Abstract
Influenza poses a significant threat to public health worldwide, particularly among vulnerable populations such as children, the elderly, immunocompromised individuals, and those with chronic diseases. It is associated with high mortality and morbidity rates. Neuraminidase inhibitors play a crucial role in influenza treatment by mitigating the risk of complications and death. However, the genetic variability of the influenza virus enables the emergence of drug-resistant mutations. This review focuses on the search for new compounds that are not analogous to sialic acid, aiming to inhibit the activity of viral neuraminidase in vitro, viral replication in cell cultures, or animal models. Influenza virus strains that have been reported in the literature present specific mutations that generate resistance to neuraminidase inhibitors. Since these inhibitors bear structural resemblance to sialic acid, the predominant location for these mutations is the enzyme’s active site. Consequently, exploring alternative compound classes becomes imperative to circumvent this interaction pattern. These compounds will introduce diverse molecular frameworks, serving as foundational structures for further development through rational drug design, thereby engendering novel antiviral agents targeting influenza. The potential prospects for developing novel influenza antivirals based on these findings are discussed.
1. Introduction
Influenza is an acute respiratory infection caused by a negative-strand RNA virus belonging to the Orthomyxoviridae family [1]. Influenza viruses are highly contagious, and symptoms can be mild or severe. Certain populations, such as children, individuals with chronic diseases, immunocompromised individuals, pregnant women, and the elderly, are more susceptible to experiencing higher morbidity and mortality rates [2]. According to the World Health Organization (WHO), the annual incidence of influenza virus infections ranges from 3 to 5 million, resulting in 290,000 to 650,000 deaths worldwide [3].
Influenza is categorized into four types: A, B, C, and D. In terms of human health, influenza A and B viruses are the most significant, causing annual epidemics. Influenza A viruses have the potential to cause pandemics, with some outbreaks resulting in millions of deaths. Influenza A viruses are further classified into subtypes based on the antigenic reactivity of their two surface proteins: hemagglutinin (HA) and neuraminidase (NA). There are 18 different hemagglutinin subtypes (H1–H18) and 11 different neuraminidase subtypes (N1-N11). On the other hand, influenza B viruses are not subdivided into subtypes but are classified into two lineages: Yamagata and Victoria [4].
Vaccination plays a crucial role in preventing influenza; however, antiviral medications are essential for the treatment of established influenza infections. The timely administration of antivirals can shorten the duration of the illness, reduce hospitalization rates, and prevent complications and death [5,6].
Several viral proteins have served as primary targets for the development of antiviral drugs. The M2 protein has been targeted by drugs such as amantadine and rimantadine (adamantanes), although their use has declined due to widespread resistance among circulating influenza viruses. The neuraminidase protein (NA) has been targeted by drugs like oseltamivir and zanamivir, which are still in use. More recently, drugs targeting components of viral RNA polymerase, such as baloxavir marboxil (targeting the polymerase acidic domain—PA), favipiravir, and pimodivir, have been approved. However, adamantanes are no longer recommended because most circulating influenza viruses are resistant, while NA and PA inhibitors are still used, but there are different proportions of viruses in populations that show resistance or low sensitivity [7,8].
Due to the emergence of resistance, there is a need to search for new drugs for influenza treatment. While there have been numerous investigations reporting potential new molecules, most of them are still in the preclinical stage and their effectiveness as drugs has not been fully established.
The main neuraminidase inhibitors are designed as sialic acid analogs [8]. However, there is ample evidence indicating the inhibitory activity of non-analog molecules to sialic acid [9,10]. These compounds present a potential advantage, as these non-analog inhibitors may not face the same limitations regarding resistance observed with sialic acid analogs [11]. Nevertheless, this hypothesis needs to be tested once non-sialic acid analogs are widely used.
For these reasons, the objective of the present study was to conduct a systematic review to gather information on molecules with evidence of in vitro inhibitory activity against influenza virus neuraminidase, as reported in peer-reviewed articles. In this article, we provide a summary of the data on the most promising molecules, which could serve as a starting point for their further development and application in the field of antiviral therapy against influenza.
