Abstract
This article presents a comprehensive study on the formulation and physicochemical characterization of a novel terpenocannabinoid-functionalized hemp oil emulsifier (AMCana-Oil) for potential applications in topical anti-inflammatory, antinociceptive, and wound healing treatments. The emulsifier exhibits interesting properties, meets international acidity index requirements, and has a room temperature density comparable to liquid oils. The prepared emulsifier (AMCana-Oil), AMCana-Oil (10% TC) and AMCana-Oil (20% TC), contains a diverse array of cannabinoids, including cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC), alongside bioactive compounds such as benzenepropanoic acid and oleamide. Physicochemical properties of AMCana-Oil and MCana-Oil (20% TC) were found, respectively, as follows: density value of 0.9872 ± 0.001 mg/mL: g/cm³ and 0.9882 ± 0.002 mg/mL: g/cm³; an acidity index of 1.599 ± 0.002 mgKOH/g and 1.605 ± 0.001 mgKOH/g; an average peroxide value encompassing a range from 12.982 ± 0.351 to 23.320 ± 0.681 (mEq O2/kg), and a K of 1.575 ± 0.004 and 1.535 ± 0.0039, which underscore the fluidity, stability, and quality of emulsifiers studied. Preliminary pharmacological examinations reveal significant antioxidant, anti-inflammatory, antinociceptive, and wound healing potentials. Moreover, in silico predictions confirm the safety profile of the prepared emulsifiers. These findings emphasize the multifaceted nature of the terpenocannabinoid-functionalized emulsifier, paving the way for its potential applications in topical formulations.
1. Introduction
The therapeutic potential of hemp oil and its constituent compounds has garnered substantial attention due to their well-documented pharmacological activities, including anti-inflammatory and antinociceptive effects [1,2]. Besides cannabinoids, the emerging significance of terpenes as crucial bioactive components has further fueled research endeavors, prompting their exploration into innovative emulsifiers aimed at enhancing the bioavailability and effectiveness of hemp oil-based products [3,4].
It is important to note that recent advancements in emulsifier technology have significantly enhanced the development of thematic formulations [5]. Traditional emulsifiers often present problems such as instability, low bioavailability, and limited effectiveness in delivering active compounds to specific sites [6]. However, to overcome these limitations, innovative approaches have emerged, such as the development of emulsifiers based on plant and hemp oils [7,8]. These innovative emulsifiers benefit from the synergistic effects of natural constituents to enhance the therapeutic potential of topical formulations. According to recent studies, emulsifiers are crucial for optimizing the distribution of bioactive substances across the epidermal barrier [9]. Emulsifiers can be designed with specific physicochemical properties to improve the penetration and retention of active substances by adjusting physicochemical parameters like stability, surface charge, and particle size. Additionally, advances in emulsifier design have enabled the development of multifunctional formulations that can deliver therapeutic agents while also providing hydrating, soothing, or protective effects to the skin [10].
Accordingly, this study aimed to perform a comprehensive investigation into the formulation and in-depth physicochemical characterization of an innovative terpenocannabinoid-functionalized hemp oil emulsifier. The main objective of this emulsifier is to optimize the targeted delivery of terpenocannabinoids through topical applications, thereby offering a promising opportunity for skin therapeutics. By harnessing the unique composition and properties of the emulsifier, this approach aims to enhance the transdermal penetration of active compounds, enabling precise and effective interventions for inflammatory conditions and pain management.
Beyond its formulation and stability assessment, this research thoroughly examines the emulsifier’s physicochemical proprieties, phytochemical composition, and cutaneous pharmacological activities. In order to complete these analyses, safety and stability evaluations are conducted via in silico predictions. Through this multidimensional analysis, the study aims to illuminate the potential of this novel emulsifier for topical applications, particularly in anti-inflammatory and antinociceptive therapies. Moreover, the exploration extends to the domains of antioxidant activity and wound healing, establishing an integrated framework that highlights the interconnectedness of these indices with the overarching objectives of the study. Finally, the results and ensuing discussions will furnish a meticulous analysis of the amassed data, providing insights into the emulsifier’s potential as a versatile platform for advancing topical therapies, encompassing inflammation, pain, and the overall well-being of the skin.
2. Material and Methods
2.1. Plant Material
The hemp (Cannabis sativa L.) utilized in this study originates from a farm situated in Ighajdaren Al Hoceima, Northern Morocco (X = 34.810234 N, Y = 4.660386 W). The selected hemp variety is renowned for its elevated terpenocannabinoid content, particularly the presence of cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC). Notably, this specific Cannabis variety is associated with a unique code voucher: “RAB113319”. Additionally, the GenBank accession number “BankIt2726356 Cannabis OR372636” corresponds to its nucleotide sequence, further corroborating its genetic authenticity and providing a distinct reference for its identification. The cultivation process was meticulously executed under traditional conditions used in the north region of Morocco. At the point of ideal maturity, the hemp plants underwent harvesting and drying using traditional air-drying techniques.
This study focused on two distinct components of the hemp plant: seeds were used to extract hemp oil, while the flower powder (trichomes) was utilized to prepare the terpenocannabinoid fraction.
2.2. Animal Material
A total of 25 female and male mice (30–35 g) and 25 female and male rats (200–250 g) were employed as the animal model for these studies. The mice were used for anti-nociceptive and anti-inflammatory activities, and rats were used for wound healing activity and toxicity.
The animals underwent initial weighing and were then randomly housed in individual polypropylene plastic cages, in a temperature-controlled environment with a 12/12 h light/dark cycle and free access to water and food. For each experiment, the animals were divided into 5 groups, each consisting of 5 mice/rat, except for the toxicity study, which involved 4 groups: group 1: Negative control; group 2: Positive control; group 3: Hemp oil Emulsifier (10% TC: Terpenocannabinoid Extract); group 4: Hemp oil Emulsifier (20% TC); Group 5: Hemp oil Emulsifier (0% TC).
During the experiments, all animal handling and protocols strictly adhered to ethical guidelines and regulations to ensure the welfare and well-being of the mice. The institutional ethical committee of care and use of the laboratory animals at the Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdallah Fez University, Morocco, reviewed and approved the present study #04/2019/LBEAS. The care and the use of animals were subjected to the institutional ethical committee according to the directive EEC/86/EEC of the European community (Union, 1986).
2.3. Preparation of Terpenocannabinoid-Functionalized Hemp Oil Emulsifier
The hemp oil was extracted from hemp seeds using a dynamic cold maceration method with hexane, under controlled agitation, for a 24 h cycle (n = 3) at room temperature. The resulting extract was then concentrated under a vacuum at a temperature below 37 °C to obtain the hemp oil. The obtained oil was stored in an amber flask in a dark place under 4 °C to preserve its stability.
For the extraction of the terpenocannabinoid fraction from Cannabis flower powder, a maceration process with agitation was carried out for a single 24 h cycle (n = 1) using a mixture of diethyl ether and petroleum ether. The resulting terpenocannabinoid extract was concentrated under a vacuum at a temperature below 37 °C, lyophilized, and stored appropriately.
The formulation of the terpenocannabinoid-functionalized hemp oil emulsifier involved the combination of a known concentration of the extract (prepared at 10% and 20%, respectively) with an aliquot of the hemp oil. The emulsifier formulation was achieved using ultrasonic waves to ensure effective homogenization and dispersion of the terpenocannabinoid extract within the hemp oil.
2.4. Physicochemical Characterization
In each test, all instruments were previously calibrated in accordance with the manufacturer’s specifications. In accordance with test procedures, measurements were taken in triplicate and averaged (n = 3) to ensure statistical reliability. The results were presented as mean values ± standard deviation (SD).
2.4.1. Density
The density of the emulsifier was determined using the density meter DS7800-Krüss. The assessments were conducted under controlled temperature conditions, ranging from 293 K to 296 K (20 °C to 23 °C), and at atmospheric pressure [11].
