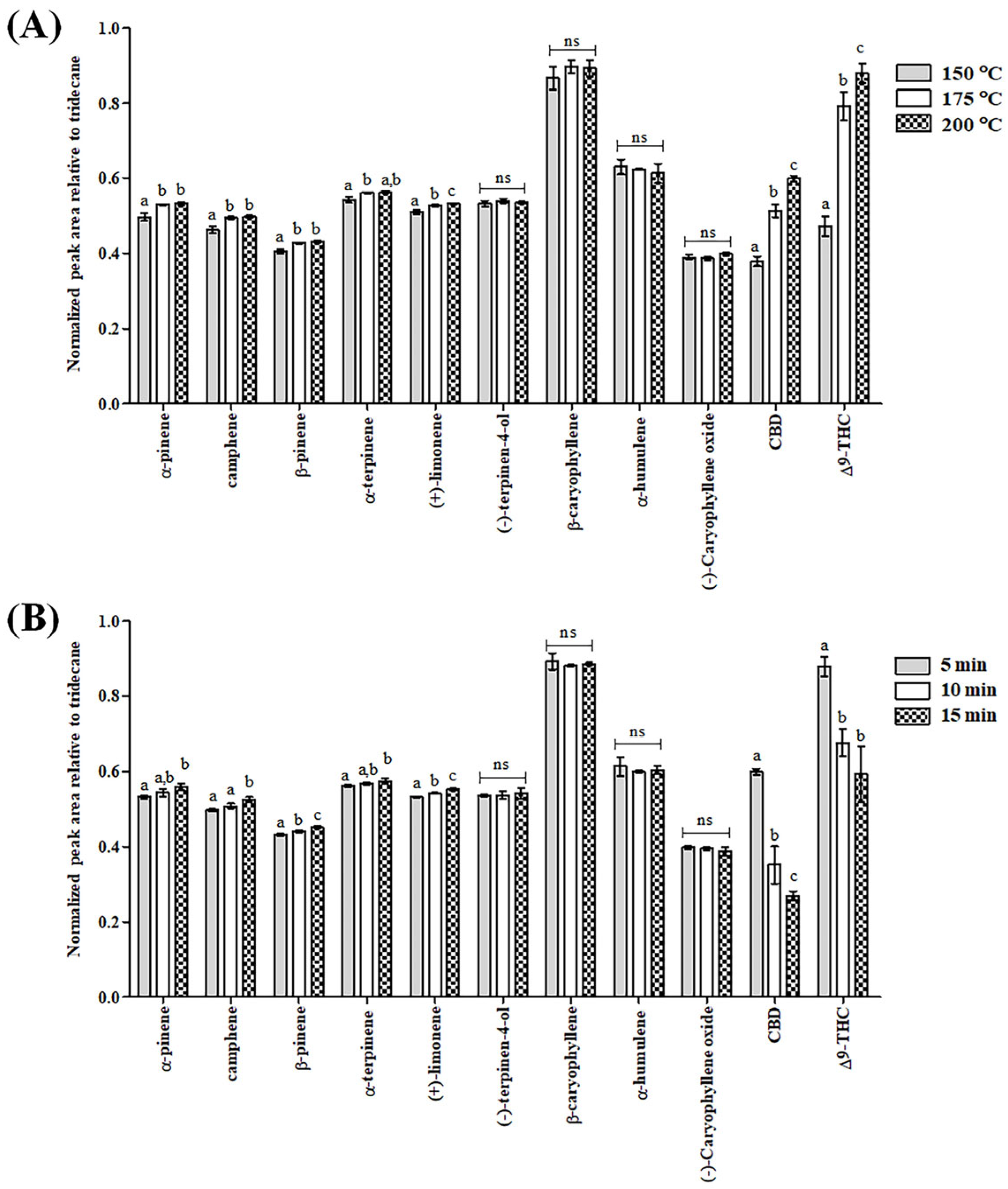
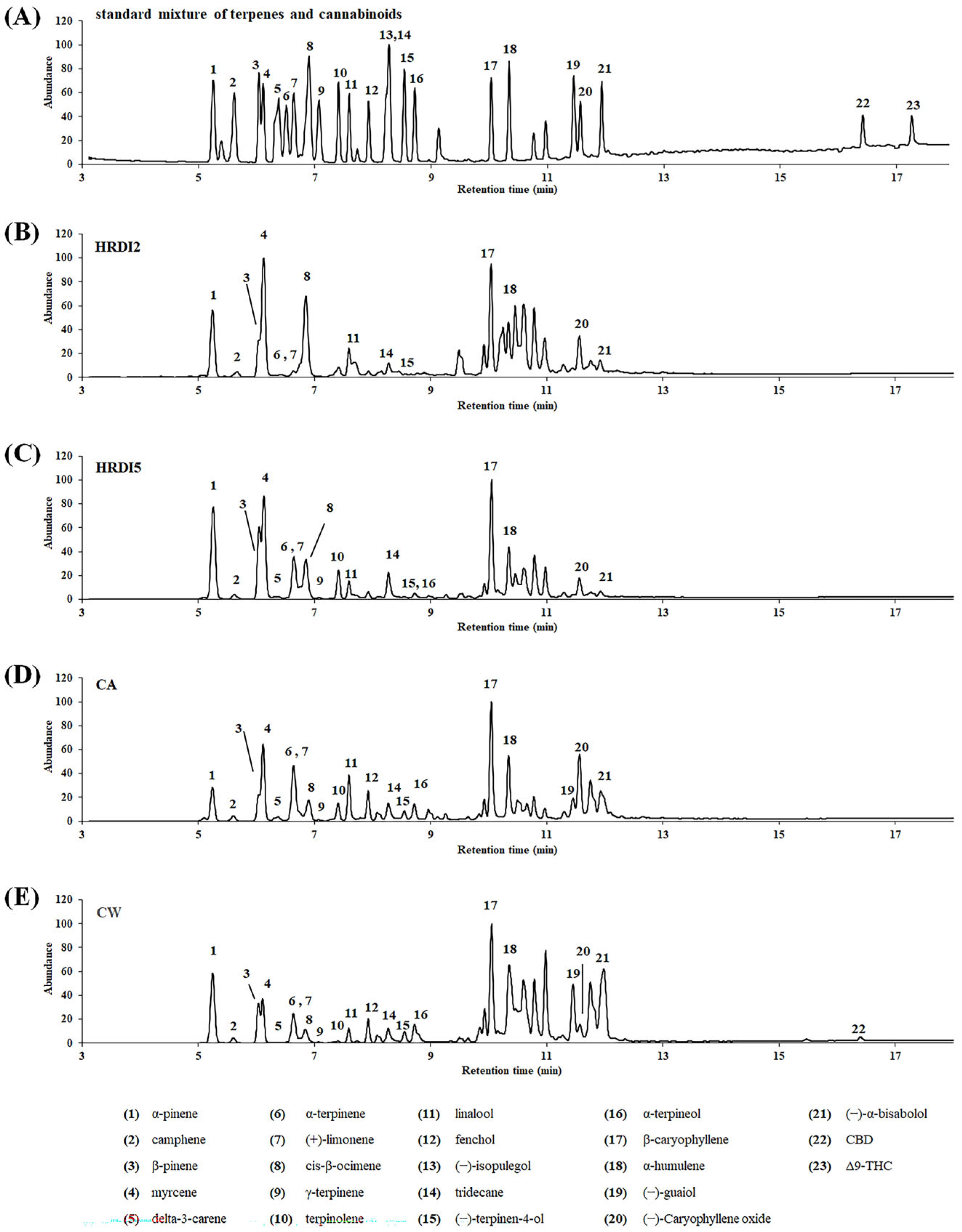
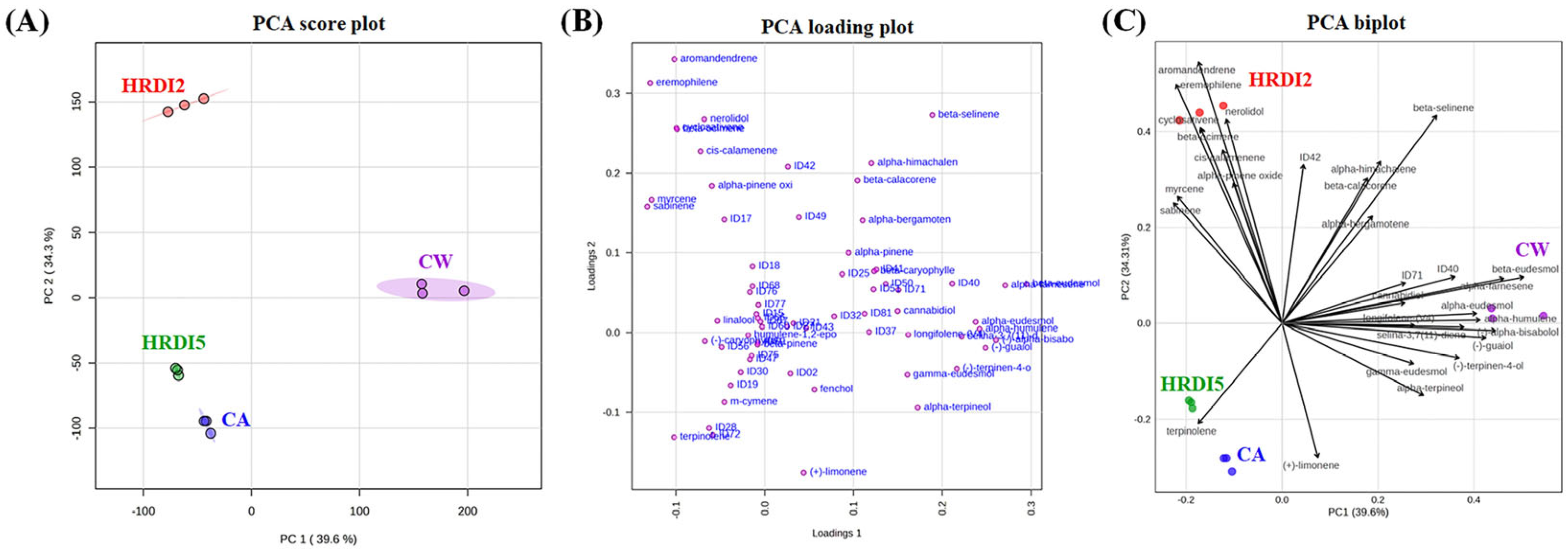
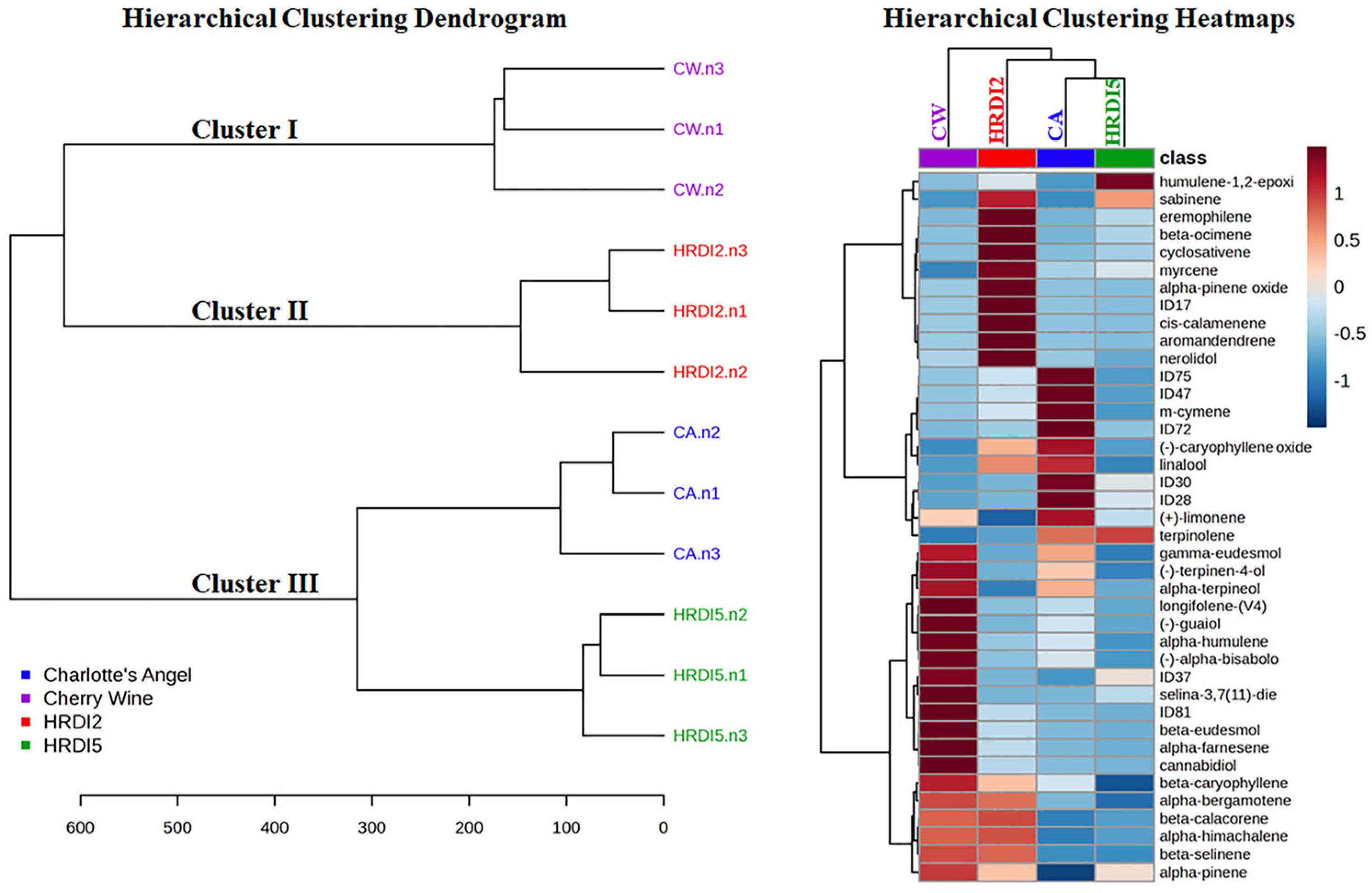
| Compound | Time Window (min) | Formula | Exact Masses | Quantification Ion (m/z) | Qualifier Ions (m/z) |
|---|---|---|---|---|---|
| α-pinene | 3.0–5.8 | C10H16 | 136.13 | 93 | 43, 91, 93, 121 |
| camphene | 3.0–5.8 | C10H16 | 136.13 | 93 | 43, 91, 93, 121 |
| β-pinene | 5.8–6.3 | C10H16 | 136.13 | 93 | 69, 91, 92, 93 |
| myrcene | 5.8–6.3 | C10H16 | 136.13 | 69 | 69, 91, 92, 93 |
| delta-3-carene | 6.3–7.3 | C10H16 | 136.13 | 93 | 67, 91, 93, 121 |
| α-terpinene | 6.3–7.3 | C10H16 | 136.13 | 121 | 67, 91, 93, 121 |
| (+)-limonene | 6.3–7.3 | C10H16 | 136.13 | 67 | 67, 91, 93, 121 |
| cis-β-ocimene | 6.3–7.3 | C10H16 | 136.13 | 91 | 67, 91, 93, 121 |
| γ-terpinene | 6.3–7.3 | C10H16 | 136.13 | 93 | 67, 91, 93, 121 |
| terpinolene | 7.3–7.8 | C10H16 | 136.13 | 121 | 71, 93, 121, 136 |
| Linalool | 7.3–7.8 | C10H18O | 154.14 | 93 | 71, 93, 121, 136 |
| Fenchol | 7.8–8.1 | C10H18O | 154.14 | 93 | 59, 93, 121 |
| (−)-isopulegol | 8.1–8.4 | C10H18O | 154.14 | 121 | 43, 57, 71, 121 |
