Inhibitory Effect of Mistletoe Ointment on DNCB-Induced Atopic Dermatitis in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparation of Korean Mistletoe Extract (KME) Ointment
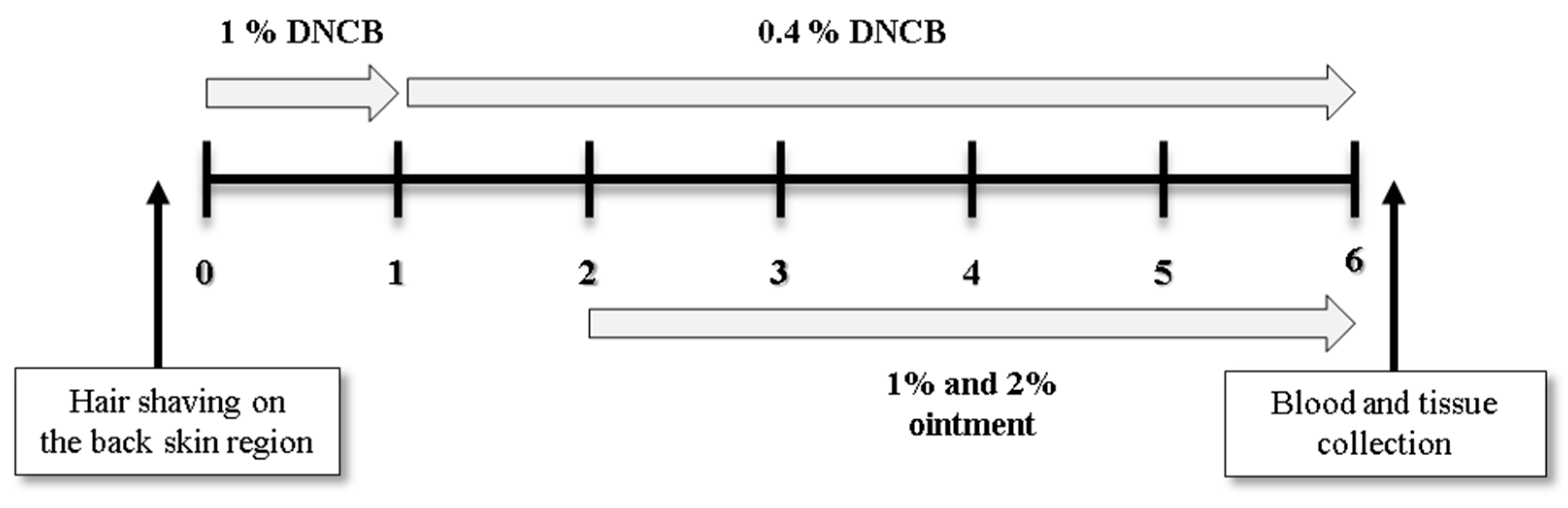
2.3. The Triggering and Treatment of AD-Like Cutaneous Lesion in Mice
2.4. Measurement of the Weight of Mice and Their Spleens
2.5. Measurement of the Scratching Behavior Frequency
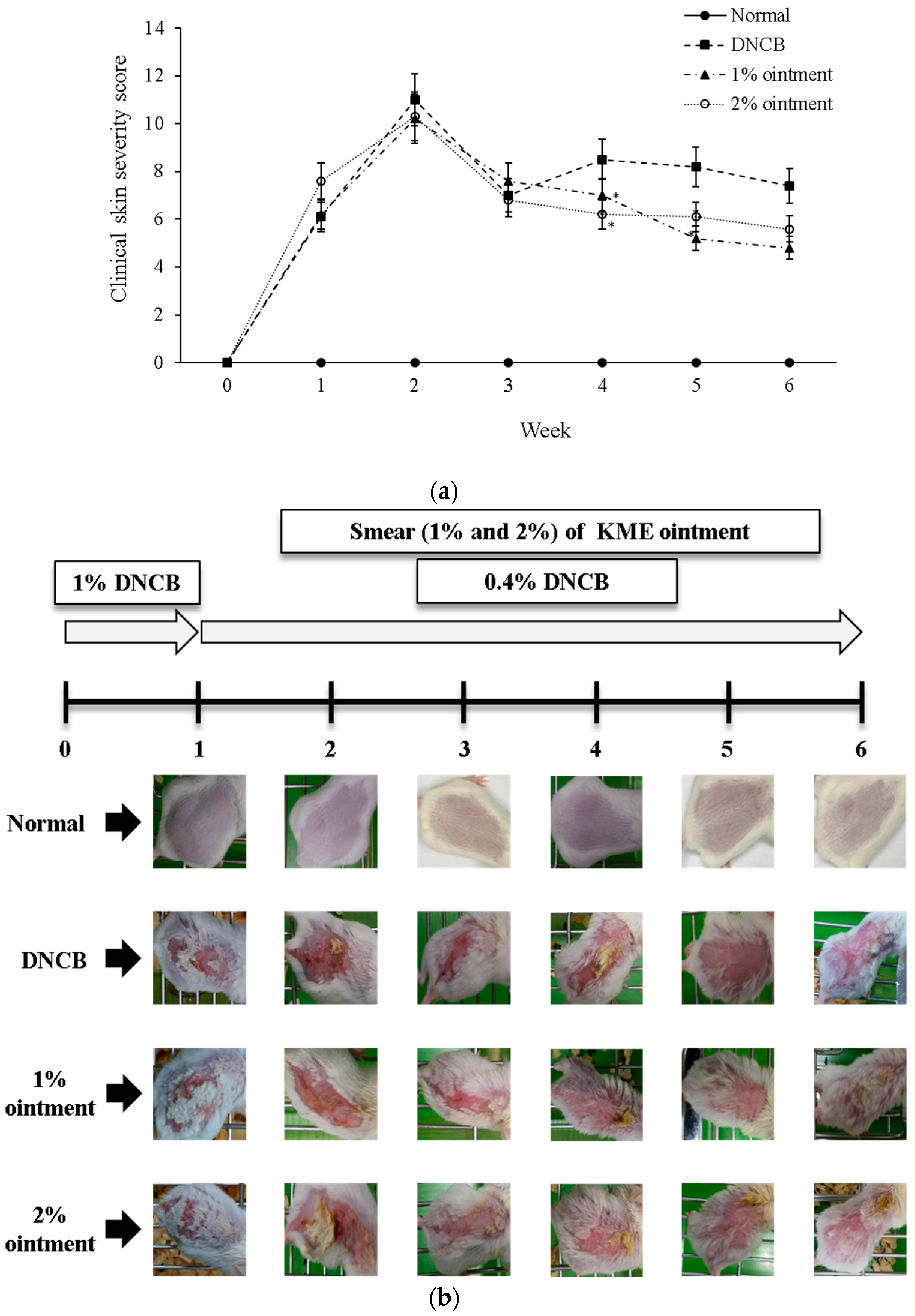
2.6. Evaluation of the Cutaneous Lesion
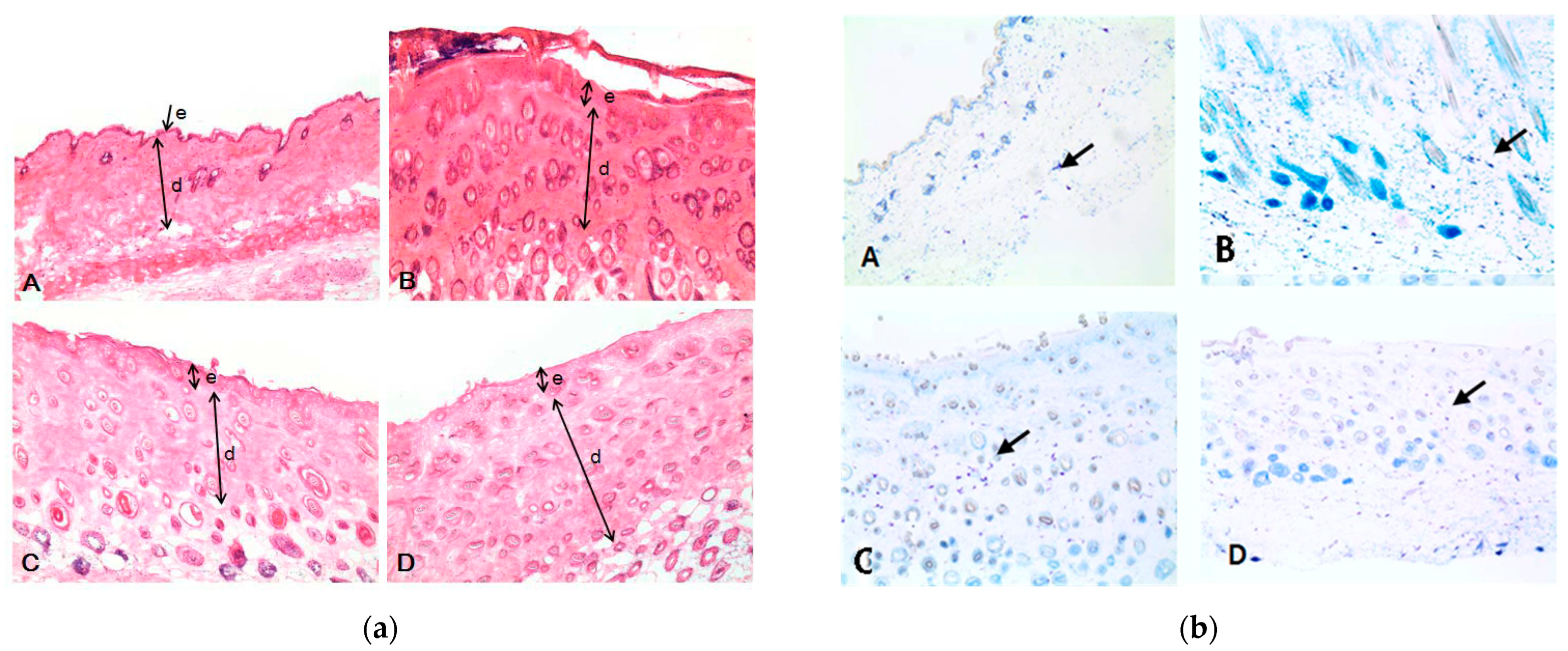
2.7. Histological Examination of Epidermal and Dermal Layers
2.8. Measurement of the Total Serum IgE and Cytokine Levels
2.9. Statistical Analysis
3. Results
3.1. KME Ointment’s Impact on the Weight and Spleen Size of Mice
3.2. Observations in Macroscopic Change
3.3. KME Ointment’s Effect on the Frequency of Scratching Behavior
3.4. Histopathological Observations Change
3.5. KME Ointment’s Effect on the Total Serum of IL-4, IL-5, TNF-α, IFN-γ, and IgE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, M.M.; Bruckner, A.L. Atopic dermatitis: Skin-directed management. Pediatrics 2014, 134, e1735–e1744. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Robertson, C.; Stewart, A.; Aït-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Björkstén, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for treatment of atopic eczema (Atopic dermatitis) Part II. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26, 1176–1193. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, H.K.; Park, S.H.; Jang, S.; Park, K.S.; Song, K.H.; Lee, S.K.; Kim, T. Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-ĸB signaling in vitro. Phytomed. Int. J. Phytother. Phytopharm. 2022, 104, 154318. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, D.; Jabara, H.H.; Arai, K.; Geha, R.S. Induction of human IgE synthesis requires interleukin 4 and T/B cell interactions involving the T cell receptor/CD3 complex and MHC class II antigens. J. Exp. Med. 1989, 169, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, G.; Maggi, E.; Parronchi, P.; Chrétien, I.; Tiri, A.; Macchia, D.; Ricci, M.; Banchereau, J.; De Vries, J.; Romagnani, S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J. Immunol. 1988, 140, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef]
- Tang, M.; Kemp, A.; Varigos, G. IL-4 and interferon-gamma production in children with atopic disease. Clin. Exp. Immunol. 1993, 92, 120–124. [Google Scholar] [CrossRef]
- Tsianakas, A.; Luger, T.A. The anti-IL-4 receptor alpha antibody dupilumab: Facing a new era in treating atopic dermatitis. Expert Opin. Biol. Ther. 2015, 15, 1657–1660. [Google Scholar] [CrossRef]
- Ilves, T.; Harvima, I.T. Decrease in chymase activity is associated with increase in IL-6 expression in mast cells in atopic dermatitis. Acta Derm. Venereol. 2015, 95, 411–416. [Google Scholar] [CrossRef]