2.4.2. Acid Value
The acid value, a crucial parameter indicating the content of free fatty acids resulting from the hydrolysis of triglycerides, was determined to assess the chemical composition of the hemp oil and terpenocannabinoid-functionalized hemp oil emulsifier. The acid value was conventionally expressed in terms of oleic acid content (g/100 g of oil).
The experimental procedure for acid value determination followed a validated method described in the literature [12,13]. Briefly, a sample aliquot of the emulsifier was dissolved in a solvent mixture. Free fatty acids present in the sample were titrated using an ethanolic solution of potassium hydroxide (KOH) of 0.1 N. To initiate titration, 5 g of the emulsifier was dissolved in 30 mL of an ethanol/ether mixture (v/v) and titrated with the ethanolic KOH solution. The titration process was conducted with continuous agitation and monitored visually. A 1% phenolphthalein solution in ethanol was used as an indicator, resulting in a characteristic color change from colorless to pink upon neutralization of the free fatty acids.
The acid value (AV) was calculated using the following equation:
where
AV= ((V × N × 56.1)) ⁄ m
V is the volume of titrated KOH ethanolic solution (mL);
N is the exact normality of the ethanolic solution of KOH (N);
m is the sample weight (g).
2.4.3. Peroxide Indicator
The peroxide indicator (PI) evaluates peroxide content as milliequivalents of active oxygen per kilogram of product (mEq O2/kg). It measures freshness through iodine release from potassium iodide oxidation. This process involves treating the lipid sample in a solution of acetic acid and chloroform with a potassium iodide solution, followed by titration of the liberated iodine using a solution of sodium thiosulfate [14,15]. A standardized protocol was used: 1 g of the emulsifier was mixed with 10 mL of chloroform, 15 mL of acetic acid and 1 mL of potassium iodide. After agitation, the solution rested in the dark at room temperature for 5 min. Then, 75 mL distilled water was added, and iodine was titrated with 0.01 N sodium thiosulfate using starch as an indicator.
The PI (mEq O2/kg) was calculated:
where
PI = ((V × T × 8000) ⁄ m)
V is the volume of sodium thiosulfate solution used for the test, corrected based on the results of the blank test (mL);
T is the exact titer of the sodium thiosulfate solution used (mL);
m is the mass of the test sample (g).
2.4.4. Potassium Value (K Value)
The potassium value (K Value) serves as a key indicator of natural fatty substance quality, particularly related to unsaturated fatty acid oxidation. The method involves UV spectrophotometric analysis, focusing on conjugated diene and triene structures [16].
For this study, a sample of the terpenocannabinoid-functionalized hemp oil emulsifier (0.25 g) was dissolved in cyclohexane (25 mL). UV absorbance readings were taken at 270 nm using a quartz cuvette and UV/Visible spectrophotometer V series from J.P Selecta. The K value (Ecm1%) was calculated using the formula:
where
K = Aλ ⁄ (C × S)
Aλ is the absorbance at wavelength λ;
C is the concentration of the solution (mg/100 mL);
S is the optical path length (1 cm).
2.5. Phytochemical Characterization
Phytochemical characterization of the hemp oil emulsifier (AMCana-Oil) and the terpenocannabinoid fraction (TC) required specific derivatization steps prior to analysis by gas chromatography-mass spectrometry (GC-MS/MS, TQ) [17,18]. The aim of this analysis was to elucidate the composition and content of various bioactive components present in the terpenocannabinoid-functionalized hemp oil emulsifier. Derived samples were analyzed by Shimadzu-GCMS-TQ8040 NX (Shimadzu, Kyoto, Japan) triple quadrupole gas chromatograph equipped with a mass spectrometer and triple quadrupole detector. Chromatographic separation was performed using an Apolar RTxi-5 Sil MS capillary column (30 m × 0.25 mm ID × 0.25 µm) and Helium as the carrier gas where the temperature program started at 50 °C for 2 min, followed by a temperature gradient increase of 5 °C/min to 160 °C for 28 min. The gradient was then continued up to 280 °C for a further 20 min. A volume of 1.5 µL was injected in the splitless mode, and the analysis time was set at 50 min. The ion source temperature was maintained at 200 °C, while the interface and injection temperatures were set at 280 °C and 250 °C, respectively. It should be noted that this method was designed for the needs of this study and is subject to ongoing validation.
2.6. Pharmacological Properties
2.6.1. Antioxidant Activity
The antioxidant capacity of the hemp oil and the terpenocannabinoid-functionalized hemp oil emulsifier was assessed through in vitro evaluations utilizing four distinct assays:
2,2-diphenylpicrylhydrazyl (DPPH) Method
The DPPH method involved adding 100 µL of each extract solution to 750 µL of a methanolic solution of DPPH (0.004%) [19]. After 30 min of incubation at room temperature, the absorbance was measured at 517 nm. The percentage of DPPH inhibition was calculated using the following equation:
where
PI(%) = ((A0 − A) ⁄ A0) ×100
PI is the percentage of inhibition;
A0 is the absorbance of the DPPH of negative control (NC);
A is the absorbance of DPPH of the sample.
IC50 values were obtained from the inhibition percentage graph against extract concentration.
Reducing Power Test (FRAP)
For the FRAP test, 500 µL of phosphate buffer (0.2 M, pH = 6.6), 500 µL of potassium ferricyanide (1%), and 100 µL of different sample concentrations dissolved in methanol were combined. After incubating at 50 °C for 20 min, 500 µL of aqueous TCA solution (10%), 500 µL of distilled water, and 100 µL of 0.1% FeCl3 were added. The absorbance was measured at 700 nm, and results were expressed as 50% effective concentration (EC50) [20].
Total Antioxidant Capacity (TAC) Test
For the TAC test, 25 µL of the extract was mixed with a reagent solution containing 0.6 M sulfuric acid (H2SO4), 28 mM sodium phosphate (Na2HPO4), and 4 mM ammonium molybdate ((NH4)2MoO4). The mixture was then incubated at 95 °C for 90 min. After incubation, the absorbance was measured at 695 nm using a spectrophotometer V series from J.P Selecta. The total antioxidant capacity was determined by comparing the absorbance of the extract with a standard curve of ascorbic acid. The results were expressed as micrograms of ascorbic acid equivalent per gram of extract (µg EAA/g extract), providing an assessment of the extract’s antioxidant potential based on the behavior of ascorbic acid under the same conditions [21].
Beta-Carotene Bleaching Assay
The Beta-Carotene Bleaching Inhibition Test, as adapted from Ozsoy et al. (2008) [22], was conducted to evaluate the ability to inhibit the bleaching of beta-carotene using a beta-carotene/linoleic acid model. Linoleic acid (10 μL) and Tween 80 (100 μL) were introduced into a flask, followed by the addition of beta-carotene solution (1 mL, prepared by dissolving 2 mg of beta-carotene powder in 10 mL chloroform). The chloroform was removed using rotary evaporation at 40 °C. To the residue, 30% hydrogen peroxide (25 mL) was gradually added, creating a stable emulsion through vigorous shaking. From this emulsion, 2.5 mL was combined with 100 μL of diluted extract or a reference antioxidant (ascorbic acid) at 35 mg/mL concentration. A negative control was established using the same emulsion with methanol. The mixture was well mixed, and the absorbance was measured at 470 nm immediately (t0) against a blank emulsion lacking beta-carotene. Covered tubes were placed in a 50 °C water bath, and absorbance readings were taken at various intervals (0 min, 25 min, 50 min, 75 min, 100 min, and 120 min) until beta-carotene was no longer visible in the control sample (approximately 120 min). Antioxidant activity was assessed based on the inhibition of beta-carotene bleaching, calculated using the following formula:
where
AA% = (A_E⁄A_PC) × 100
AA% is the percentage of antioxidant activity.
AE is the absorbance after 120 min with negative control.
APC is the absorbance after 120 min of the positive control.