| tridecane | 8.1–8.4 | C13H28 | 184.22 | 57 | 43, 57, 71, 121 |
| (−)-terpinen-4-ol | 8.4–9.0 | C10H18O | 154.14 | 111 | 59, 93, 111, 121 |
| α-terpineol | 8.4–9.0 | C10H18O | 154.14 | 93 | 59, 93, 111, 121 |
| β-caryophyllene | 9.5–10.2 | C15H24 | 204.19 | 133 | 133, 161, 189 |
| α-humulene | 10.2–10.5 | C15H24 | 204.19 | 93 | 80, 93, 121, 147 |
| (−)-guaiol | 11.2–11.8 | C15H26O | 222.20 | 161 | 59, 79, 121, 161 |
| (−)-caryophyllene oxide | 11.2–11.8 | C15H24O | 220.18 | 79 | 59, 79, 121, 161 |
| (−)-α-bisabolol | 11.8–12.4 | C15H26O | 222.20 | 69 | 69, 105, 119 |
| CBD | 15.0–23.0 | C21H30O2 | 314.22 | 231 | 231, 299, 310, 314 |
| Δ9-THC | 15.0–23.0 | C21H30O2 | 314.22 | 299 | 231, 299, 310, 314 |
| Compound | LOD (µg/mL) | LOQ (µg/mL) | RT (min) | r2 | Regression Equation | %RSD | Average % Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intraday (n = 3) | Interday (n = 9) | Spiked (µg/mL) | ||||||||||||
| Standard (µg/mL) | Standard (µg/mL) | |||||||||||||
| 0.8 | 8 | 20 | 0.8 | 8 | 20 | 0.8 | 8 | 20 | ||||||
| α-pinene | 0.025 | 0.1 | 5.20 | 0.998 | y = 0.0109x − 0.0003 | 2.0 | 1.6 | 5.2 | 8.4 | 13.6 | 5.9 | 96.5 | 88.7 | 88.8 |
