Development and Characterization of Bigels for the Topical Delivery of Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bigel Formulation
2.3. Rheological Characterization
2.3.1. Small Amplitude Oscillatory Shear Flow (SAOS) Methodology
2.3.2. Application of the Principle of Time-Temperature Superposition (TTS)
2.4. Microscopy Methodology
2.5. Determination of the pH Value Methodology
2.6. Curcumin Content
Differential Scanning Calorimetry Methodology
2.7. Curcumin Release Mechanisms
2.8. In Vitro Permeation Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Bigels Obtention
3.2. Formulation of the Curcumin-Loaded Bigels
3.3. Small Amplitude Oscillatory Shear Flow (SAOS)
3.4. Master Curves Arising from Time-Temperature Superposition (TTS)
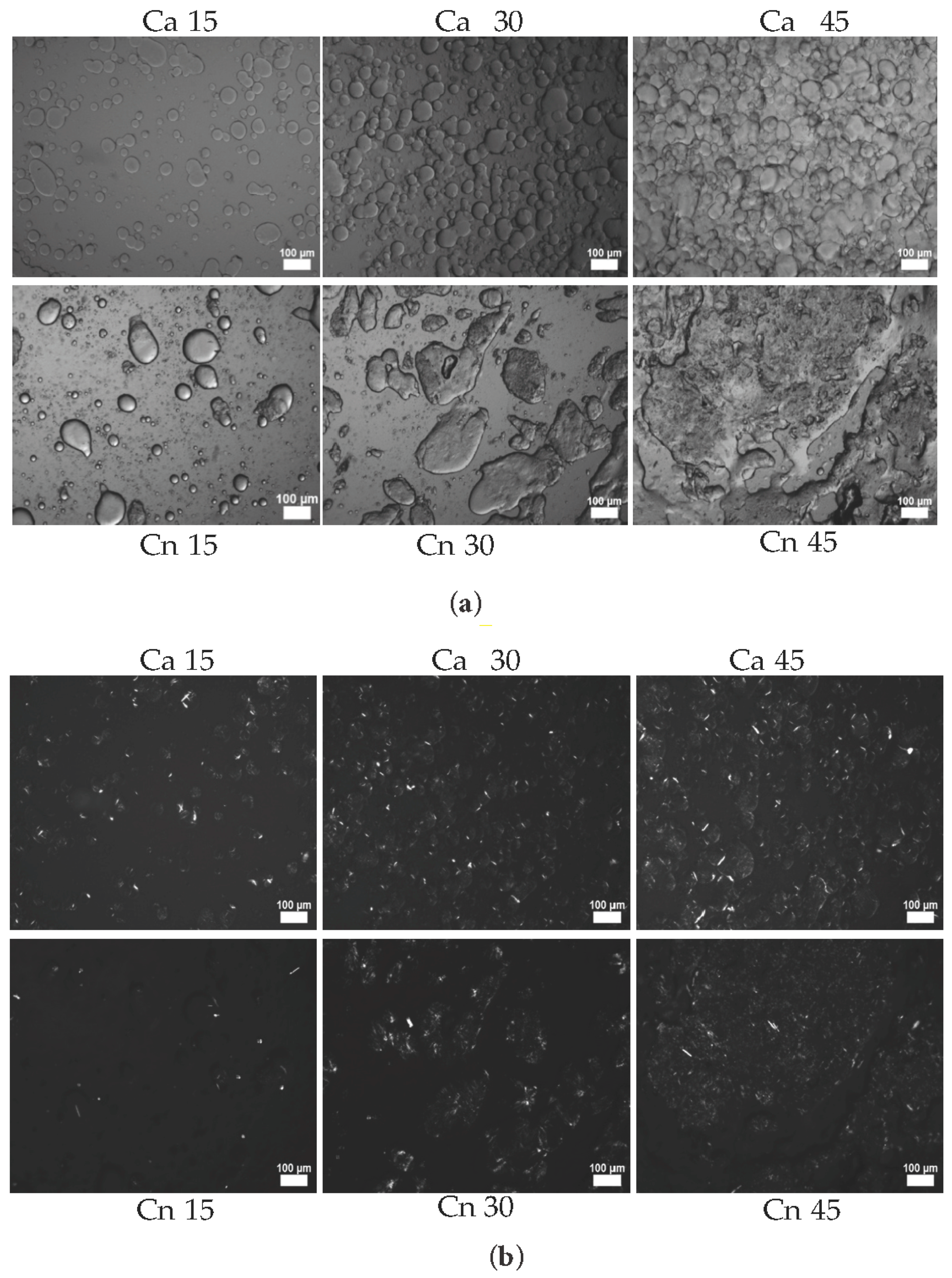
3.5. Microscopy
3.6. Determination of the pH Value
3.7. Determination of the Curcumin Content
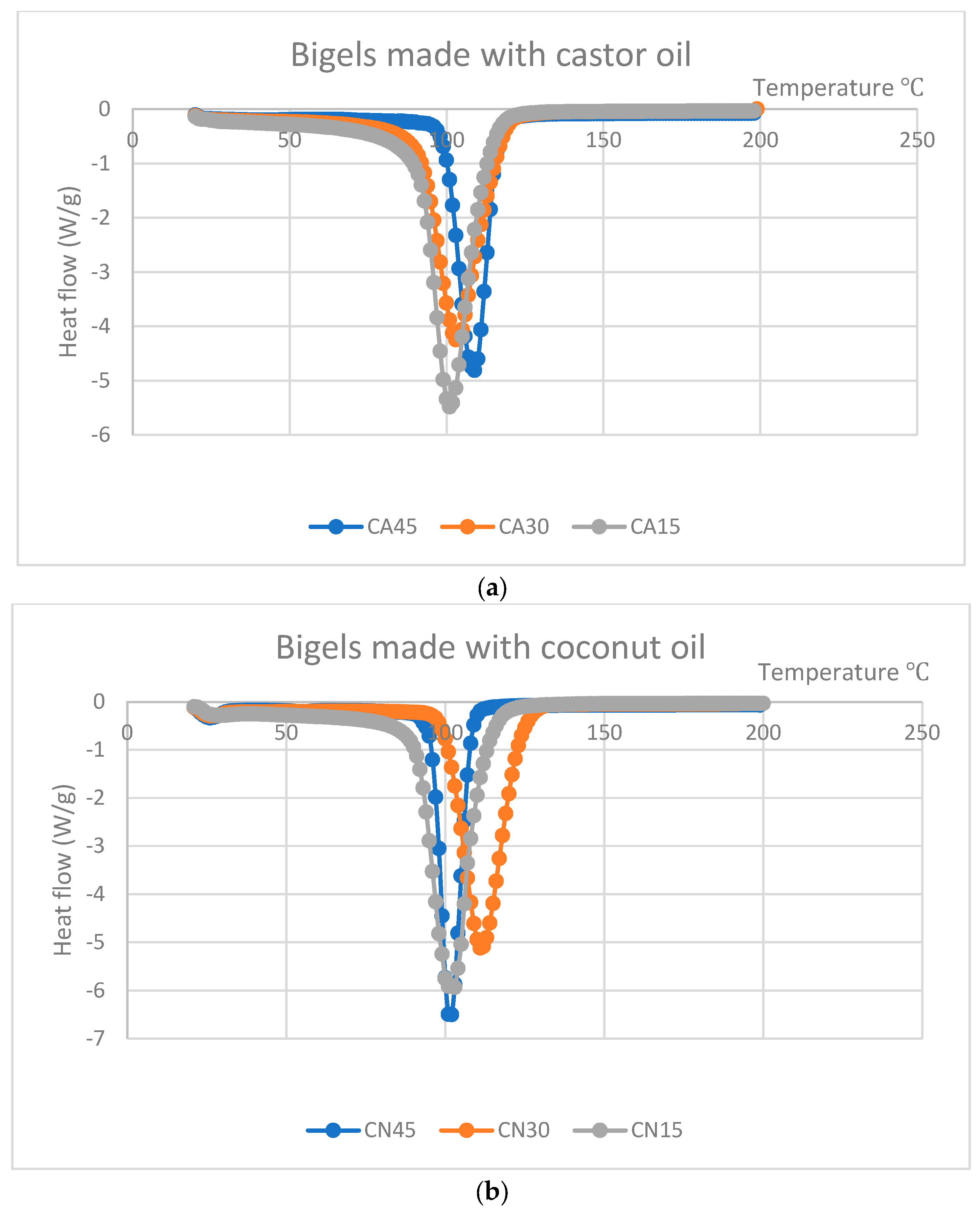
3.8. Differential Scanning Calorimetry
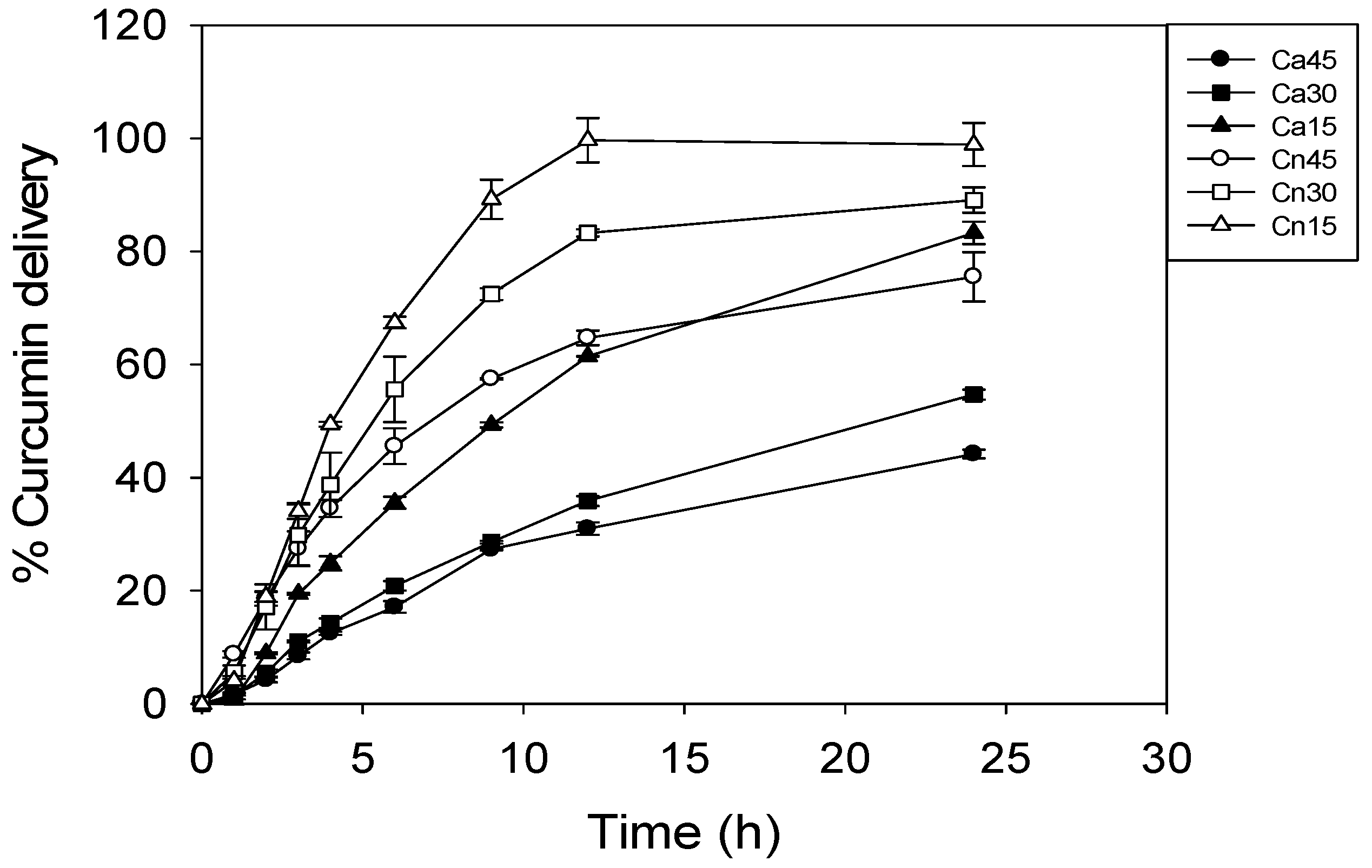
3.9. Mechanisms of Curcumin Release
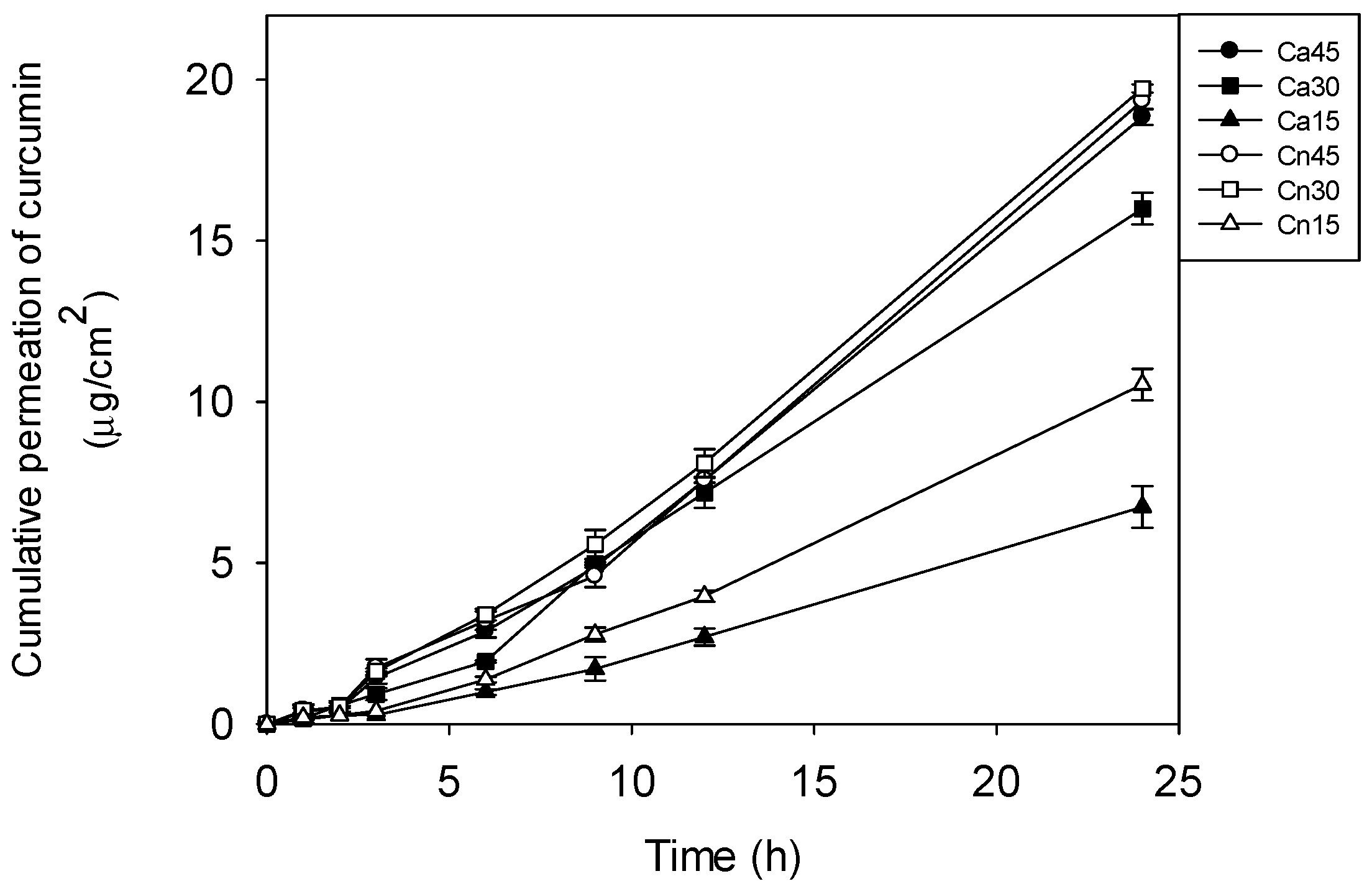
3.10. Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, K.Y.; Ho, C.T.; Pan, M.H. The therapeutic potential of curcumin and its related substances in turmeric: From raw material selection to application strategies. J. Food Drug Anal. 2023, 31, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin formulations for better bioavailability: What we learned from clinical trials thus far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to improve the solubility of curcumin from turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsionand emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Pandey, K.U.; Dalvi, S.V. Understanding stability relationships among three curcumin polymorphs. Adv. Powder Technol. 2019, 30, 266–276. [Google Scholar] [CrossRef]
- Alves Barroso, L.; Grossi Bovi Karatay, G.; Dupas Hubinger, M. Effect of Potato Starch Hydrogel:Glycerol Monostearate Oleogel Ratio on the Physico-Rheological Properties of Bigels. Gels 2022, 8, 694. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef]