2.6.2. Anti-Inflammatory Activity
After skin administration of the different doses of the formulated emulsifier (TC: 0%, 10%, 20%) to the mice, the paw volume was measured initially before the injection of 0.5% carrageenan into the left paw of the rats. The paw volume was then periodically measured at 3, 4, 5, and 6 h after the injection. The inhibitory activity was calculated using the following equation:
where
PI = (1 − ((a − x) ⁄ (b − y))) × 100
PI is the percent inhibition.
a is the average paw volume of the treated rats after carrageenan injection.
x is the average paw volume of the treated rats before the injection.
b is the average paw volume of the control rats after carrageenan injection.
y is the average paw volume of the control rats before the injection.
2.6.3. Antinociceptive Activity
This activity was conducted following two methods: Acetic acid-induced pain was conducted following the method described by Farzaei et al. (2013) [23]. After skin administration of the different doses of the formulated emulsifier (TC: 0%, 10%, 20%) to the mice, one hour later, an intraperitoneal injection of 1% acetic acid was performed at a rate of 10 mL/kg. After 10 min, writhing movements were counted over a period of 30 min. And we compare the results between the negative control group, positive control group, and the different groups to assess the topical analgesic effect of the extracts. The formalin-induced pain protocol involved the subcutaneous injection of formalin into the hind paw of the mice. The procedure followed established guidelines [24], and observations were made over a specified period (30 min).
2.6.4. Wound Healing Activity
For evaluating wound healing activity, we employed an excision wound model following the Morton and Malone protocol (1972) [25]. Animals belonging to five groups (1: AMCana-Oil (0% TC); 2: AMCana-Oil (10% TC); 3: AMCana-Oil (20% TC); 4–5: positive and negative controls) received relevant topical treatments. Anesthesia was induced through intraperitoneal injection of thiopental (60 mg/kg body weight), followed by shaving the dorsal area using an electric clipper. The shaved area was sanitized with 70% surgical alcohol. Subsequently, the marked wound area was excised using a heated plate, and the wound size was immediately measured. Topical treatments were administered once daily until complete healing. The gradual reduction in wound size for each animal was periodically monitored on days 0, 4, 7, 9, 11, and 14. The Percentage Contraction in Wound (PCW) was calculated using the following formula:
where
PCW(%) = (WSD0 − WSDn) ⁄ WSD0) × 100
WSD0 is the wound surface on day 0;
WSDn is the wound surface on day n.
Additionally, after achieving complete epithelialization, wound tissues were excised and preserved in 10% buffered formalin for 24 h. Histopathological analysis of the regenerated tissue was performed, following steps similar to the histopathological analysis of the liver and kidneys. An examination focused on identifying histological changes has been scheduled.
2.6.5. Subacute Toxicity
For the subacute toxicity test, four groups of mice underwent a 28 days skin administration of the formulated emulsifier (TC: 0%, 10%, 20%). Dosages were determined based on previous pharmacological investigations and animal body weight, following guidelines from Directive No. 410 (OECD 2020) [26] for subacute toxicity assessments. Throughout the four-week period, all animals were subject to daily weight monitoring and toxicity symptom evaluation. At the test’s conclusion, the animals were anesthetized using intraperitoneal pentobarbital injection (30 mg/kg). Blood samples were collected via cardiac puncture using EDTA-containing tubes for biochemical parameter analysis, including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea, and creatinine (CREA). In addition, skin histological examinations were conducted to assess tissue morphology and identify any potential histopathological changes [27].
2.6.6. Molecular Docking Analysis
The process involved preparing the ligands derived from the molecules found in the formulated terpenocannabinoid-functionalized hemp oil emulsifier (AMCana-Oil (% TC)). These molecules were sourced from the PUBCHEM database in .SDF format. The LigPrep tool from Schrödinger Software version 11.5 was then utilized, along with the OPLS3 force field, to ensure proper ligand preparation. To create a comprehensive set of ligands, each individual ligand was adjusted for ionization state selection at a pH range of 7.0 ± 2.0. This led to the creation of 32 stereoisomers for every molecule [28].
The three-dimensional structure of 5-Lipoxygenase (PDB ID: 3V99) was used to assess anti-inflammatory activity [29], cyclooxygenase-2 (prostaglandin synthase-2) (PDB ID: 6COX) to assess antinociceptive activity [30], and crystal structure of human casein kinase 1 (PDB ID: 6GZD) and crystal structure of glycogen synthase kinase 3 (PDB ID: 1Q5K) to evaluate wound healing activity [31]. These structures were directly sourced from the protein data bank. The optimization process encompassed tasks such as the addition of hydrogen atoms, completion of bond orders, removal of water molecules, assignment of hydrogen bonds, adjustment of the potential of receptor atoms, and energy minimization. This was achieved using the OPLS3 force field [28].
The Qik-Prop module of the Schrödinger software was used to describe the pharmacokinetic and physicochemical properties of fatty acids and active terpenes and cannabinoids detected in Cannabis oil such as molecular weight (MW), predicted skin permeability (log Kp), and polar surface area (PSA) [5].
In silico toxicity study was performed using the ProTox-II web server developed by Drwal et al. (2014) in which parameters such as the median lethal dose (LD50) in mg/Kg and toxicity class have been studied [32]. Skin sensitization was performed by in vitro cell response (http://predskin.labmol.com.br/), a web tool used for early skin sensitization [33].
2.7. Statistical Analysis
Using the SPSS software version 23.0, a one-way analysis of variance (ANOVA) test was used to complete the statistical evaluation. The significance of the differences between means was then determined using the Tukey test, with a significance threshold of p < 0.05. Subsequently, the data were displayed as the average ± standard error, obtained from three independent measurements.
3. Results and Discussion
3.1. Physicochemical Properties
The physicochemical characterization of hemp oil emulsifier that is provided here sheds light on the inherent properties of our emulsifier and opens up the door to its possible therapeutic uses, which include impacts on cutaneous toxicity, wound healing, anti-inflammatory, and antinociceptive properties. The density of the studied samples (AMCana-Oil and AMCana-Oil (20% TC) emulsifier) is a crucial indicator of their fluidity and compactness. This density is dependent on their fatty acid composition, minor components and temperature (Fakhri & Qadir, 2011). Our findings, showcasing an average density of 0.9872 ± 0.001 mg/mL: g/cm³ for AMCana-Oil and 0.9882 ± 0.002 mg/mL: g/cm³ for AMCana-Oil (20% TC), align with the liquid state of these oils at room temperature. Additionally, the marginal difference between the densities of AMCana-Oil and AMCana-Oil (20% TC) suggests that the addition of the 20% THC component does not significantly alter the overall density of the oil. This is in accordance with previous research by Anwar F. et al. (2006) [1], where similar density values were reported for hemp oils.
The acidity index, an essential parameter reflecting oil quality of fatty acids and acidity, showed values of around 1.605 ± 0.001 mgKOH/g for the emulsifier AMCana-Oil (20% TC) and 1.599 ± 0.002 mgKOH/g for the hemp oil studied AMCana-Oil (0% TC). This low acidity index indicates that the emulsifiers are suitable for a variety of applications as they will be stable over a long period of time and protect against rancidity and peroxidation. The emulsification process and the addition of the terpenocannabinoid fraction (TC) to the prepared Cannabis seed oil (AMCana-Oil) did not influence the acidity value and this could be attributed to their wealth of antioxidant compounds. It corresponds to the values recommended by the World Health Organization (ALINORM 68/11-1967, Revised 2003) [11] and the Food and Agriculture Organization of the United Nations (CODEX STAN 210-1999, Revised 2017) [34].
The peroxide value, or PV, is a measure of how much lipid oxidation has occurred. Significant PV values indicate significant levels of oxidative rancidity in the oils, as well as low or absent antioxidant levels. The obtained PV, encompassing a range from 12.982 ± 0.351 to 23.320 ± 0.681 (mEq O2/kg) for AMCana-Oil (0% TC) and AMCana-Oil (20% TC), unveil varying oxidation levels attributed to different treatments. These levels provide insights into the oils’ susceptibility to oxidation and potential stability. Similar observations were reported by Mitrea L. et al. (2022) [35] who investigated peroxide values in natural oils subjected to oxidative stress.