2. Materials and Methods
2.1. Systematic Review
A comprehensive search for full-text articles was conducted on PubMed (https://www.ncbi.nlm.nih.gov/pubmed; last access date was 10 December 2021) to identify articles that reported molecules with neuraminidase inhibition activity against any type or subtype of influenza virus. The search terms used as keywords were “neuraminidase”, “influenza A”, and “inhibitors”. Only studies published in English were included in the review. The selection of relevant articles was based on the following criteria: original articles published from January 2015 to June 2021; the inclusion of information stating that the studied molecules were not sialic acid analogs and were of high purity; the presentation of protocols for neuraminidase inhibition analysis using influenza A viral neuraminidase; and reported values of the inhibitory concentration 50% (IC50) below 100 µM.
2.2. Data Analysis
Non-original or incomplete articles were excluded from the analysis. The database search, study screening, and primary data analysis were independently conducted by three coauthors. Any discrepancies or inconsistencies that arose during the process were resolved through group discussions, leading to a final consensus on the data. The information extracted from the selected articles was categorized and organized based on the publication year, with articles arranged in descending order. A table was created, including the article name, author, compound type, IUPAC name, compound ID, structure, subtype or strain of the studied influenza virus, and values of IC50 (inhibitory concentration 50%), Ki (inhibition constant), CC50 (50% cytotoxic concentration), selectivity index (SI), or EC50 (50% effective concentration). The table can be found in the Supplementary Materials.
3. Results
Description of Included Studies
Initially, a total of 992 articles were identified through an original search conducted using the specified keywords as described in the methodology. After removing duplicates and applying the selection criteria, 31 articles were deemed relevant for extracting the desired information. To provide a visual representation of the article selection process, a flow chart was constructed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. Figure 1 and Table 1 present a summary of the number of articles included and discarded at each stage of the analysis process.
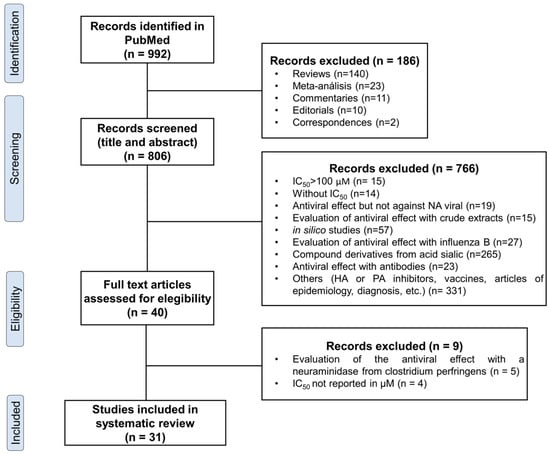
Figure 1.
Flow diagram of literature search and study selection.

Table 1.
Selected active compounds with neuraminidase inhibition activity and in vitro/in vivo infection inhibition.
Among the 31 selected articles, a total of 192 compounds were identified as inhibitors of viral neuraminidase (NA) activity at concentrations below 100 μM. Notably, seven of these compounds exhibited nanomolar potency, with IC50 values ranging from 20 nM to 100 nM. It is noteworthy that the order of magnitude in which these molecules exhibit their activity is in the nanomolar range, similar to what has been observed for oseltamivir and zanamivir, which are currently used as antivirals. Among the selected compounds, four belong to the dihydrofurocoumarin derivative class, one is a diazenylaryl sulfonic acid compound, and two are polyphenolic compounds (Figure 2 and Figure 3, Table 1). Additionally, we identified 11 compounds that exhibited significant inhibitory activity against viral replication in vitro. Out of these, 10 compounds demonstrated an EC50 below 0.1 μM, while the remaining compound had an EC50 of 0.2 μM. Furthermore, three compounds underwent testing in animal experiments, indicating their potential for further development (Figure 2, Table 1). In the studies involving the most promising compounds (Table 1), the majority centered on the human serotype H1N1, with only one compound tested against H3N2. Compound 114 was specifically tested in vitro against different mutants of the H1N1 subtype that are known to exhibit resistance to oseltamivir and zanamivir. It is important to note that there is no available evidence on the selected compounds regarding non-human viral subtypes, such as avian or porcine strains, at nanomolar (nM) concentrations.
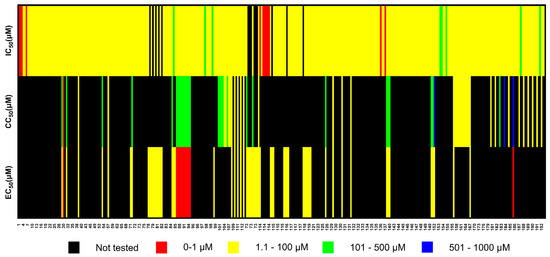
Figure 2.