- Martins, E.; Reis, R.L.; Silva, T.H. In Vivo Skin Hydrating Efficacy of Fish Collagen from Greenland Halibut as a High-Value Active Ingredient for Cosmetic Applications. Mar. Drugs 2023, 21, 57. [Google Scholar] [CrossRef]
- Chao, E.; Li, J.; Duan, Z.; Fan, L. Bigels as emerging biphasic systems: Properties, applications, and prospects in the food industry. Food Hydrocoll. 2024, 154, 110089. [Google Scholar] [CrossRef]
- Almeida, I.F.; Fernandes, A.R.; Fernandes, L.; Pena Ferreira, M.R.; Costa, P.C.; Bahia, M.F. Moisturizing Effect of Oleogel/Hydrogel Mixtures. Pharm. Dev. Technol. 2008, 13, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Qureshi, D.; Nayak, S.K.; Pal, K. Bigels. In Polymeric Gels; Woodhead Publishing: Sawston, UK, 2018; pp. 265–282. [Google Scholar]
- Yang, J.; Zheng, H.; Mo, Y.; Gao, Y.; Mao, L. Structural characterization of hydrogel-oleogel biphasic systems as affected by oleogelators. Food Res. Intl. 2022, 158, 111536. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Corradini, M.G.; Joye, I.J. Bigels as delivery systems: Potential uses and applicability in food. Gels 2023, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Dangi, M.; Setya, S.; Talegaonkar, S. Bigels: A comprehensive review of properties, preparation techniques, applications in pharmaceuticals, food, cosmetic products, and emerging patents. J. Dispers. Sci. Technol. 2025, 1–31. [Google Scholar] [CrossRef]
- Pérez-Salas, J.L.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Rosas-Flores, W.; González-Laredo, R.F.; Medina-Torres, L.; Gallegos-Infante, J.A. Effect of storage time on the mechanical and microstructural properties of bigels based on xanthan gum and castor oil. J. Am. Oil Chem. Soc. 2023, 100, 699–709. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Wróblewska, M.; Winnicka, K.; Wieczorek, P.; Majewski, P.; Celińska-Janowicz, K.; Tomczyk, M. Novel gel formulations as topical carriers for the essential oil of Bidens tripartita for the treatment of candidiasis. Molecules 2018, 23, 2517. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Silva, S.S.; Reis, R.L. Challenges and opportunities on vegetable oils derived systems for biomedical applications. Biomater. Adv. 2022, 134, 112720. [Google Scholar] [CrossRef]