The K value, reflective of the presence of unsaturated compounds like conjugated trienes and oxidation products, implies the existence of unique chemical compositions within the emulsifier. Specifically, the obtained values for AMCana-Oil (0% TC) and AMCana-Oil (20% TC) are 1.575 ± 0.004 and 1.535 ± 0.0039, respectively, aligning with the findings of Anwar F. et al. (2006) [1].
These comparative analyses underline the importance of our physicochemical results in the wider context of plant-derived oils and investigate the influence of the addition of the terpenocannabinoid fraction (TC) to Cannabis seed vegetable oil (AMCana-Oil), as well as that of the entire process of refining and formulating the emulsifier AMCana-Oil (20% TC). The insights gained from our investigations, in conjunction with existing research, collectively contribute to a global understanding of Cannabis sativa L. seed oils, shaping their potential applications in cosmetic and therapeutic contexts.
3.2. Active Compound Composition and Fatty Acid Characterization
The chemical identification of different prepared emulsifier’s volatiles was determined by GC/MSMS and presented in Table 1. The intricate profile of active compounds and fatty acids detected (Table 1) in the studied AMCana-Oil (0% TC), AMCana-Oil (10% TC) and AMCana-Oil (20% TC) is of paramount importance in understanding the potential therapeutic properties of Cannabis sativa L. seed oils. The identification of specific fatty acids, such as palmitic acid and stearic acid, aligns with the well-documented presence of saturated fatty acids in hemp oils [36,37]. Moreover, the bioactive compounds observed in AMCana-Oil encompass a diverse array of chemical classes, including alkylbenzenes and fatty derivatives. Notably, the presence of benzenepropanoic acid, oleionitril, oleamide, and monopalmitin suggests the potential for antioxidant and anti-inflammatory effects [38], contributing to the oils’ multifaceted therapeutic potential.

Table 1.
Fatty acids and active terpenes and cannabinoids detected in the cannabis oil studied (AMCana-Oil) and the terpenocannabinoid fraction (TC), respectively, by analysis via GC-MS-MS (TQ).
In the terpenocannabinoid fraction (TC), the presence of a terpene caryophylene oxide and a various cannabinoid, including cannabidivarin (CBDV), delta-9-tetrahydrocannabivarin (THCV), cannabidiol (CBD), and delta-9-tetrahydrocannabinol (THC), underscores the rich cannabinoid diversity of the studied Cannabis variety. These findings collectively emphasize the complex and dynamic nature of the formulated AMCana-Oil (10–20% TC) emulsifier and its potential applications in a wide range of therapeutic contexts.
3.3. Pharmacological Properties Determination
3.3.1. Antioxidant Activity
Concerning the antioxidant activity, as demonstrated in Table 2, it has been evaluated using Total Antioxidant Capacity (TAC), DPPH Radical Scavenging Activity (IC50), Ferric Reducing Antioxidant Power (FRAP), and the Beta-Carotene Bleaching Assay. These assays provide comprehensive insights into the ability of AMCana-Oil formulations (0% TC, 10% TC, 20% TC) to counteract oxidative stress and neutralize free radicals.

Table 2.
Antioxidant power evaluation results of AMCana-Oil (0% TC) Emulsifier, AMCana-Oil (10% TC) Emulsifier, AMCana-Oil (20% TC) Emulsifier and STANDARDS (PC: Positive control) by TAC, DPPH and FRAP tests. Data are means ± confidence intervals (95%) from three replicates, and values with different letters (a, b and c) are significantly different according to Tukey’s HSD test.
The results from the antioxidant evaluation suggest that higher concentrations of AMCana-Oil formulations, especially the 20% TC variant, display superior antioxidant activity, evident from the elevated Total Antioxidant Capacity (TAC), lower DPPH IC50 values indicating enhanced radical scavenging, and higher Ferric Reducing Antioxidant Power (FRAP). These findings highlight the potential of AMCana-Oil formulations to function as effective antioxidants. The robust antioxidant performance, especially the emulsifier with 20% TC, suggests promising prospects for these formulations in mitigating oxidative stress. This activity is attributed to the richness of Cannabis in bioactive molecules, particularly THC and CBD, which possess significant antioxidant properties [39]. The interaction of these cannabinoids with oxidative processes underscores the potential therapeutic benefits of AMCana-Oil in combating oxidative stress and promoting overall well-being.
3.3.2. Wound Healing Activity
The wound healing ability of Cannabis oil formulations (0%, 10% and 20%) and positive control (Biafine: Trolamine (0.7%)) was examined in Wistar rats. Figure 1 presents the differences in the rate of wound healing as a function of time in the groups of rats treated for 3 weeks. The images show the healing process in which illustrates the progression of the healing.
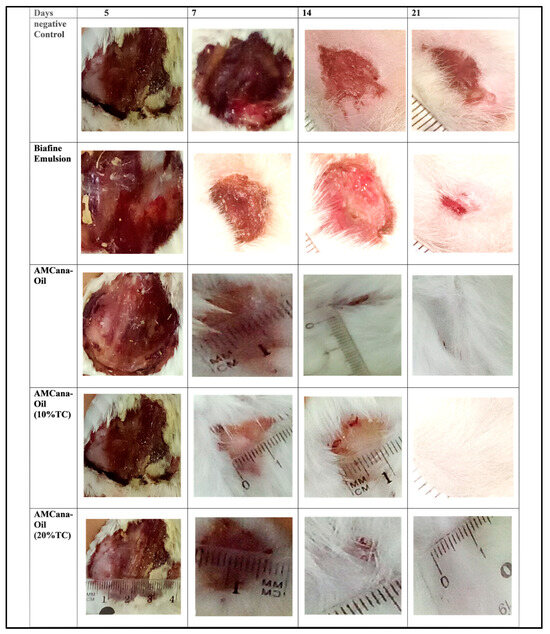
Figure 1.
Images (X1800) of the healing process in treated rats by AMCana-Oil (0% TC) Emulsifier, AMCana-Oil (10% TC) Emulsifier, AMCana-Oil (20% TC) Emulsifier and Biafine emulsion (PC: Positive control).
Upon comparing these images with the negative control, it is evident that burns treated with Cannabis-based formulations (0%, 10%, and 20%) as well as Trolamine (0.7%) exhibit improvement over time. However, the results of the AMCana-Oil (20% TC) consistently demonstrate superior healing outcomes compared to the positive control. A previous study highlighted the potential bioactive compounds in Cannabis sativa L. that contribute to its wound healing, such as Cannabidiol (CBD) with various therapeutic effects, and Dronabinol (THC) with potential benefits. These bioactive compounds collectively play a role in enhancing wound healing efficacy by promoting anti-inflammatory effects, accelerating re epithelialization, and enhancing granulation tissue formation [40,41].
3.3.3. Toxicity Subacute
The subacute toxicity of Cannabis oil formulations (0%, 10% and 20%) and the negative control (NaCl 0.9%) was examined in Wistar rats. During the treatment period, the weight of the rats was measured, and after 28 days of treatment, the change in weight was calculated. After 28 days of treatment, blood and organs were collected for analysis of biochemical parameters, and histological sections were also taken. All the results are shown in Table 3 and Figure 2 and Figure 3.

Table 3.
Effect of negative control, AMCana-Oil emulsifier (0% TC), AMCana-Oil emulsifier (10% TC) and AMCana-Oil emulsifier (20% TC) on biochemical parameters in male and female mice treated for 28 days. Data are means ± confidence intervals (95%) from three replicates, and values with different letters (a, b and c) are significantly different according to Tukey’s HSD test.
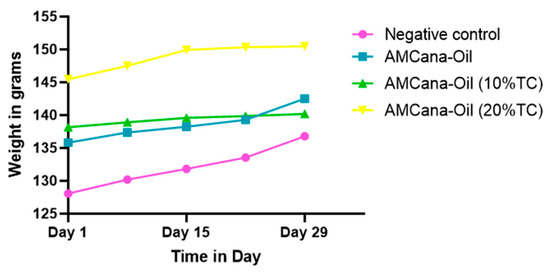
Figure 2.