Heat map of influenza virus inhibitors reported in 31 selected articles. The numbers on the x-axis correspond to the numeration of all compounds, which can be found in Supplementary Table S1. It is important to note that some compounds may appear more than once on the heat map, depending on whether they were tested with different viral subtypes or if the same compound was tested in different studies. IC50: half maximal inhibitory concentration, CC50: half maximal cytotoxic concentration, EC50: half maximal effective concentration.
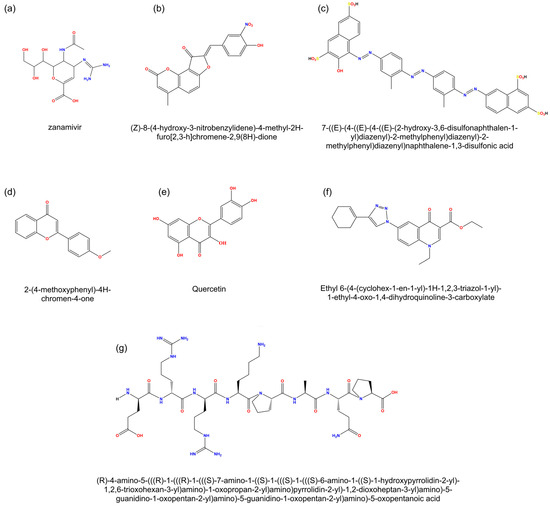
Figure 3.
Molecular structures of selected compounds. (a) zanamivir, a commercial antiviral; (b,c) compounds selected for their high inhibitory capacity in vitro (IC50 < 1 μM); (d–f) compounds selected for their inhibitory activity against viral replication (EC50 < 0.1 μM); (g) compound select for in vivo studies.
4. Discussion
4.1. Compounds with IC50 < 1 μM In Vitro
Neuraminidase inhibitors (NAIs) play a crucial role in preventing the release and spread of viral progeny by blocking the function of the neuraminidase enzyme. These inhibitors are designed to mimic the structure of the viral substrate, sialic acid. In vitro evaluation of NA sensitivity to NAIs is commonly performed using enzyme inhibition tests with fluorescent or chemiluminescent substrates [12,13]. Determining the inhibitory concentration 50 (IC50) through these tests allows for the assessment of new compounds with inhibitory activity against viral neuraminidase. This methodology enables comparisons between different viral subtypes and strains, including those with mutations that reduce susceptibility to NAIs. Identifying compounds that can act on viruses with existing resistance to current antivirals is of particular importance [14,15,16].
It is important to evaluate the functionality of the compounds mentioned with both group 1 (N1, N4, N5, and N8) and group 2 (N2, N3, N6, N7, and N9) neuraminidase (NA) subtypes. Group 1 NA presents a cavity within the active site called “cavity 150”, while in group 2, NA is absent. This conformational difference affects the susceptibility of the compounds towards NA. Most of the selected compounds were analyzed only with H1N1 subtypes, and it is unknown if they also affect N2 subtypes. Another adjacent cavity in the active site, cavity 430, has also been identified [17,18,19]. Both cavities have been used as anchoring sites for relatively large compounds, as analyzed in Table 1, which prevent or modify the interaction of the substrate with the active site. Therefore, these compounds have certain characteristics that enable them to modify or prevent access to the active site of the substrate, such as steric effects, functional groups for electrostatic interactions, and the ability to form hydrogen bonds or hydrophobic interactions with amino acid residues within the active site or in cavities 430 or 150 in group 1 NA. These interactions stabilize the compound–NA interaction, achieving the objective of inhibiting enzymatic activity [20].
Only compound 114 was evaluated in viruses with characteristic neuraminidase mutations, such as H274Y, N294S, Q136L, or I427Q/M. These mutations are known to result in reduced susceptibility to NAIs, especially oseltamivir [21]. It was observed that the compound maintained its inhibitory capacity regardless of the presence of these mutations. This is because most of the mutations are found within the active site and the scaffold, so the strategy of using cavities 150 and 430, away from the active site, to anchor the compound allows it to circumvent such viral defense mechanisms.