- Machado, M.; Costa, E.M.; Silva, S.; Sousa, S.C.; Gomes, A.M.; Pintado, M. Enhancing medium-chain fatty acid delivery through bigel technology. Gels 2024, 10, 738. [Google Scholar] [CrossRef]
- Pénzes, T.; Csóka, I.; Erős, I. Rheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogels. Rheol. Acta 2004, 43, 457–463. [Google Scholar] [CrossRef]
- Kumar, M.; Shanthi, N.; Mahato, A.K.; Soni, S.; Rajnikanth, P.S. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon 2019, 5, e01688. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and multi-component organogels: An overview from rheological perspective. Food Hydrocoll. 2021, 111, 111–119. [Google Scholar] [CrossRef]
- Lupi, F.R.; Shakeel, A.; Greco, V.; Rossi, C.O.; Baldino, N.; Gabriele, D. A rheological and microstructural characterisation of bigels for cosmetic and pharmaceutical uses. Mat. Sci. Eng. C 2016, 69, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Jinugu, M.E.; Thareja, P. Rheology and Extrusion Printing of κ-Carrageenan/Olive Oil Emulsion Gel Tablets with Varying Surface Area to Volume Ratios for Release of Vitamin C and Curcumin. Langmuir 2024, 40, 16069–16084. [Google Scholar] [CrossRef]
- Lupi, F.R.; Greco, V.; Baldino, N.; de Cindio, B.; Fischer, P.; Gabriele, D. The effects of intermolecular interactions on the physical properties of organogels in edible oils. J. Colloid Interface Sci. 2016, 483, 154–164. [Google Scholar] [CrossRef]
- Mata-Mota, J.D.; Gallegos-Infante, J.A.; Pérez-Martínez, J.D.; Rocha-Guzmán, N.E.; González-Laredo, R.F. Effect of hydrogel/oleogel ratio, speed and time of mixing, on the mechanical properties of bigel materials and the application of Cox-Merz rule. Food Mater. Res. 2023, 3, 24. [Google Scholar] [CrossRef]
- Nakano, T. Applicability condition of time–temperature superposition principle (TTSP) to a multi-phase system. Mech. Time Depend. Mater. 2013, 17, 439–447. [Google Scholar] [CrossRef]