| camphene | 0.025 | 0.1 | 5.60 | 0.9982 | y = 0.0071x − 0.0003 | 2.7 | 1.5 | 4.9 | 5.1 | 11.2 | 9.6 | 95.3 | 103.8 | 96.6 |
| β-pinene | 0.025 | 0.1 | 6.00 | 0.9981 | y = 0.0105x − 0.001 | 3.2 | 6.0 | 1.3 | 6.8 | 12.4 | 4.5 | 87.5 | 105.9 | 101.5 |
| myrcene | 0.1 | 0.5 | 6.11 | 0.9977 | y = 0.005x − 0.0017 | 2.2 | 7.6 | 1.6 | 7.3 | 13.1 | 6.7 | 86.8 | 106.7 | 87.3 |
| delta-3-carene | 0.025 | 0.1 | 6.30 | 0.9993 | y = 0.0099x − 0.0023 | 10.9 | 4.5 | 0.8 | 11.6 | 12.6 | 9.7 | 104.8 | 111.7 | 111.4 |
| α-terpinene | 0.1 | 0.5 | 6.51 | 0.9977 | y = 0.0027x − 0.0002 | 5.2 | 6.1 | 3.5 | 12.4 | 10.3 | 6.2 | 88.7 | 101.6 | 98.4 |
| (+)-limonene | 0.1 | 0.5 | 6.60 | 0.9971 | y = 0.004x − 0.0004 | 3.4 | 5.3 | 0.9 | 13.7 | 6.4 | 5.1 | 97.8 | 90.4 | 100.6 |
| cis-β-ocimene | 0.1 | 0.5 | 6.84 | 0.997 | y = 0.009x − 0.0015 | 5.4 | 4.7 | 1.2 | 12.6 | 12.1 | 10.1 | 92.4 | 96.0 | 101.0 |
| γ-terpinene | 0.1 | 0.5 | 7.07 | 0.9968 | y = 0.0043x − 0.0005 | 7.2 | 5.6 | 2.4 | 10.9 | 5.4 | 3.5 | 103.4 | 111.8 | 95.1 |
| terpinolene | 0.1 | 0.5 | 7.40 | 0.9964 | y = 0.0033x − 0.0004 | 3.0 | 5.0 | 0.3 | 8.0 | 4.7 | 3.3 | 87.8 | 90.0 | 97.6 |
| linalool | 0.1 | 0.5 | 7.60 | 0.9988 | y = 0.0034x − 0.0015 | 4.2 | 5.0 | 5.5 | 9.5 | 10.2 | 4.3 | 85.1 | 103.5 | 95.9 |