4.2. Compounds with EC50 < 1 μM In Vitro
Ten of the eleven compounds were described by Chintakrindi et al. in 2019, of which six are derived from flavones (ID 86–91) and three from aurones (ID 92–94), while the tenth is quercetin (ID 95), a polyphenolic flavonoid. These compounds demonstrated the inhibition of both NA activity in vitro (with IC50 values ranging from 7.75 to 45.36 μM) and the replication of the influenza A/Pune/2009 (H1N1) virus. The inhibition of viral replication was determined by observing reduced activity of viral hemagglutinin (HA) [22].
The eleventh compound, described by Boechat et al. (2015), was an oxoquinoline derivative. This compound exhibited activity against the NA of influenza viruses A/WA/10/2008 WT (H1N1) and A/WA/01/2007 WT (H3N2). Importantly, it also demonstrated inhibitory effects against the NA of two oseltamivir-resistant strains of the virus: A/TX/12/2007 E119V (H3N2) and A/FL/21/2008 H275Y (H1N1). The compound displayed IC50 values ranging from 2.6 to 19.9 μM for the tested strains. Furthermore, the compound exhibited an EC50 value of 0.20 ± 0.01 μM for inhibiting viral replication, along with a selectivity index of 2830, indicating low toxicity in the cell culture. The specific viral strain used in this study was not specified [23].
The mentioned 11 compounds examined exhibit lower EC50 values (inhibition of viral replication) than IC50 values (viral NA inhibition), indicating that these compounds possess additional mechanisms of inhibition against the influenza virus beyond NA inhibition. Given that hemagglutinin and neuraminidase both recognize sialic acid as their ligand, certain molecules have been identified to inhibit both glycoproteins. These compounds not only decrease viral replication by blocking the release of virions through neuraminidase inhibition but also impede virion entry into target cells by preventing hemagglutinin binding to sialic acid. Furthermore, multi-target compounds such as epigallocatechin-3-O-gallate (EGCG) have been discovered, capable of inhibiting the activity of neuraminidase, hemagglutinin, and RNA-dependent RNA polymerase concurrently [24,25,26].
Quercetin is a compound previously known for its inhibitory properties against influenza virus. It has been shown to not only directly inhibit viral NA in a noncompetitive manner [27] but also affect viral replication through other pathways, particularly in the initial stages [22,28]. One proposed mechanism suggests that quercetin can interact with viral hemagglutinin (HA) and inhibit viral–cell fusion [27]. Furthermore, other compounds included in this review, such as the derivatives of quercetin, flavonoids, and polyphenols, have been described to possess additional important properties, including antioxidant and anti-inflammatory activities, alongside their antiviral activity [29,30,31].
For some compounds included in the works analyzed in this review, clinical studies have been carried out in groups of people with different conditions, in which the pharmacokinetics and pharmacodynamics as well as the effectiveness of those compounds and their main metabolites have been studied. Such is the case of quercetin, which, at doses of 1000 mg/day for 12 weeks, did not induce significant side effects and was able to reduce the time and severity of illness in a group of low-risk people with upper respiratory tract infection; curiously, this effect was not observed in the group of patients with high risk [32]. The above invites us to continue studying this type of compound and their mechanisms of action in clinical studies, especially under different treatment and dosage schemes, to understand and improve their pharmacological effects. A relevant area of opportunity is to improve the formulation to increase the bioavailability of the compounds. In the case of quercetin, the design of a formulation administered orally has been achieved that facilitates obtaining plasma levels up to 20 times higher in humans and without notable side effects, thus facilitating the effective use of this flavonoid to treat various diseases in humans [33].
When designing a drug against influenza, it is crucial to consider not only the ability of the molecule to inhibit NA and viral replication but also its cytotoxicity. The 11 compounds discussed in this section have relatively high CC50 values (ranging from 115.4 to 566 μM), comparable to the CC50 of oseltamivir (321 μM) [23]. However, it is important to note that toxicity may vary in an in vivo model, and the antiviral and anti-inflammatory effects may also differ. Therefore, further research is necessary for these compounds, and exploring the derivatives or analogs of these molecules could help identify compounds with enhanced antiviral potential and reduced toxicity.