- Ahmed, J. Time–temperature superposition principle and its application to biopolymer and food rheology. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 209–241. [Google Scholar]
- Nickerson, M.T.; Paulson, A.T.; Speers, R.A. Time–temperature studies of gellan polysaccharide gelation in the presence of low, intermediate and high levels of co-solutes. Food Hydrocoll. 2004, 18, 783–794. [Google Scholar] [CrossRef]
- Susan-Resiga, D. Application of the time-temperature superposition principle to concentrated magnetic nanofluids. Rom. Rep. Phys. 2015, 67, 890–914. [Google Scholar]
- Nickerson, M.T.; Paulson, A.T.; Speers, R.A. A time–temperature rheological approach for examining food polymer gelation. Trends Food Sci. Technol. 2004, 15, 569–574. [Google Scholar] [CrossRef]
- Pranali, S.; Charushila, S.; Sayali, C.; Namrata, M. Design and Characterisation of Emulgel of an Antifungal drug. J. Pharm. Sci. Res. 2019, 11, 2357–2361. [Google Scholar]
- Kim, Y.J.; Kim, B.K.; Lee, M.H. Improving curcumin retention in oil-in-water emulsions coated by chitosan and their disperse stability exposed to thermal treatments. J. Food Eng. 2022, 319, 110918. [Google Scholar] [CrossRef]
- Scomoroscenco, C.; Teodorescu, M.; Nistor, C.L.; Gifu, I.C.; Petcu, C.; Banciu, D.D.; Banciu, A.; Cinteza, L.O. Preparation and In Vitro Characterization of Alkyl Polyglucoside-Based Microemulsion for Topical Administration of Curcumin. Pharmaceutics 2023, 15, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Serna, I.E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Cháirez-Ramírez, M.H.; Rosas-Flores, W.; Pérez-Martínez, J.D.; González-Laredo, R.F. Water-in-oil organogel based emulsions as a tool for increasing bioaccessibility and cell permeability of poorly water-soluble nutraceuticals. Food Res. Int. 2019, 120, 415–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Vershkov, B.; Davidovich-Pinhas, M. The effect of preparation temperature and composition on bigel performance as fat replacers. Food Funct. 2023, 14, 3838–3848. [Google Scholar] [CrossRef]
