Combined Effect of Sertraline and Capecitabine on Breast Cancer Cell Lines In Vitro and In Silico Evidence for Synergistic Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Cell Viability Assay
2.3. Trypan Blue
2.4. Neutral Red Assay
2.5. CompuSyn Combination Analysis
2.6. Investigation of Caspase-3-8 and 9 Enzyme Amounts
2.7. DNA Fragmentation
2.8. Investigation of mTOR Amount
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results
3.1. Cytotoxic Effect of Sertraline and Capecitabine
3.2. Synergistic Effect of the Combination of Sertraline and Capecitabine
3.3. Colorimetric Protease (Caspase-3, -8, -9) and Cellular DNA Fragmentation Assay
3.4. Colorimetric Autophagy (mTOR) Assay
3.5. Docking Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Crupi, R.; Drago, F.; Spina, E. Metabolic Drug Interactions Between Antidepressants and Anticancer Drugs: Focus on Selective Serotonin Reuptake Inhibitors and Hypericum Extract. Curr. Drug. Metab. 2011, 12, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Katirtzoglou, N.A.; Syrigos, K.N. Capecitabine: An Overview of the Side Effects and Their Management. Anticancer. Drugs 2008, 19, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Rodin, G.; Lloyd, N.; Katz, M.; Green, E.; Mackay, J.A.; Wong, R.K.S. The Treatment of Depression in Cancer Patients: A Systematic Review. Support Care Cancer 2007, 15, 123–136. [Google Scholar] [CrossRef]
- Smith, H.R. Depression in Cancer Patients: Pathogenesis, Implications and Treatment (Review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Duberstein, P.R. Depression and Cancer Mortality: A Meta-Analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, R.; Mercolini, L.; Saracino, M.A.; Raggi, M.A. Selective Serotonin Reuptake Inhibitors (SSRIs): Therapeutic Drug Monitoring and Pharmacological Interactions. Curr. Med. Chem. 2012, 19, 1846–1863. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Härtter, S. Pharmacokinetics of Selective Serotonin Reuptake Inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef]
- Amini Khodashahri, F.; Zargarnezhad, M.; Sajadi, R.; Khodashahri, A. The Cytotoxic Effects of Sertraline on Ovarian (A2780) Cancer Cells in Vitro. J. Biol. Stud. 2022, 5, 120–127. [Google Scholar] [CrossRef]
- He, L.; Fu, Y.; Tian, Y.; Wang, X.; Zhou, X.; Ding, R.-B.; Qi, X.; Bao, J. Antidepressants as Autophagy Modulators for Cancer Therapy. Molecules 2023, 28, 7594. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-L.; Chou, C.-T.; Cheng, J.-S.; Chang, H.-T.; Liang, W.-Z.; Kuo, C.-C.; Chen, I.-L.; Tseng, L.-L.; Shieh, P.; Wu, R.-F.; et al. Effect of Fluoxetine on [Ca2+]i and Cell Viability in OC2 Human Oral Cancer Cells. Chin. J. Physiol. 2014, 57, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, H.; Liu, Y.; Li, X.; Xu, B.; Li, L.; Jin, W. HTR1A Inhibits the Progression of Triple-Negative Breast Cancer via TGF-β Canonical and Noncanonical Pathways. Adv. Sci. 2022, 9, 2105672. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pan, C.; Xu, W.; Li, J.; Zhu, X. Leonurine Promotes Cisplatin Sensitivity in Human Cervical Cancer Cells through Increasing Apoptosis and Inhibiting Drug-Resistant Proteins. Drug Des. Devel. Ther. 2020, 14, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Barar, J.; Hejazi, M.S.; Samadi, N. Doxorubicin Changes Bax /Bcl-XL, Caspase-8 and 9 in Breast Cancer Cells. Adv. Pharm. Bull. 2015, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; Zhang, Y.; Zhang, Y.X.; Jiang, Z.Y.; Yang, H.; Jiang, L.; Yang, B.; Tong, J.C. Helicid Reverses the Effect of Overexpressing NCALD, Which Blocks the SGC/CGMP/PKG Signaling Pathway in the CUMS-Induced Rat Model. J. Healthc. Eng. 2021, 2021, 7168397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.H.; Motoo, Y.; Sawabu, N.; Minamoto, T. Effect of Gemcitabine on the Expression of Apoptosis-Related Genes in Human Pancreatic Cancer Cells. World J. Gastroenterol. 2006, 12, 1597. [Google Scholar] [CrossRef]
- Chen, L.J.; Hsu, T.C.; Chan, H.L.; Lin, C.F.; Huang, J.Y.; Stewart, R.; Tzang, B.S.; Chen, V.C.H. Protective Effect of Escitalopram on Hepatocellular Carcinoma by Inducing Autophagy. Int. J. Mol. Sci. 2022, 23, 9247. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Antidepressant Drug Sertraline against Human Cancer Cells. Biomolecules 2022, 12, 1513. [Google Scholar] [CrossRef]
- Malard, F.; Jacquet, E.; Nhiri, N.; Sizun, C.; Chabrier, A.; Messaoudi, S.; Dejeu, J.; Betzi, S.; Zhang, X.; Thureau, A.; et al. Revisiting the Molecular Interactions between the Tumor Protein TCTP and the Drugs Sertraline/Thioridazine. ChemMedChem 2022, 17, e202100528. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, S.L.; Kampen, K.R.; Rinaldi, G.; Gupta, P.; Planque, M.; Louros, N.; Heylen, E.; De Cremer, K.; De Brucker, K.; Vereecke, S.; et al. Repurposing the Antidepressant Sertraline as SHMT Inhibitor to Suppress Serine/Glycine Synthesis–Addicted Breast Tumor Growth. Mol. Cancer Ther. 2021, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Giacopuzzi, E.; Pain, O.; Fabbri, C.; Magri, C.; Minelli, A.; Lewis, C.M.; Gennarelli, M. Investigating an in Silico Approach for Prioritizing Antidepressant Drug Prescription Based on Drug-Induced Expression Profiles and Predicted Gene Expression. Pharmacogenom. J. 2021, 21, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bin Kanner, Y.; Teng, Q.-X.; Ganoth, A.; Peer, D.; Wang, J.-Q.; Chen, Z.-S.; Tsfadia, Y. Cytotoxicity and Reversal Effect of Sertraline, Fluoxetine, and Citalopram on MRP1- and MRP7-Mediated MDR. Front. Pharmacol. 2023, 14, 1290255. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.W.; Zhang, Y. DockRMSD: An Open-Source Tool for Atom Mapping and RMSD Calculation of Symmetric Molecules through Graph Isomorphism. J. Cheminform. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sharma, R.; Mishra, A.K.; Singh, K.R. Prevalence and Psychobiological Correlates of Depression Among Breast Cancer Patients. Indian. J. Surg. Oncol. 2021, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Matuo, R.; Sousa, F.G.; Escargueil, A.E.; Grivicich, I.; Garcia-Santos, D.; Chies, J.A.B.; Saffi, J.; Larsen, A.K.; Henriques, J.A.P. 5-Fluorouracil and Its Active Metabolite FdUMP Cause DNA Damage in Human SW620 Colon Adenocarcinoma Cell Line. J. Appl. Toxicol. 2009, 29, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Övey, İ.S.; Güler, Y. Apoptotic Efficiency of Capecitabine and 5-Fluorouracil on Human Cancer Cells through TRPV1 Channels. Indian. J. Biochem. Biophys. 2020, 57, 64–72. [Google Scholar]
- Shen, M.; Pan, H.; Chen, Y.; Xu, Y.H.; Yang, W.; Wu, Z. A Review of Current Progress in Triple-Negative Breast Cancer Therapy. Open Med. 2020, 15, 1143–1149. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus Capecitabine versus Lapatinib plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer (PHOEBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Terranova-Barberio, M.; Roca, M.S.; Zotti, A.I.; Leone, A.; Bruzzese, F.; Vitagliano, C.; Scogliamiglio, G.; Russo, D.; D’Angelo, G.; Franco, R.; et al. Valproic Acid Potentiates the Anticancer Activity of Capecitabine in Vitro and in Vivo in Breast Cancer Models via Induction of Thymidine Phosphorylase Expression. Oncotarget 2016, 7, 7715–7731. [Google Scholar] [CrossRef] [PubMed]
- Kaya Çakir, H.; Eroglu, O. In Vitro Anti-Proliferative Effect of Capecitabine (Xeloda) Combined with Mocetinostat (MGCD0103) in 4T1 Breast Cancer Cell Line by Immunoblotting. Iran J. Basic Med. Sci. 2021, 24, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Khvatova, G.I.; Semeikin, A.V. Molecular-Biological Problems of Drug Design and Mechanism of Drug Action: Comparative Cytotoxicity of Capecitabine and Xeloda on Cultured MCF-7, HT-12, and Rat Thymocytes. Pharm. Chem. J. 2011, 44, 651–653. [Google Scholar] [CrossRef]
- Pitts, T.M.; Simmons, D.M.; Bagby, S.M.; Hartman, S.J.; Yacob, B.W.; Gittleman, B.; Tentler, J.J.; Cittelly, D.; Ormond, D.R.; Messersmith, W.A.; et al. Wee1 Inhibition Enhances the Anti-Tumor Effects of Capecitabine in Preclinical Models of Triple-Negative Breast Cancer. Cancers 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.-H. 5-Fluorouracil Combined with Apigenin Enhances Anticancer Activity through Induction of Apoptosis in Human Breast Cancer MDA-MB-453 Cells. Oncol. Rep. 2009, 22, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Abd El-Alim, A.E.-A.F.; El-Hafeez, M.A.; Metwally, M.M.M.; Khamis, T.; Galal, A.A.A. Baicalein Prevents Capecitabine-Induced Heart Damage in Female Wistar Rats and Enhances Its Anticancer Potential in MCF-7 Breast Cancer Cells. Life Sci. 2023, 319, 121523. [Google Scholar] [CrossRef] [PubMed]