In the field of flavonoids, Liu et al. (2008) compared the effects of various types of flavonoids (aurones, flavones, isoflavones, flavanones, and flavanes) and identified aurones and flavones as the compounds with the most significant inhibitory effects against the influenza virus [34]. Furthermore, Chintakrindi et al., supported by bioinformatic analysis, found that among the studied flavones, para-substitution on the phenyl ring was preferred over meta-substitution for H1N1-NA inhibition. They also observed that methoxy substitution on the phenyl rings of flavone and aurone was favored over nitro and chloro substitution for H1N1-NA inhibition. These modifications enable the compounds to bind to cavities 150 or 430 in the active site of group 1 NAs, as discussed in the in vitro IC50 analysis [22]. In other hand, Boechat et al. found that the 4-oxoquinoline moiety is important for viral NA inhibition in the studied oxoquinoline derivatives, while the triazole ring and cyclohexenyl radical are required for antiviral activity [23].
4.3. In Vivo Studies
Out of the initial 192 molecules included in the search, only three molecules have progressed to being studied in murine models. Two of these molecules, puerarin (ID 71) and chlorogenic acid (CHA; ID 73), were derived from plants, while the third molecule, the octapeptide errKPAQP (ID 79), was synthesized in the lab [35,36,37].
Puerarin was tested in a murine model of lethal infection with H1N1 influenza virus, and it demonstrated up to 70% protection when administered at a dosage of 200 mg/kg/day. Puerarin prolonged the survival rate and improved the lung index, showing a similar effect to oseltamivir (10 mg/kg/day). Puerarin is hypothesized to act as a neuraminidase (NA) blocker, inhibiting IAV (influenza A virus) in both cellular and animal models, and it may also play a role in antiviral activity following virus adsorption [35].
On the other hand, CHA (administered at a dosage of 100 mg/kg/day) was evaluated in a murine model infected with influenza A/PuertoRico/8/1934 (H1N1) and A/Beijing/32/92 (H3N2) viruses. The treatment with CHA induced up to 60% and 50% survival rates, respectively, protecting mice from weight loss, inflammation, and lung tissue damage caused by influenza virus infection. Comparatively, oseltamivir at the same concentration of 100 mg/kg/day yielded survival rates of 70% against both viruses. CHA also exhibited the ability to inhibit in vitro NA activity, particularly against N1, and it decreased the secretion of IL-6 and TNF-α induced by influenza virus infection [36].
The third molecule, the peptide errKPAQP (capital letters represent the natural L-amino acids, while lowercase letters represent the unnatural D-amino acids), was designed to replicate interactions observed between oseltamivir and viral neuraminidase. This peptide (administered at a dosage of 1 mg/kg/day) showed promising results in infected mice, providing 90% protection against death, similar to oseltamivir (50 mg/kg/day). The peptide also led to weight recovery in infected mice, restored lung structure to normal, and reduced the secretion of proinflammatory cytokines IL-6, TNF-α, and IFN-γ four days after infection. Biocompatibility tests demonstrated that errKPAQP exhibited low hemolytic activity, minimal cytotoxicity, and good pharmacokinetic characteristics, such as stability in serum and cellular uptake. These findings indicate that the errKPAQP peptide has the potential to be used as a therapeutic agent for influenza virus infection in vivo [37].
In vivo studies allow for the evaluation of the global or systemic effects of compounds. While some molecules may inhibit influenza virus replication in vitro and in vivo, they can also have direct effects on the biological system being evaluated. These molecules may possess additional properties, such as antioxidant, anti-inflammatory, antiapoptotic, and anti-autophagic activities, among others. These properties can influence the overall outcome in an in vivo model.
5. Conclusions
Currently, the effectiveness of antivirals against the influenza virus is compromised due to its genetic variability, resulting in biochemical and pathogenic changes. This poses a significant challenge in controlling the disease on a global scale. The emergence of resistance to NA inhibitors, such as oseltamivir and zanamivir, is particularly concerning as it renders compounds that are similar or derived from these ineffective against the mutated strains. Therefore, there is a pressing need to identify suitable candidates that utilize alternative mechanisms to bypass these mutations and exhibit efficacy at low concentrations, while minimizing or eliminating toxic effects. Additionally, these candidates should demonstrate favorable pharmacokinetic characteristics. Although this task may seem daunting, the use of bioinformatic tools can facilitate the process by integrating experimental data and guiding the modification or generation of new derivatives that possess these desired attributes, similar to the case of the errKPAQP peptide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92020033/s1, Table S1: Non-Analog Compounds to sialic acid as Inhibitors of Influenza Virus Neuraminidase.