- Dabbaghi, M.; Namjoshi, S.; Panchal, B.; Grice, J.E.; Prakash, S.; Roberts, M.S.; Mohammed, Y. Viscoelastic and deformation characteristics of structurally different commercial topical systems. Pharmaceutics 2021, 13, 1351–1359. [Google Scholar] [CrossRef]
- Hamed, R.; AbuRezeq, A.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Bigels as delivery systems of bioactive fatty acids present in functional edible oils: Coconut, avocado, and pomegranate. Gels 2023, 9, 349. [Google Scholar] [CrossRef]
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid gels: Influence of oleogel/hydrogel ratio on rheological and textural properties. Food Res. Int. 2019, 116, 1298–1305. [Google Scholar] [CrossRef]
- Martins, A.J.; Guimarães, A.; Fuciños, P.; Sousa, P.; Venâncio, A.; Pastrana, L.M.; Cerqueira, M.A. Food-grade bigels: Evaluation of hydrogel: Oleogel ratio and gelator concentration on their physicochemical properties. Food Hydrocoll. 2023, 143, 108893. [Google Scholar] [CrossRef]
- Zampouni, K.; Sideris, N.; Tsavdaris, E.; Katsanidis, E. On the structural and mechanical properties of mixed coconut and olive oil oleogels and bigels. Int. J. Biol. Macromol. 2024, 268, 131942. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Thickening properties of several NCO-functionalized cellulose derivatives in castor oil. Chem. Eng. Sci. 2015, 134, 260–268. [Google Scholar] [CrossRef]
- Habibi, A.; Kasapis, S.; Truong, T. Effect of hydrogel particle size embedded into oleogels on the physico-functional properties of hydrogel-in-oleogel (bigels). LWT 2022, 163, 113501. [Google Scholar] [CrossRef]
- Capodagli, J.; Lakes, R. Isothermal viscoelastic properties of PMMA and LDPE over 11 decades of frequency and time: A test of time–temperature superposition. Rheol. Acta 2008, 47, 777–786. [Google Scholar] [CrossRef]
- Meneses, A.; Naya, S.; Francisco-Fernández, M.; López-Beceiro, J.; Gracia-Fernández, C.; Tarrío-Saavedra, J. TTS package: Computational tools for the application of the Time Temperature Superposition principle. Heliyon 2023, 9, e15816. [Google Scholar] [CrossRef]