| fenchol | 0.1 | 0.5 | 7.90 | 0.9963 | y = 0.001x − 0.0002 | 8.9 | 1.2 | 9.4 | 8.7 | 13.9 | 7.4 | 90.4 | 106.2 | 100.4 |
| (−)-terpinen-4-ol | 0.1 | 0.5 | 8.50 | 0.9984 | y = 0.0041x − 0.0017 | 2.0 | 3.5 | 4.1 | 7.8 | 7.8 | 5.6 | 86.3 | 96.7 | 88.0 |
| α-humulene | 0.025 | 0.1 | 10.34 | 0.9985 | y = 0.0096x − 0.001 | 2.5 | 4.6 | 5.3 | 8.8 | 8.8 | 5.5 | 100.5 | 102.0 | 93.4 |
| (−)-guaiol | 0.1 | 0.5 | 11.45 | 0.9948 | y = 0.0048x − 0.0015 | 0.8 | 11.0 | 7.8 | 8.3 | 8.6 | 5.2 | 95.2 | 87.6 | 90.3 |
| (−)-caryophyllene oxide | 0.1 | 0.5 | 11.56 | 0.995 | y = 0.0022x − 0.0004 | 2.3 | 6.8 | 1.1 | 6.5 | 8.3 | 7.7 | 101.1 | 94.7 | 92.5 |
| (−)-α-bisabolol | 0.1 | 0.5 | 11.93 | 0.9941 | y = 0.0029x − 0.0013 | 0.8 | 5.6 | 6.5 | 11.8 | 12.6 | 4.0 | 93.1 | 115.5 | 96.9 |
| 8 | 20 | 40 | 8 | 20 | 40 | 8 | 20 | 40 | ||||||
| (−)-isopulegol | 0.5 | 1 | 8.23 | 0.9972 | y = 0.0014x − 0.0013 | 7.8 | 6.4 | 2.5 | 7.7 | 8.7 | 3.1 | 98.3 | 94.2 | 104.1 |
| α-terpineol | 0.5 | 1 | 8.71 | 0.9946 | y = 0.0041x − 0.0015 | 3.8 | 5.2 | 3.6 | 4.3 | 3.7 | 3.8 | 86.9 | 99.1 | 96.0 |