Author Contributions
Conceptualization, L.M.-D. and G.S.-L.; writing—original draft preparation, L.M.-D., C.J.-M., V.S.-M. and G.S.-L.; writing—review and editing, L.M.-D., C.J.-M., V.S.-M. and G.S.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar]
- Keilman, L.J. Seasonal Influenza (Flu). Nurs. Clin. N. Am. 2019, 54, 227–243. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Katzen, J.; Kohn, R.; Houk, J.L.; Ison, M.G. Early Oseltamivir After Hospital Admission Is Associated With Shortened Hospitalization: A 5-Year Analysis of Oseltamivir Timing and Clinical Outcomes. Clin. Infect. Dis. 2019, 69, 52–58. [Google Scholar] [CrossRef]
- Campbell, A.P.; Tokars, J.I.; Reynolds, S.; Garg, S.; Kirley, P.D.; Miller, L.; Yousey-Hindes, K.; Anderson, E.J.; Oni, O.; Monroe, M.; et al. Influenza Antiviral Treatment and Length of Stay. Pediatrics 2021, 148, e2021050417. [Google Scholar] [CrossRef]
- Takashita, E. Influenza Polymerase Inhibitors: Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2021, 11, a038687. [Google Scholar] [CrossRef]
- Holmes, E.C.; Hurt, A.C.; Dobbie, Z.; Clinch, B.; Oxford, J.S.; Piedra, P.A. Understanding the Impact of Resistance to Influenza Antivirals. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef]
- Grienke, U.; Schmidtke, M.; von Grafenstein, S.; Kirchmair, J.; Liedl, K.R.; Rollinger, J.M. Influenza neuraminidase: A druggable target for natural products. Nat. Prod. Rep. 2012, 29, 11–36. [Google Scholar] [CrossRef]
- Yu, G.; Fang, D. Evaluation of Neuraminidase Inhibitory Activity of Compounds and Extracts from Traditional Medicines by HPLC-FLD. Int. J. Anal. Chem. 2021, 2021, 6694771. [Google Scholar] [CrossRef]
- Marquez-Dominguez, L.; Reyes-Leyva, J.; Herrera-Camacho, I.; Santos-Lopez, G.; Scior, T. Five Novel Non-Sialic Acid-Like Scaffolds Inhibit In Vitro H1N1 and H5N2 Neuraminidase Activity of Influenza a Virus. Molecules 2020, 25, 4248. [Google Scholar] [CrossRef]
- Potier, M.; Mameli, L.; Belisle, M.; Dallaire, L.; Melancon, S.B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal. Biochem. 1979, 94, 287–296. [Google Scholar] [CrossRef]
- Buxton, R.C.; Edwards, B.; Juo, R.R.; Voyta, J.C.; Tisdale, M.; Bethell, R.C. Development of a sensitive chemiluminescent neuraminidase assay for the determination of influenza virus susceptibility to zanamivir. Anal. Biochem. 2000, 280, 291–300. [Google Scholar] [CrossRef]
- Zambon, M.; Hayden, F.G.; Global Neuraminidase Inhibitor Susceptibility, N. Position statement: Global neuraminidase inhibitor susceptibility network. Antivir. Res. 2001, 49, 147–156. [Google Scholar] [CrossRef]
- Lackenby, A.; Besselaar, T.G.; Daniels, R.S.; Fry, A.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Leang, S.K.; Lee, R.T.C.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors and status of novel antivirals, 2016-2017. Antivir. Res. 2018, 157, 38–46. [Google Scholar] [CrossRef]
- Marathe, B.M.; Leveque, V.; Klumpp, K.; Webster, R.G.; Govorkova, E.A. Determination of neuraminidase kinetic constants using whole influenza virus preparations and correction for spectroscopic interference by a fluorogenic substrate. PLoS ONE 2013, 8, e71401. [Google Scholar] [CrossRef]
- Russell, R.J.; Haire, L.F.; Stevens, D.J.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.J.; Skehel, J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef]
- von Itzstein, M. The war against influenza: Discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 2007, 6, 967–974. [Google Scholar] [CrossRef]