- Tian, W.; Huang, Y.; Song, Z.; Yu, Y.; Liu, J.; Cao, Y.; Xiao, J. Flexible control of bigel microstructure for enhanced stability and flavor release during oral consumption. Food Res. Int. 2023, 174, 113606. [Google Scholar] [CrossRef]
- Agarwal, M.; Sachdeva, M.; Sharma, P.K. Development and Characterization of novel bigel of loxoprofen for topical drug delivery. J. Drug Deliv. Ther. 2022, 12, 46–54. [Google Scholar] [CrossRef]
- Raytthatha, N.E.N.S.I.; Vyas, J. Development of bigels containing antifungal agent for vaginal infection. Int. J. Pharm. Pharm. Sci. 2022, 14, 38–42. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Li, G.; Du, D. Rheological properties of fish skin collagen solution: Effects of temperature and concentration. Korea-Aust. Rheo J. 2010, 22, 119–127. [Google Scholar]
- Evageliou, V.; Kasapis, S.; Hember, M.W. Vitrification of κ-carrageenan in the presence of high levels of glucose syrup. Polymer 1998, 39, 3909–3917. [Google Scholar] [CrossRef]
- Ferry, J.D. Dependence of viscoelastic behavior on temperature and pressure (Ch. 11). In Viscoelastic Properties of Polymers, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1980; p. 225. [Google Scholar]
- Kasapis, S.; Sworn, G. Separation of the variables of time and temperature in the mechanical properties of high sugar/polysaccharide mixtures. Biopolym. Orig. Res. Biomol. 2000, 53, 40–45. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; de Cindio, B. Olive oil and hyperthermal water bigels for cosmetic uses. J. Colloid Interface Sci. 2015, 459, 70–78. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Wang, Y.; Rogers, M.A.; Cao, Y.; Lan, Y. Microstructure and physical properties of novel bigel-based foamed emulsions. Food Hydrocoll. 2023, 134, 108097. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm.-Venereol. 2013, 93, 234–242. [Google Scholar] [CrossRef]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in healthy skin and in atopic eczema. BioMed Res. Int. 2014, 1, 436921. [Google Scholar] [CrossRef]
- Farmacopea de los Estados Unidos Mexicanos 13.0, 2021, Vol I, pp. 1004–1006. Available online: https://farmacopea.org.mx/online (accessed on 19 January 2024).