- Wińska, P.; Karatsai, O.; Staniszewska, M.; Koronkiewicz, M.; Chojnacki, K.; Rędowicz, M.J. Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with P38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line. Int. J. Mol. Sci. 2020, 21, 6234. [Google Scholar] [CrossRef] [PubMed]
- Garbar, C.; Mascaux, C.; Giustiniani, J.; Merrouche, Y.; Bensussan, A. Chemotherapy Treatment Induces an Increase of Autophagy in the Luminal Breast Cancer Cell MCF7, but Not in the Triple-Negative MDA-MB231. Sci. Rep. 2017, 7, 7201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pan, Y. Fluorouracil Induces Autophagy-Related Gastric Carcinoma Cell Death through Beclin-1 Upregulation by MiR-30 Suppression. Tumor Biol. 2016, 37, 15489–15494. [Google Scholar] [CrossRef]
- Li, J.; Hou, N.; Faried, A.; Tsutsumi, S.; Kuwano, H. Inhibition of Autophagy Augments 5-Fluorouracil Chemotherapy in Human Colon Cancer in Vitro and in Vivo Model. Eur. J. Cancer 2010, 46, 1900–1909. [Google Scholar] [CrossRef]
- Milczarek, M.; Wiktorska, K.; Mielczarek, L.; Koronkiewicz, M.; Dąbrowska, A.; Lubelska, K.; Matosiuk, D.; Chilmonczyk, Z. Autophagic Cell Death and Premature Senescence: New Mechanism of 5-Fluorouracil and Sulforaphane Synergistic Anticancer Effect in MDA-MB-231 Triple Negative Breast Cancer Cell Line. Food Chem. Toxicol. 2018, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The Therapeutic Potential of MTOR Inhibitors in Breast Cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Q.; Li, X.; Li, D.; Fan, M.; Ren, Z.; Bryant, M.; Mei, N.; Ning, B.; Guo, L. The Role of Hepatic Cytochrome P450s in the Cytotoxicity of Sertraline. Arch. Toxicol. 2020, 94, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Chinnapaka, S.; Bakthavachalam, V.; Munirathinam, G. Repurposing Antidepressant Sertraline as a Pharmacological Drug to Target Prostate Cancer Stem Cells: Dual Activation of Apoptosis and Autophagy Signaling by Deregulating Redox Balance. Am. J. Cancer Res. 2020, 10, 2043–2065. [Google Scholar] [PubMed]
- Chen, S.; Xuan, J.; Wan, L.; Lin, H.; Couch, L.; Mei, N.; Dobrovolsky, V.N.; Guo, L. Sertraline, an Antidepressant, Induces Apoptosis in Hepatic Cells Through the Mitogen-Activated Protein Kinase Pathway. Toxicol. Sci. 2014, 137, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Drinberg, V.; Bitcover, R.; Rajchenbach, W.; Peer, D. Modulating Cancer Multidrug Resistance by Sertraline in Combination with a Nanomedicine. Cancer Lett. 2014, 354, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Peng, R.-K.; Guo, C.-L.; Li, A.; Yang, X.-M.; Zeng, R.; Li, Y.-L.; Gu, J.; Ouyang, Q. Discovery of Sertraline and Its Derivatives Able to Combat Drug-Resistant Gastric Cancer Cell via Inducing Apoptosis. Bioorg. Med. Chem. Lett. 2021, 41, 127997. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, W.D.; Hallett, R.M.; Girgis-Gabardo, A.; Bojovic, B.; Dvorkin-Gheva, A.; Aarts, C.; Dias, K.; Bane, A.; Hassell, J.A. Serotonergic System Antagonists Target Breast Tumor Initiating Cells and Synergize with Chemotherapy to Shrink Human Breast Tumor Xenografts. Oncotarget 2017, 8, 32101–32116. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, S.; Beery, E.; Israeli, M.; Uziel, O.; Lahav, M.; Fenig, E.; Gil-Ad, I.; Weizman, A.; Nordenberg, J. In Vitro Novel Combinations of Psychotropics and Anti-Cancer Modalities in U87 Human Glioblastoma Cells. Int. J. Oncol. 2010, 37, 1043–1051. [Google Scholar] [CrossRef]
- Cava, C.; Castiglioni, I. Integration of Molecular Docking and In Vitro Studies: A Powerful Approach for Drug Discovery in Breast Cancer. Appl. Sci. 2020, 10, 6981. [Google Scholar] [CrossRef]
- Ghanem, A.; Emara, H.A.; Muawia, S.; Abd El Maksoud, A.I.; Al-Karmalawy, A.A.; Elshal, M.F. Tanshinone IIA Synergistically Enhances the Antitumor Activity of Doxorubicin by Interfering with the PI3K/AKT/MTOR Pathway and Inhibition of Topoisomerase II: In Vitro and Molecular Docking Studies. New J. Chem. 2020, 44, 17374–17381. [Google Scholar] [CrossRef]
- Sánchez-Castillo, A.; Heylen, E.; Hounjet, J.; Savelkouls, K.G.; Lieuwes, N.G.; Biemans, R.; Dubois, L.J.; Reynders, K.; Rouschop, K.M.; Vaes, R.D.W.; et al. Targeting Serine/Glycine Metabolism Improves Radiotherapy Response in Non-Small Cell Lung Cancer. Br. J. Cancer 2024, 130, 568–584. [Google Scholar] [CrossRef] [PubMed]
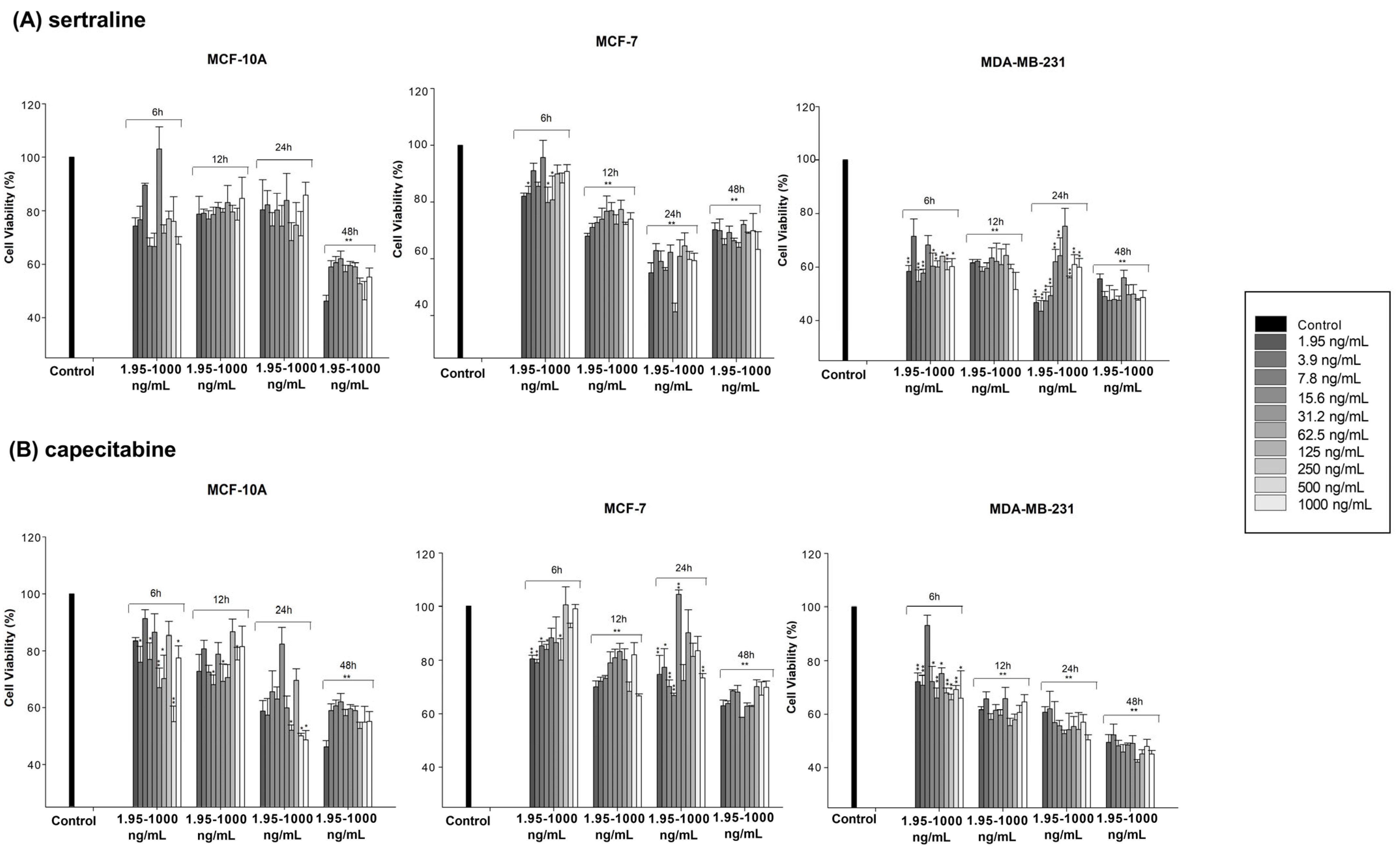





| Drug | MCF-7 | MDA-MB-231 | MCF10A |
|---|---|---|---|
| sertraline | 51.3554 ng/mL (24 h) | 16.86 ng/mL (24 h) 247.937 ng/mL (48 h) | 362.91 ng/mL (48 h) |
| capecitabine | nd | 29.48 ng/mL (24 h) 8.72 ng/mL (48 h) | 498.92 ng/mL (48 h) |
| AKT1 | AMPK | SIRT1 | LKB1 | |
|---|---|---|---|---|
| Sertraline | −9.9 | −7.9 | −7.5 | −7.0 |
| Co-crystallized ligands | −13.8 a | −9.1 b | −7.2 c | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, S.O.; Korkut, A.; Aydemir, E. Combined Effect of Sertraline and Capecitabine on Breast Cancer Cell Lines In Vitro and In Silico Evidence for Synergistic Interaction. Sci. Pharm. 2024, 92, 38. https://doi.org/10.3390/scipharm92030038
Gul SO, Korkut A, Aydemir E. Combined Effect of Sertraline and Capecitabine on Breast Cancer Cell Lines In Vitro and In Silico Evidence for Synergistic Interaction. Scientia Pharmaceutica. 2024; 92(3):38. https://doi.org/10.3390/scipharm92030038
Chicago/Turabian StyleGul, Serap Ozkaya, Alaaddin Korkut, and Esra Aydemir. 2024. "Combined Effect of Sertraline and Capecitabine on Breast Cancer Cell Lines In Vitro and In Silico Evidence for Synergistic Interaction" Scientia Pharmaceutica 92, no. 3: 38. https://doi.org/10.3390/scipharm92030038
APA StyleGul, S. O., Korkut, A., & Aydemir, E. (2024). Combined Effect of Sertraline and Capecitabine on Breast Cancer Cell Lines In Vitro and In Silico Evidence for Synergistic Interaction. Scientia Pharmaceutica, 92(3), 38. https://doi.org/10.3390/scipharm92030038