- Tao, J.; Wang, H.; Wang, W.; Mi, N.; Zhang, W.; Wen, Q.; Ouyang, J.; Liang, X.; Chen, M.; Guo, W.; et al. Binding mechanism of oseltamivir and influenza neuraminidase suggests perspectives for the design of new anti-influenza drugs. PLoS Comput. Biol. 2022, 18, e1010343. [Google Scholar] [CrossRef]
- Evteev, S.; Nilov, D.; Polenova, A.; Svedas, V. Bifunctional Inhibitors of Influenza Virus Neuraminidase: Molecular Design of a Sulfonamide Linker. Int. J. Mol. Sci. 2021, 22, 13112. [Google Scholar] [CrossRef]
- Hoffmann, A.; Richter, M.; von Grafenstein, S.; Walther, E.; Xu, Z.; Schumann, L.; Grienke, U.; Mair, C.E.; Kramer, C.; Rollinger, J.M.; et al. Discovery and Characterization of Diazenylaryl Sulfonic Acids as Inhibitors of Viral and Bacterial Neuraminidases. Front. Microbiol. 2017, 8, 205. [Google Scholar] [CrossRef]
- Chintakrindi, A.S.; Gohil, D.J.; Chowdhary, A.S.; Kanyalkar, M.A. Design, synthesis and biological evaluation of substituted flavones and aurones as potential anti-influenza agents. Bioorg. Med. Chem. 2020, 28, 115191. [Google Scholar] [CrossRef]
- Boechat Fda, C.; Sacramento, C.Q.; Cunha, A.C.; Sagrillo, F.S.; Nogueira, C.M.; Fintelman-Rodrigues, N.; Santos-Filho, O.; Riscado, C.S.; Forezi Lda, S.; Faro, L.V.; et al. 1,2,3-Triazolyl-4-oxoquinolines: A feasible beginning for promising chemical structures to inhibit oseltamivir-resistant influenza A and B viruses. Bioorg. Med. Chem. 2015, 23, 7777–7784. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.P.; Yu, Q.; Yang, M.B.; Wang, D.M.; Jia, T.W.; He, H.J.; He, Y.; Xiao, H.X.; Iyer, S.S.; et al. Multivalent S-sialoside protein conjugates block influenza hemagglutinin and neuraminidase. Carbohydr. Res. 2016, 435, 68–75. [Google Scholar] [CrossRef]
- Sacramento, C.Q.; Marttorelli, A.; Fintelman-Rodrigues, N.; de Freitas, C.S.; de Melo, G.R.; Rocha, M.E.; Kaiser, C.R.; Rodrigues, K.F.; da Costa, G.L.; Alves, C.M.; et al. Aureonitol, a Fungi-Derived Tetrahydrofuran, Inhibits Influenza Replication by Targeting Its Surface Glycoprotein Hemagglutinin. PLoS ONE 2015, 10, e0139236. [Google Scholar] [CrossRef]
- da Silva-Junior, E.F.; Silva, L.R. Multi-target Approaches of Epigallocatechin-3-O-gallate (EGCG) and its Derivatives against Influenza Viruses. Curr. Top. Med. Chem. 2022, 22, 1485–1500. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Mehrbod, P.; Abdalla, M.A.; Fotouhi, F.; Heidarzadeh, M.; Aro, A.O.; Eloff, J.N.; McGaw, L.J.; Fasina, F.O. Immunomodulatory properties of quercetin-3-O-alpha-L-rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complement. Altern. Med. 2018, 18, 184. [Google Scholar] [CrossRef]
- Mehrbod, P.; Hudy, D.; Shyntum, D.; Markowski, J.; Los, M.J.; Ghavami, S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules 2020, 11, 10. [Google Scholar] [CrossRef]
- Kumar, P.; Khanna, M.; Srivastava, V.; Tyagi, Y.K.; Raj, H.G.; Ravi, K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp. Lung Res. 2005, 31, 449–459. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome(R), a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Liu, A.L.; Wang, H.D.; Lee, S.M.; Wang, Y.T.; Du, G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef]
- Wang, H.X.; Zeng, M.S.; Ye, Y.; Liu, J.Y.; Xu, P.P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2021, 35, 324–336. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef]
- Chen, J.; Feng, S.; Xu, Y.; Huang, X.; Zhang, J.; Chen, J.; An, X.; Zhang, Y.; Ning, X. Discovery and characterization of a novel peptide inhibitor against influenza neuraminidase. RSC Med. Chem. 2020, 11, 148–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).