- Contreras-Ramírez, J.I.; Gallegos-Infante, J.A.; Pérez-Martínez, J.D.; Dibildox-Alvarado, E.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rosas-Flores, W. Influence of vegetable oil, monoglycerides and polyglycerol polyricinoleate into the physical stability of organogel-emulsion (w/o) systems. SN Appl. Sci. 2020, 2, 1343. [Google Scholar] [CrossRef]
- Saleel, C.A. A review on the use of coconut oil as an organic phase change material with its melting process, heat transfer, and energy storage characteristics. J. Therm. Anal. Calorim. 2022, 147, 4451–4472. [Google Scholar] [CrossRef]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Tang, C.; Li, Y. Crystallization behavior and physical properties of monoglycerides-based oleogels as function of oleogelator concentration. Foods 2023, 12, 345. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.G.; Lazaridou, A.; Katsanidis, E. Crystalline microstructure and physicochemical properties of olive oil oleogels formulated with monoglycerides and phytosterols. LWT 2022, 154, 112815. [Google Scholar] [CrossRef]
- Valoppi, F.; Calligaris, S.; Barba, L.; Šegatin, N.; Poklar Ulrih, N.; Nicoli, M.C. Influence of oil type on formation, structure, thermal, and physical properties of monoglyceride-based organogel. Eur. J. Lipid Sci. Technol. 2017, 119, 150–159. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Zhou, H.; Vicente, A.A.; Pinheiro, A.C.; McClements, D.J. Plant-based bigels for delivery of bioactive compounds: Influence of hydrogel: Oleogel ratio and protein concentration on their physicochemical properties. Food Hydrocoll. 2024, 150, 109721. [Google Scholar] [CrossRef]
- Calligaris, S.; Valoppi, F.; Barba, L.; Pizzale, L.; Anese, M.; Conte, L.; Nicoli, M.C. Development of transparent curcumin loaded microemulsions by phase inversion temperature (PIT) method: Effect of lipid type and physical state on curcumin stability. Food Biophys. 2017, 12, 45–51. [Google Scholar] [CrossRef]
- Jahromi, L.P.; Ghazali, M.; Ashrafi, H.; Azadi, A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; Sánchez-Burgos, J.A.; Gomes, C.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Bernad-Bernad, M.J.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E. Morphological and release characterization of nanoparticles formulated with poly (dl-lactide-co-glycolide)(PLGA) and lupeol: In vitro permeability and modulator effect on NF-κB in Caco-2 cell system stimulated with TNF-α. Food Chem. Toxicol. 2015, 85, 2–9. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical Modelling of Drug Release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Viljoen, J.M.; Cowley, A.; du Preez, J.; Gerber, M.; du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Vigato, A.A.; Querobino, S.M.; de Faria, N.C.; Candido, A.C.B.B.; Magalhães, L.G.; Cereda, C.M.S.; Tófoli, G.R.; Campos, E.V.R.; Machado, I.P.; Fraceto, L.F.; et al. Physico-Chemical Characterization and Biopharmaceutical Evaluation of Lipid-Poloxamer-Based Organogels for Curcumin Skin Delivery. Front. Pharmacol. 2019, 10, 451560. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Dhal, K.; Patel, P.; Ravi, P.; Kundu, S.; Tappa, K. Current biomedical applications of 3d-printed hydrogels. Gels 2023, 10, 8. [Google Scholar] [CrossRef]



| Codes | |||
|---|---|---|---|
| Materials | CaOG | CnOG | HG |
| Myverol | 6 | 6 | - |
| Castor oil | 94 | - | - |
| Coconut oil | - | 94 | - |
| Xanthan Gum | - | - | 2 |
| Water | - | - | 96.5 |
| Geogard Ultra | 1.5 | ||
| Code | CaOG15 (%) | CaOG30 (%) | CaOG45 (%) | CnOG15 (%) | CnOG30 (%) | CnOG45 (%) | |
|---|---|---|---|---|---|---|---|
| Materials | |||||||
| HG | 85 | 70 | 55 | 85 | 70 | 55 | |
| CaOG | 15 | 30 | 45 | - | - | - | |
| CnOG | - | - | - | 15 | 30 | 45 | |
| Sample | Retardation Activation Energy kcal/mol | Relaxation Activation Energy kcal/mol |