- Hu, W.; Yang, X.; Zhe, C.; Zhang, Q.; Sun, L.; Cao, K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW264.7 macrophage cells. Pharmacol. Rep. 2011, 63, 781–789. [Google Scholar] [CrossRef]
- Fan, G.W.; Zhang, Y.; Jiang, X.; Zhu, Y.; Wang, B.; Su, L.; Cao, W.; Zhang, H.; Gao, X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation 2013, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Drucker, A.M.; Lebwohl, M.; Silverberg, J.I. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, 733–740.e711. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.A.; Patel, P.M.; Wilson, C.; Wang, H.; Ashack, K.A. Complementary and alternative medicine treatments for common skin diseases: A systematic review and meta-analysis. JAAD Int. 2021, 2, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, M.R.; Lee, G.S.; An, W.G.; Cho, S.I. Effect of Sophora flavescens Aiton extract on degranulation of mast cells and contact dermatitis induced by dinitrofluorobenzene in mice. J. Ethnopharmacol. 2012, 142, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.E.; Lyu, S.Y. Anti-inflammatory and Anti-oxidative Effects of Korean Red Ginseng Extract in Human Keratinocytes. Immune Netw. 2011, 11, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, H.K.; N’deh KP, U.; Choi, Y.J.; Fan, M.; Kim, E.K.; Chung, K.H.; An, A.J.H. Inhibitory Effect of Centella asiatica Extract on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Nutrients 2020, 12, 411. [Google Scholar] [CrossRef]