Change in rat weight (in grams) over time (in days) for different treatments: negative control, AMCana-Oil (0% TC) Emulsifier, AMCana-Oil (10% TC) Emulsifier and AMCana-Oil (20% TC).
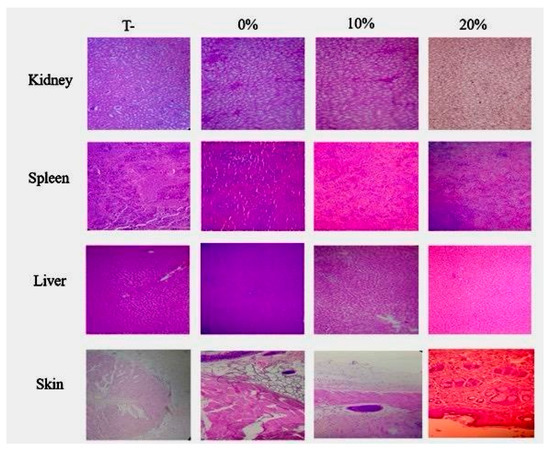
Figure 3.
Histological sections of liver, kidney, and spleen from negative control, AMCana-Oil (0% TC) Emulsifier, AMCana-Oil (10% TC) Emulsifier, and AMCana-Oil (20% TC).
Concerning the weight of the treated animals, as shown in the graph, there was an increase in weight over time for both the negative control group and the treated groups, indicating that the treatment was normal and non-toxic. The comprehensive evaluation of biochemical parameters in rats treated with varying concentrations of Cannabis formulations (0%, 10%, and 20%) reveals subtle variations in Urea, Creatinine, ASAT, ALAT, and protein levels compared to the control group. While Urea and Creatinine show slight changes, what stands out is the steadfast adherence of ASAT and ALAT levels to the normal range, remaining below the triple threshold for abnormality. This good consistency in liver enzyme levels suggests a potential safety profile of the Cannabis formulations.
To further this study, a histological examination of vital organs—liver, spleen, kidneys, and skin—was realized in order to complete the biochemical parameters. As depicted in Figure 3, the absence of any signs of toxicity in these organs further strengthens the overall safety profile of the Cannabis formulations.
This multidisciplinary approach, combining biochemical analysis with histological understandings, underscores the need for a complete understanding of potential therapeutic effects and any associated risks. These collective results contribute significantly to delineating the nuanced interplay between Cannabis compounds and physiological markers, reinforcing the importance of further research in this domain.
It is crucial to contextualize these findings within the broader literature. An earlier study highlighting the toxic effects of Cannabis through oral administration at a 10% dose underscores the significance of route-dependent toxicity [42,43]. This comparison accentuates the importance of understanding how the mode of administration influences the potential adverse effects of Cannabis formulations.
3.3.4. Anti-Inflammatory Activity
Anti-inflammatory activity was assessed by measuring the percentage inhibition of inflammation in mice after cutaneous application the formulated Cannabis-based emulsifier (TC: 0%, 10%, 20%) and positive control (flammazine1%). Table 4 shows the results of this evaluation, indicating the degree of inhibition at different times. At time zero (t0) after carrageenan injection, with no treatment at this stage, all animals gave a normal response by expressing inflammation through swelling of their right planetary paws at the site of injection. As the observation progressed, a clear trend of increasing inhibition was seen for the various formulations as the observation progressed (Table 4). Notably, the emulsifier AMCana-Oil (20% TC) showed the highest inhibition percentages after 6 h of treatment (97.059% ± 4.81), closely followed by the emulsifier AMCana-Oil (10% TC) (94.706% ± 14.86). These results suggest a potential anti-inflammatory effect of the studied hemp oil functionalized with terpenocannabinoids, however, with higher concentrations showing stronger inhibitory effects. Compared with the positive control, Flammazine 1%, the emulsifiers showed competitive anti-inflammatory properties. In addition, the standard deviations (SDs) associated with inhibition percentages were generally within acceptable ranges, indicating consistent results across experimental groups. Measurements of the mean inflammation diameter (mm) also followed a similar pattern, confirming the inhibitory trends observed. These results suggest that formulated emulsifiers may possess anti-inflammatory properties, warranting further investigation for their potential applications in dermatological treatments.

Table 4.
Percentage inhibition of inflammation in mice of AMCana-Oil (0% TC) Emulsifier, AMCana-Oil (10% TC) Emulsifier, AMCana-Oil (20% TC) Emulsifier and Flammazine 1% (PC: Positive control); SD: standard deviation. Data are means ± confidence intervals (95%) from three replicates and values with different letters (a, b and c) are significantly different according to Tukey’s HSD test.
These outcomes resonate with prior research highlighting the anti-inflammatory attributes of hemp-derived CBD. Notably, investigations involving hemp oil have showcased CBD’s capacity to mitigate inflammation across diverse experimental models, such as arthritis, colitis, and neuroinflammation [44]. Previous studies [45,46] have pointed towards the intricate role of CBD in curbing inflammation, notably by interfering with the nuclear factor-kappa B (NF-κB) pathway. Previous studies have highlighted CBD’s complex role in fighting inflammation, including interfering with the nuclear factor kappa B (NF-κB) pathway. Relevantly, the ability of CBD to impede the NF-κB pathway both in vitro and in vivo, coupled with its potential to suppress inflammatory markers from keratinocytes and modulate their interaction with immune cells, positions it as a promising way to combat inflammatory skin conditions [46]. The emulsifier valued in this study is composed of CBD in particular and of terpenes and other cannabinoids and has demonstrated a very significant anti-inflammatory effect so the effect of the mixture does not influence the anti-inflammatory activity of CBD and may improve it by the entourage effect.
3.3.5. Antinociceptive Activity
The obtained result in our investigation highlights the noteworthy antinociceptive potential, as evidenced by both the acetic acid-induced pain (Table 5) and the formalin-induced pain (Table 6), exhibited by various hemp oil formulations. Particularly, the 10% and 20% concentrations reveal pronounced efficacy in mitigating pain responses. This result is further underscored by the notable inhibition percentages demonstrated by these formulations when compared to the positive control group (Flammazine 1% or Paracetamol 100 mg).

Table 5.
Results of antinociceptive activity produced by acetic acid, percentage of analgesic inhibition (%±SD), average number of spasms and percentage of analgesic inhibition (number ± SD), and Flammazine 1% (PC). Data are means ± confidence intervals (95%) from three replicates and values with different letters (b and c) are significantly different according to Tukey’s HSD test.

Table 6.
Results of antinociceptive activity produced by formalin, percentage of analgesic inhibition (%±SD), average number of spasms and percentage of analgesic inhibition (number ± SD), and Flammazine 1% (PC). Data are means ± confidence intervals (95%) from three replicates and values with different letters (a and c) are significantly different according to Tukey’s HSD test.
The studied emulsifier, derived from Cannabis plant seeds and powder, boasts a rich composition of bioactive constituents, encompassing cannabinoids (notably CBD), terpenes, and others. CBD, in particular, has garnered significant attention for its potential therapeutic attributes, including its analgesic properties. Extensive prior research has elucidated CBD’s intricate interactions with the endocannabinoid system (ECS) [47], a pivotal regulatory network governing various physiological processes, notably pain perception. By modulating ECS receptors, CBD may influence pain sensation and temper inflammatory responses, thereby manifesting potential analgesic effects [46].
The findings of our study align with the emerging body of evidence elucidating the potential of hemp oil formulations, especially those enriched with CBD, as promising candidates for addressing nociceptive sensations. These outcomes hold implications for the development of novel analgesic interventions, potentially providing an alternative avenue for managing pain-related conditions. Nevertheless, further investigations are imperative to comprehensively understand the mechanistic underpinnings and therapeutic implications of hemp-derived compounds in the context of pain management.