|---|---|---|
| Ca45 | 142.69 | 6.56 |
| Ca30 | 98.73 | 0 |
| Ca15 | 23.10 | 0 |
| Cn45 | 132.01 | 4 |
| Cn30 | 66.85 | 0 |
| Cn15 | 47.51 | 0 |
| Sample | Curcumin Content (%) | pH |
|---|---|---|
| Ca15 | 101.48 ± 4.30 a | 4.85 ± 0.19 abc |
| Ca30 | 98.08 ± 1.93 a | 4.61 ± 0.03 ac |
| Ca45 | 102.09 ± 4.25 a | 4.58 ± 0.08 ac |
| Cn15 | 101.77 ± 2.4 a | 4.64 ± 0.03 ac |
| Cn30 | 96.87 ± 0.54 a | 4.73 ± 0.02 b |
| Cn45 | 102.38 ± 1.64 a | 4.81 ± 0.21 abc |
| Tmax °C | Enthalpy J/g | ||||||
|---|---|---|---|---|---|---|---|
| Peak | 1 | 2 | 3 | 1 | 2 | 3 | |
| Sample | |||||||
| Ca15 | - | 44.98 ± 0.66 a | 106.68 ± 1.41 a | - | 0.17 ± 0.03 a | 1475.50 ± 85.56 c | |
| Ca30 | - | 45.00 ± 0.18 a | 107.68 ± 1.38 a | - | 0.35 ± 0.04 b | 1324.00 ± 82.02 b | |
| Ca45 | - | 45.16 ± 0.28 a | 109.49 ± 3.71 a | - | 0.60 ± 0.03 c | 988.90 ± 76.51 a | |
| Cn15 | 27.27 ± 0.12 a | 54.23 ± 0.35 b | 104.89 ± 2.33 a | 3.43 ± 0.08 a | 0.20 ± 0.02 a | 1654.50 ± 41.72 d | |
| Cn30 | 27.65 ± 0.04 a | 54.37 ± 0.48 b | 109.84 ± 0.97 a | 4.33 ± 0.17 b | 0.28 ± 0.04 b | 1367.50 ± 43.14 b | |
| Cn45 | 27.34 ± 0.21 a | 54.67 ± 0.24 b | 104.95 ± 3.66 a | 9.78 ± 0.05 c | 0.55 ± 0.03 c | 962.55 ± 11.39 a | |
| Zero Order | First Order | Higuchi | Korsmeyer-Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca15 | 4.12 ± 0.04 | 0.83 | 0.07 ± 0.00 | 0.99 | 15.61 ± 0.04 | 0.89 | 9.50 ± 0.53 | 0.70 ± 0.02 | 0.95 |
| Ca30 | 2.57 ± 0.05 | 0.92 | 0.04 ± 0.00 | 0.99 | 9.60 ± 0.22 | 0.89 | 5.02 ± 0.29 | 0.76 ± 0.01 | 0.98 |
| Ca45 | 2.15 ± 0.04 | 0.88 | 0.03 ± 0.00 | 0.96 | 8.11 ± 0.16 | 0.88 | 4.64 ± 0.14 | 0.73 ± 0.01 | 0.96 |
| Cn15 | 9.53 ± 0.26 | 0.87 | 0.19 ± 0.01 | 0.97 | 26.73 ± 0.65 | 0.90 | 17.17 ± 0.53 | 0.73 ± 0.02 | 0.96 |
| Cn30 | 5.08 ± 0.07 | 0.22 | 0.013 ± 0.01 | 0.97 | 20.28 ± 0.61 | 0.87 | 12.23 ± 0.63 | 0.80 ± 0.01 | 0.96 |
| Cn45 | 4.20 ± 0.09 | 0.52 | 0.08 ± 0.00 | 0.98 | 16.88 ± 0.13 | 0.94 | 10.01 ± 1.20 | 0.74 ± 0.04 | 0.98 |
| Bigel | Jss (µg cm−2 h−1) | T Lag (h) | Q24 (µg/cm2) | Qret |
|---|---|---|---|---|
| Ca 15 | 0.32 ± 0.03 a | 3.41 ± 0.06 bc | 6.73 ± 0.64 a | 23.16 ± 0.44 c |
| Ca 30 | 0.75 ± 0.01 c | 2.71 ± 0.11 ab | 15.99 ± 0.49 c | 15.93 ± 1.03 a |
| Ca 45 | 0.85 ± 0.02 d | 2.44 ± 0.22 ab | 18.84 ± 0.25 d | 18.91 ± 0.49 a |
| Cn 15 | 0.52 ± 0.04 b | 3.64 ± 0.47 c | 10.54 ± 0.49 b | 34.79 ± 0.85 d |
| Cn 30 | 0.88 ± 0.01 d | 2.05 ± 0.22 a | 19.72 ± 0.13 d | 20.60 ± 0.71 b |
| Cn 45 | 0.86 ± 0.02 cd | 2.35 ± 0.25 a | 19.34 ± 0.26 d | 20.37 ± 0.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peréz-Salas, J.L.; Moreno-Jiménez, M.R.; Medina-Torres, L.; Rocha-Guzmán, N.E.; Bernad-Bernad, M.J.; González-Laredo, R.F.; Gallegos-Infante, J.A. Development and Characterization of Bigels for the Topical Delivery of Curcumin. Sci. Pharm. 2025, 93, 28. https://doi.org/10.3390/scipharm93030028
Peréz-Salas JL, Moreno-Jiménez MR, Medina-Torres L, Rocha-Guzmán NE, Bernad-Bernad MJ, González-Laredo RF, Gallegos-Infante JA. Development and Characterization of Bigels for the Topical Delivery of Curcumin. Scientia Pharmaceutica. 2025; 93(3):28. https://doi.org/10.3390/scipharm93030028
Chicago/Turabian StylePeréz-Salas, Juan Luis, Martha Rocío Moreno-Jiménez, Luis Medina-Torres, Nuria Elizabeth Rocha-Guzmán, María Josefa Bernad-Bernad, Rubén Francisco González-Laredo, and José Alberto Gallegos-Infante. 2025. "Development and Characterization of Bigels for the Topical Delivery of Curcumin" Scientia Pharmaceutica 93, no. 3: 28. https://doi.org/10.3390/scipharm93030028
APA StylePeréz-Salas, J. L., Moreno-Jiménez, M. R., Medina-Torres, L., Rocha-Guzmán, N. E., Bernad-Bernad, M. J., González-Laredo, R. F., & Gallegos-Infante, J. A. (2025). Development and Characterization of Bigels for the Topical Delivery of Curcumin. Scientia Pharmaceutica, 93(3), 28. https://doi.org/10.3390/scipharm93030028