| β-caryophyllene | 0.5 | 1 | 10.03 | 0.9985 | y = 0.0025x + 0.0022 | 3.6 | 5.9 | 0.6 | 9.8 | 7.0 | 4.7 | 104.3 | 97.4 | 93.8 |
| CBD | 1 | 5 | 16.40 | 0.9939 | y = 0.003x − 0.0143 | 8.7 | 2.7 | 3.8 | 10.2 | 5.3 | 3.6 | 106.2 | 94.7 | 87.7 |
| Δ9-THC | 1 | 5 | 17.26 | 0.9975 | y = 0.0006x − 0.0013 | 2.5 | 7.0 | 3.1 | 4.3 | 13.4 | 8.0 | 100.0 | 89.2 | 113.3 |
| Compound | Average %w/w ± SD, n = 3 | |||
|---|---|---|---|---|
| HRDI2 | HRDI5 | CA | CW | |
| α-pinene | 2.35 ± 0.78 | 1.65 ± 0.04 | 0.99 ± 0.20 | 5.08 ± 0.98 |
| camphene | 0.05 ± 0.00 | 0.12 ± 0.02 | 0.23 ± 0.00 | 0.24 ± 0.00 |
| β-pinene | 0.92 ± 0.37 | 0.63 ± 0.01 | 0.53 ± 0.10 | 1.35 ± 0.31 |
| myrcene | 15.35 ± 1.80 | 2.84 ± 0.04 | 7.00 ± 1.54 | 2.38 ± 0.54 |
| delta-3-carene | ND | 0.10 ± 0.00 | 0.15 ± 0.02 | 0.03 ± 0.00 |
| α-terpinene | 0.01 ± 0.00 | 0.08 ± 0.00 | 0.13 ± 0.02 | 0.14 ± 0.02 |
| (+)-limonene | 0.15 ± 0.04 | 0.38 ± 0.01 | 2.71 ± 0.59 | 1.18 ± 0.28 |
| cis-β-ocimene | 2.65 ± 0.96 | 0.10 ± 0.01 | 0.21 ± 0.04 | 0.14 ± 0.03 |
| γ-terpinene | ND | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.02 |
| terpinolene | ND | 0.33 ± 0.01 | 0.71 ± 0.15 | 0.11 ± 0.02 |
| linalool | 0.43 ± 0.10 | 0.16 ± 0.01 | 1.57 ± 0.30 | 0.42 ± 0.08 |