3.3.6. Molecular Docking
5-Lipoxygenase (5-LOX) is a crucial enzyme involved in the biosynthesis of leukotrienes, which are potent inflammatory mediators [48]. By inhibiting the activity of 5-LOX, the production of leukotrienes is decreased, leading to a reduction in inflammation. This mechanism is particularly relevant in the context of anti-inflammatory strategies and therapies. In our in silico study, Benzenepropanoic acid and Cannabichromene were the most active molecules against 5-lipoxygenase with a glide score of −5.715 and −4.906 kcal/mol, respectively (Table 7).

Table 7.
Docking results with ligands in different receptors.
Moreover, cyclooxygenase-2 (COX-2) is an enzyme responsible for producing prostaglandins, which are lipid molecules involved in various physiological processes, including inflammation and pain perception [49]. The inhibition of COX-2 is associated with antinociceptive activity by reducing the production of prostaglandins responsible for pain and inflammation. All fatty acids and active terpenes and cannabinoids detected in the AMCana-Oil (% TC) by GC-MS-MS (TQ) showed remarkable antinociceptive activity. Cannabidiol and Caryophyllene oxide were the most active molecules against COX-2 with a glide score of −8.243 and −7.816 kcal/mol (Table 7).
In wound healing, the Wnt/β-catenin signaling pathway is crucial and involves several proteins, including glycogen synthase kinase-3β (GSK-3β) and casein kinase-1 (CK1) [30]. Benzenepropanoic acid and delta-9-Tetrahydrocannabivarin were the most active molecules against Casein Kinase 1 with a glide score of −5.540 and −4.741 kcal/mol. While Cannabigerol and Cannabielsoin acid were the most active molecules against Glycogen synthase kinase 3 with a glide sore of −7.339 and −7.019 kcal/mol, respectively (Table 7).
The 2D and 3D interaction of Cannabigerol in the active site of Glycogen synthase kinase 3 showed the formation of a single hydrogen bond between the hydroxyl group and the VAL 135 residue.
Benzenepropanoic acid established a single hydrogen bond with the LYS 232 residue and salt bridged with the LYS 225 residue in the active site of Casein Kinase 1 (Figure 4 and Figure 5).
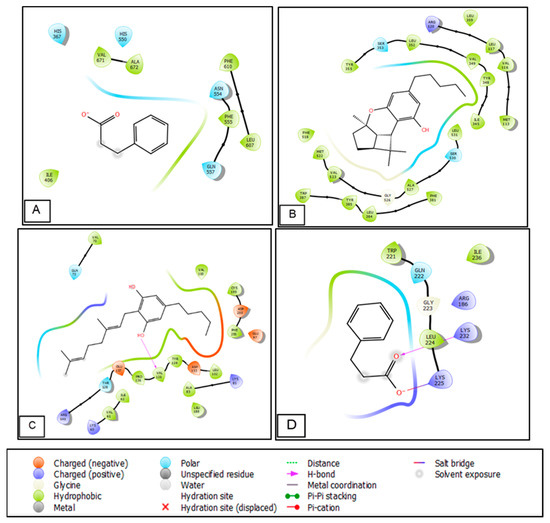
Figure 4.
Two-dimensional diagrams of ligands interactions with the active sites. (A) Benzenepropanoic acid interactions with the active site of 5-lipoxygenase. (B) Cannabidiol interactions with the active site of cycloxygenase-2. (C) Cannabigerol interactions with the active site of Glycogen synthase kinase 3. (D) Benzenepropanoic interactions with the active site of Casein Kinase 1.
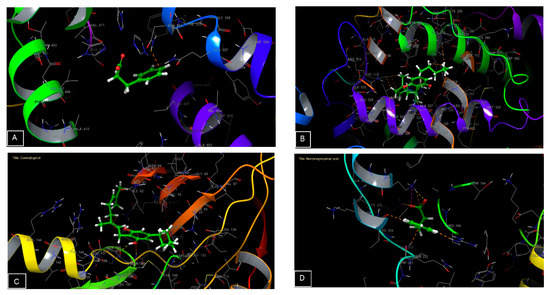
Figure 5.
Three-dimensional diagrams of ligands interactions with the active sites. (A) Benzenepropanoic acid interactions with the active site of 5-lipoxygenase. (B) Cannabidiol interactions with the active site of cycloxygenase-2. (C) Cannabigerol interactions with the active site of Glycogen synthase kinase 3. (D) Benzenepropanoic interactions with the active site of Casein Kinase 1.
The compounds identified in AMCana-Oil (% TC) emulsifier, underwent toxicity assessment using ProTox-II [50]. Following the globally standardized chemical labeling classification system (GHS), toxicity was categorized into six levels: Category 1: LD50 ≤ 5 (lethal if ingested); Category 2: 5 < LD50 ≤ 50 (lethal if swallowed); Category 3: 50 < LD50 ≤ 300 (toxic if ingested); Category 4: 300 < LD50 ≤ 2000 (harmful if ingested); Category 5: 2000 < LD50 ≤ 5000 (potential harm if ingested); Category 6: LD50 > 5000 (non-toxic).
Table 8 shows the predicted LD50 values of the compounds palmitic acid, stearic acid, oleamide, 4,5,7-tris(1,1-dimethylethyl)-3,4-dihydro-1,4-epoxynaphthalene-1(2H)- methanol, 7,9-di-tert-butyl-1-oxaspiro [4.5]deca-6,9-diene-2,8-dione, cannabidivarin, delta-9-Tetrahydrocannabivarin, cannabipinol, cannabidiol, cannabichromene, cannabicoumaronone, cannabielsoin acid, delta-9-tetrahydrocannabinol and cannabigerol ranging from 482 to 2000 mg/kg belong to toxicity class 4 according to the OECD guidelines for the testing of chemicals. While the LD50 of other molecules ranging from 2300 to 13,500 mg/kg belong to toxicity classes 5 and 6.

Table 8.
ADME properties and skin sensitization tests of fatty acids and active terpenes and cannabinoids detected in the terpenocannabinoid-functionalized hemp oil emulsifier (AMCana-Oil + % TC).
The predicted octanol/water partition coefficient (QPlogPo/w) is a measure used in chemistry and pharmacology to estimate the distribution of a chemical compound between an organic solvent (usually octanol) and water [51]. It is an indicator of a compound’s lipophilicity or hydrophobicity, which can provide insights into its solubility, absorption, distribution, and potential biological activity. All the molecules studied presented an acceptable value (acceptable range; −2.0 to 6.5) except Tris(2,4-di-tert-butylphenyl) phosphite presented a co-efficient of 13.24. This result is in agreement with other studies in which the octanol–water partition coefficient of phytocannabinoids also presented acceptable values [52].
The anticipated skin permeability, represented by log Kp, serves as a valuable pharmacokinetic parameter for evaluating the transdermal efficacy of ligands [53]. All compounds exhibited satisfactory values of skin permeability (expressed as Kp in cm/h) falling within the acceptable range (−80 to −1.0). This result is in agreement with a study where Cannabigerol, Cannabinol, and Cannabidivarin presented acceptable skin permeability values [54].
The Direct Peptide Reactivity Assay (DPRA) is an in vitro test method used to assess the skin sensitization potential of chemicals. It is an alternative method to animal testing, designed to predict the ability of a substance to cause allergic reactions when it comes into contact with the skin. Skin sensitization is an important aspect of toxicology, as it helps to identify potential allergens and irritants that could lead to adverse health effects in humans. Palmitic Acid, Stearic acid, Benzenepropanoic acid, Oleonitrile, Oleamide, and Monopalmitin showed no skin sensitization, while the other molecules showed positive sensitization. Abchi et al. (2023) [55] carried out a study that yielded results that aligned with our own investigation into the skin sensitization of some cannabinoids examined in this study, including delta-9-tetrahydrocannabinol, cannabinol, cannabidiol, cannabigerol, and tetrahydrocannabivarin.