| fenchol | ND | ND | 0.62 ± 0.10 | 0.24 ± 0.07 |
| (−)-isopulegol | ND | ND | ND | ND |
| (−)-terpinen-4-ol | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.14 ± 0.01 | 0.11 ± 0.01 |
| α-terpineol | ND | 0.07 ± 0.00 | 0.37 ± 0.06 | 0.50 ± 0.11 |
| β-caryophyllene | 4.07 ± 1.19 | 1.03 ± 0.07 | 7.69 ± 1.55 | 11.34 ± 2.78 |
| α-humulene | 1.24 ± 0.56 | 0.32 ± 0.04 | 2.13 ± 0.45 | 3.43 ± 0.77 |
| (−)-guaiol | ND | ND | 0.55 ± 0.04 | 2.28 ± 0.53 |
| (−)-caryophyllene oxide | 0.67 ± 0.23 | 0.11 ± 0.03 | 2.02 ± 0.50 | 1.12 ± 0.22 |
| (−)-α-bisabolol | 0.38 ± 0.06 | 0.19 ± 0.00 | 0.71 ± 0.13 | 1.66 ± 0.39 |
| CBD | BQL | 0.01 ± 0.00 | ND | 0.75 ± 0.14 |
| Δ9-THC | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwangrach, N.; Mukda, S.; Temkitthawon, P.; Nuengchamnong, N.; Pinmanee, S.; Somboon, T.; Boonnoun, P.; Ingkaninan, K. Simultaneous Profiling of Terpenes and Cannabinoids in Hemp Essential Oils Using Static Headspace Gas Chromatography–Mass Spectrometry for Quality Control and Chemotype Differentiation. Sci. Pharm. 2025, 93, 27. https://doi.org/10.3390/scipharm93020027