The KeratinoSens assay focuses on assessing a chemical’s potential to cause skin sensitization by evaluating its interaction with a specific human-derived cell line, known as the KeratinoSens cell line. These cells are designed to mimic certain characteristics of human skin cells. Palmitic Acid, Stearic acid, Benzenepropanoic acid, Oleonitrile, Monopalmitin, Tris(2,4-di-tert-butylphenyl) phosphite, 7,9-Di-tert-butyl-1-oxaspiro [4.5]deca-6,9-diene -2,8-dione, Caryophylene oxide, Cannabidivarin, and Cannabidiol showed no skin sensitization, while the other molecules showed positive sensitization.
The primary aim of the h-CLAT assay is to evaluate the capability of a chemical to stimulate dendritic cells, which are immune cells found in the skin and hold a pivotal role in immune reactions. Palmitic Acid, Stearic acid, Monopalmitin, 4,5,7-Tris(1,1-dimethylethyl)-3,4-dihydro-1,4-epoxynaphthalene-1(2H)-methanol, and Cannabipinol showed no skin sensitization, while the other molecules presented a positive sensitization.
The Local Lymph Node Assay (LLNA) is an established method for assessing the potential of a substance to cause skin sensitization. Traditionally, this test has been conducted using animals, where the test substance is applied to the skin of mice, and changes in lymph nodes are observed. However, alternative methods have been developed to replace animal testing, and the Prediction LLNA refers to using these methods to predict skin sensitization potential. Using this test, palmitic acid, stearic acid, benzenepropanoic acid, oleamide, Monopalmitin, 4,5,7-Tris(1,1-dimethylethyl)-3,4-dihydro-1,4-epoxynaphthalene-1(2H)- methanol and 7,9-Di-tert-butyl-1-oxaspiro [4.5]deca-6,9-diene-2,8-dione showed no skin sensitization, while the other molecules showed positive sensitization.
Prediction HRIPT/HMT refers to an approach and risk assessment to anticipate the potential for skin irritation and skin corrosion caused by chemicals and substances. The terms “HRIPT” and “HMT” stand for “Human Repeat Insult Patch Test” and “Human Modified Draize Test,”, respectively. These tests are commonly used to evaluate the irritancy and corrosiveness of chemicals when they come into contact with the skin. Using this test, Palmitic Acid, Stearic acid, Benzenepropanoic acid, Oleonitrile, Oleamide, Monopalmitin, 4,5,7-Tris(1,1-dimethylethyl)-3,4-dihydro-1,4-epoxynaphthalene-1(2H)-methanol, and Cannabipinol showed no skin sensitization, while the other molecules showed positive sensitization.
4. Conclusions
In conclusion, this study presents a novel terpenocannabinoid-functionalized hemp oil emulsifier with promising potential for topical anti-inflammatory, antinociceptive, and wound healing applications. The intricate profile of active compounds, including fatty acids, bioactive compounds, and a diverse range of cannabinoids, underscores its potential therapeutic value. The physicochemical assessments highlight the stability and quality of the emulsifier while satisfying international standards. The emulsifier exhibits favorable physicochemical properties highlighting its fluidity and stability, and an average of peroxide values that requires meticulous handling and storage. Analysis reveals the presence of various cannabinoids, such as cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) along with bioactive compounds like benzenepropanoic acid and oleamide. Preliminary pharmacological assessments reveal significant antioxidant, anti-inflammatory, antinociceptive, and wound healing activities. Safety evaluations through in silico predictions confirm its suitability for therapeutic use. This original phyto-emulsifier emerges as a versatile tool that could revolutionize topical treatments, providing effective solutions for a range of inflammatory conditions, pain management, and skin health. Further research is warranted to explore mechanisms of action and clinical applications, enhancing the potential of these hemp-derived compounds in therapeutic and cosmetic formulations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92030036/s1.
Author Contributions
Conceptualization, N.E.B., S.E.K. and E.E.F.; methodology, A.M., H.Z. and N.E.B.; software, M.C. and Y.L.; validation, N.E.B., S.E.K., H.E.A., D.B. and E.E.F.; formal analysis, A.M., H.Z. and D.B.; investigation, A.M., H.Z. and Y.L.; data curation, A.M. and N.E.B.; writing—original draft preparation, A.M. and N.E.B.; writing—review and editing, N.E.B., S.E.K., H.E.A., D.B. and E.E.F.; visualization, N.E.B., S.E.K., H.E.A., D.B. and E.E.F.; supervision, N.E.B. and S.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The institutional ethical committee of care and use of the laboratory animals at the Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdallah Fez University, Morocco, reviewed and approved the present study #04/2019/LBEAS. The care and the use of animals were subjected to the institutional ethical committee according to the directive EEC/86/EEC of the European community (Union, 1986).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to express our sincere gratitude to Euromed University of Fes and University Sidi Mohamed Ben Abdellah of Fez, for their invaluable support and resources provided during the course of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anwar, F.; Latif, S.; Ashraf, M. Analytical Characterization of Hemp (Cannabis sativa) Seed Oil from Different Agro-Ecological Zones of Pakistan. J. Am. Oil Chem. Soc. 2006, 83, 323–329. [Google Scholar] [CrossRef]
- Kocis, P.T.; Vrana, K.E. Delta-9-Tetrahydrocannabinol and Cannabidiol Drug-Drug Interactions. Med. Cannabis Cannabinoids 2020, 3, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Borea, P.A.; Varani, K. Medicinal Chemistry, Pharmacology, and Potential Therapeutic Benefits of Cannabinoid CB2 Receptor Agonists. Chem. Rev. 2016, 116, 519–560. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How Does Cannabidiol (CBD) Influence the Acute Effects of Delta-9-Tetrahydrocannabinol (THC) in Humans? A Systematic Review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Kildaci, I.; Budama-Kilinc, Y.; Kecel-Gunduz, S.; Altuntaş, E. Linseed Oil Nanoemulsions for Treatment of Atopic Dermatitis Disease: Formulation, Characterization, In Vitro and In Silico Evaluations. J. Drug Deliv. Sci. Technol. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and Applications of Particle Stabilized Emulsions in Cosmetic Formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Bagul, V.; Gajbhiye, S. Hemp Seed Oil For Skin Care (Non-Drug Cannabis sativa L.): A Review. World J. Pharm. Res. 2020, 9, 203–210. [Google Scholar]
- Sarkar, A.K.; Sadhukhan, S. Role of Cannabis sativa L. in the Cosmetic Industry: Opportunities and Challenges. In Cannabis sativa Cultivation, Production, and Applications in Pharmaceuticals and Cosmetics. IGI Glob. 2023, 1, 81–100. [Google Scholar]
- Heinrich, K.; Heinrich, U.; Tronnier, H. Influence of Different Cosmetic Formulations on the Human Skin Barrier. Skin Pharmacol. Physiol. 2014, 27, 141–147. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef]
- Petersen, P.E. World Health Organization, ALINORM 68/11, (1967). Community Dent. Orl Epidemiol. 2003, 31, 471. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 3-25. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=111542 (accessed on 7 August 2023).
- Kuselman, I.; Shenhar, A. Uncertainty in Chemical Analysis and Validation of the Analytical Method: Acid Value Determination in Oils. Accredit. Qual. Assur. 1997, 2, 180–185. [Google Scholar] [CrossRef]
- NF T 60 220 Afnor EDITIONS. Available online: https://www.boutique.afnor.org/fr-fr/resultats?Keywords=huile+v%C3%A9g%C3%A9tale+indice+peroxyde&StandardStateIds=1 (accessed on 9 August 2023).
- AOCS CD 8b—90 Peroxido. Available online: https://toaz.info/doc-view-2 (accessed on 7 August 2023).
- Amending Regilation (EEC), N° 2568/91. Available online: https://eur-lex.europa.eu/eli/reg/1991/3682/oj/eng (accessed on 7 August 2023).