Chaiwangrach N, Mukda S, Temkitthawon P, Nuengchamnong N, Pinmanee S, Somboon T, Boonnoun P, Ingkaninan K. Simultaneous Profiling of Terpenes and Cannabinoids in Hemp Essential Oils Using Static Headspace Gas Chromatography–Mass Spectrometry for Quality Control and Chemotype Differentiation. Scientia Pharmaceutica. 2025; 93(2):27. https://doi.org/10.3390/scipharm93020027
Chicago/Turabian StyleChaiwangrach, Nathareen, Sirikan Mukda, Prapapan Temkitthawon, Nitra Nuengchamnong, Sarita Pinmanee, Thapakorn Somboon, Panatpong Boonnoun, and Kornkanok Ingkaninan. 2025. "Simultaneous Profiling of Terpenes and Cannabinoids in Hemp Essential Oils Using Static Headspace Gas Chromatography–Mass Spectrometry for Quality Control and Chemotype Differentiation" Scientia Pharmaceutica 93, no. 2: 27. https://doi.org/10.3390/scipharm93020027
APA StyleChaiwangrach, N., Mukda, S., Temkitthawon, P., Nuengchamnong, N., Pinmanee, S., Somboon, T., Boonnoun, P., & Ingkaninan, K. (2025). Simultaneous Profiling of Terpenes and Cannabinoids in Hemp Essential Oils Using Static Headspace Gas Chromatography–Mass Spectrometry for Quality Control and Chemotype Differentiation. Scientia Pharmaceutica, 93(2), 27. https://doi.org/10.3390/scipharm93020027