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive Quality Evaluation of Medical Cannabis sativa L. Inflorescence and Macerated Oils Based on HS-SPME Coupled to GC–MS and LC-HRMS (q-Exactive Orbitrap®) Approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Ali, V.; Khajuria, M.; Faiz, S.; Gairola, S.; Vyas, D. GC–MS Based Metabolomic Approach to Understand Nutraceutical Potential of Cannabis Seeds from Two Different Environments. Food. Chem. 2021, 339, 128076. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. In Vitro Antioxidant Activities of the Methanol Extracts of Five Allium Species from Turkey. Food. Chem. 2005, 92, 89–92. [Google Scholar] [CrossRef]
- Cando, D.; Morcuende, D.; Utrera, M.; Estévez, M. Phenolic-Rich Extracts from Willowherb (Epilobium hirsutum L.) Inhibit Lipid Oxidation but Accelerate Protein Carbonylation and Discoloration of Beef Patties. J. Eur. Food Res. Technol. 2014, 238, 741–751. [Google Scholar] [CrossRef]
- Mašković, P.Z.; Manojlović, N.T.; Mandić, A.I.; Mišan, A.Č.; Milovanović, I.L.; Radojković, M.M.; Cvijović, M.S.; Solujić, S.R. Phytochemical Screening and Biological Activity of Extracts of Plant Species Halacsya Sendtneri (Boiss.) Dörfl. Hem. Ind. 2012, 66, 43–51. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant Activity of Smilax excelsa L. Leaf Extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-Inflammatory and Analgesic Properties of the Leaf Extracts and Essential Oil of Lavandula Angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Morton, J.J.; Malone, M.H. Evaluation of Vulneray Activity by an Open Wound Procedure in Rats. Arch. Int. Pharmacodyn. Ther. 1972, 196, 117–126. [Google Scholar] [PubMed]
- OECD. Test No. 410: Repeated Dose Dermal Toxicity: 21/28-Day Study; Organisation for Economic Co-Operation and Development: Paris, France, 1981. [Google Scholar]
- Vanhulle, V.P.; Martiat, G.A.; Verbeeck, R.K.; Horsmans, Y.; Calderon, P.B.; Eeckhoudt, S.L.; Taper, H.S.; Delzenne, N. Cryopreservation of Rat Precision-Cut Liver Slices by Ultrarapid Freezing: Influence on Phase I and II Metabolism and on Cell Viability upon Incubation for 24 Hours. Life Sci. 2001, 68, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.M.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M.; et al. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea Calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Slighoua, M.; Mssillou, I.; Chebaibi, M.; Galvão de Azevedo, R.; Boukhira, S.; Moslova, K.; Al Kamaly, O.; Saleh, A.; Correa de Oliveira, A.; et al. Lipids Fraction from Caralluma Europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects. Separations 2023, 10, 172. [Google Scholar] [CrossRef]
- Slighoua, M.; Chebaibi, M.; Mahdi, I.; Amrati, F.E.; Conte, R.; Cordero, M.A.W.; Alotaibi, A.; Saghrouchni, H.; Agour, A.; Zair, T.; et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula Officinalis Chaix: In Vivo and In Silico Approaches. Plants 2022, 11, 3222. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Chebaibi, M.; Galvão de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma Europaea Extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Tripathi, P.; Talukdar, P.; Talapatra, S.N. In Silico Study by Using ProTox-II Webserver for Oral Acute Toxicity, Organ Toxicity, Immunotoxicity, Genetic Toxicity Endpoints, Nuclear Receptor Signalling and Stress Response Pathways of Synthetic Pyrethroids. World Sci. News 2019, 132, 35–51. [Google Scholar]
- Mahnashi, M.H.; Alshahrani, M.A.; Nahari, M.H.; Hassan, S.S.U.; Jan, M.S.; Ayaz, M.; Ullah, F.; Alshehri, O.M.; Alshehri, M.A.; Rashid, U.; et al. In-Vitro, In-Vivo, Molecular Docking and ADMET Studies of 2-Substituted 3,7-Dihydroxy-4H-Chromen-4-One for Oxidative Stress, Inflammation and Alzheimer’s Disease. Metabolites 2022, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations, Codex Stan 210e. 2015. Available online: https://www.fao.org/input/download/standards/336/CXS_210e_2015.pdf (accessed on 10 June 2024).
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The Physicochemical Properties of Five Vegetable Oils Exposed at High Temperature for a Short-Time-Interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Hemp Seed Oil: A Source of Valuable Essential Fatty Acids. Available online: http://www.internationalhempassociation.org/jiha/iha03101.html (accessed on 5 December 2020).
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceutic Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- Vieira, J.E.; Abreu, L.C.; Valle, J.R. On the Pharmacology of the Hemp Seed Oil. Pharmacology 1967, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Ghacham, S.E.; Bakali, I.E.; Zarouki, M.A.; Ali, Y.A.E.H.; Ismaili, R.; Ayadi, A.E.; Souhail, B.; Tamegart, L.; Azzouz, A. Wound Healing Efficacy of Cannabis sativa L. Essential Oil in a Mouse Incisional Wound Model: A Possible Link with Stress and Anxiety. S. Afr. J. Bot 2023, 163, 488–496. [Google Scholar] [CrossRef]
- Klein, M.; De Quadros De Bortolli, J.; Guimarães, F.S.; Salum, F.G.; Cherubini, K.; De Figueiredo, M.A.Z. Effects of Cannabidiol, a Cannabis sativa Constituent, on Oral Wound Healing Process in Rats: Clinical and Histological Evaluation. Phytother. Res. 2018, 32, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.U.; Baum, C.R. Acute Cannabis Toxicity. Pediatr. Emerg. Care 2019, 35, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Yassa, H.A.; Dawood, A.E.W.A.; Shehata, M.M.; Abdel-Hady, R.H.; Abdel Aal, K.M. Subchronic Toxicity of Cannabis Leaves on Male Albino Rats. Hum. Exp. Toxicol. 2010, 29, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and Anti-Inflammatory Effect of Cannabidiol Contributes to the Decreased Lipid Peroxidation of Keratinocytes of Rat Skin Exposed to UV Radiation. Oxid. Med. Cell. Longev. 2021, 2021, 6647222. [Google Scholar] [CrossRef]
- Bhamra, S.K.; Desai, A.; Imani-Berendjestanki, P.; Horgan, M. The Emerging Role of Cannabidiol (CBD) Products; a Survey Exploring the Public’s Use and Perceptions of CBD. Phytother. Res. 2021, 35, 5734–5740. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. Extract and Cannabidiol Inhibit in Vitro Mediators of Skin Inflammation and Wound Injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Marzo, V.D. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Haeggström, J.Z. Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Investig. 2018, 128, 2680–2690. [Google Scholar] [CrossRef]
- Seibert, K.; Zhang, Y.; Leahy, K.; Hauser, S.; Masferrer, J.; Perkins, W.; Lee, L.; Isakson, P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA 1994, 91, 12013–12017. [Google Scholar] [CrossRef]
- Banerjee, P.; Ulker, O.C. Combinative ex vivo studies and in silico models ProTox-II for investigating the toxicity of chemicals used mainly in cosmetic products. Toxicol. Mech. Methods 2022, 32, 542–548. [Google Scholar] [CrossRef]
- Ogata, K.; Hatakeyama, M.; Nakamura, S. Effect of atomic charges on octanol—Water partition coefficient using alchemical free energy calculation. Molecules 2018, 23, 425. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Pecoraro, B.; Marco, T.; Ewelina, H.; Victoria, H.; Anna, M.A.; Matthew, T. Predicting skin permeability by means of computational approaches: Reliability and caveats in pharmaceutical studies. J. Chem. Inf. Model 2019, 59, 1759–1771. [Google Scholar] [CrossRef]
- Akanji, T. An in vitro Investigation of the Stability and Permeability of Phytocannabinoids for Skin Care Formulations. Master’s Thesis, University of Rhode Island, Kingston, RI, USA, 2021. [Google Scholar]
- Abchir, O.; Daoui, O.; Nour, H.; Yamari, I.; Elkhattabi, S.; Errougui, A.; Chtita, S. Exploration of Cannabis constituents as potential candidates against diabetes mellitus disease using molecular docking, dynamics simulations and ADMET investigations. Sci. Afr. 2023, 21, 1745. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).