- Fan, P.; Yang, Y.; Liu, T.; Lu, X.; Huang, H.; Chen, L.; Kuang, Y. Anti-atopic effect of Viola yedoensis ethanol extract against 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin dysfunction. J. Ethnopharmacol. 2021, 280, 114474. [Google Scholar] [CrossRef]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef]
- Khwaja, T.A.; Dias, C.B.; Pentecost, S. Recent studies on the anticancer activities of mistletoe (Viscum album) and its alkaloids. Oncology 1986, 43 (Suppl. S1), 42–50. [Google Scholar] [CrossRef]
- Büssing, A.; Suzart, K.; Bergmann, J.; Pfüller, U.; Schietzel, M.; Schweizer, K. Induction of apoptosis in human lymphocytes treated with Viscum album L. is mediated by the mistletoe lectins. Cancer Lett. 1996, 99, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Szurpnicka, A.; Zjawiony, J.K.; Szterk, A. Therapeutic potential of mistletoe in CNS-related neurological disorders and the chemical composition of Viscum species. J. Ethnopharmacol. 2019, 231, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Choi, S.H.; Park, W.B. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharmacal Res. 2002, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J.; Yoo, Y.C.; Kang, T.B.; Song, S.K.; Lee, K.B.; Her, E.; Song, K.S.; Kim, J.B. Antitumor activity of the Korean mistletoe lectin is attributed to activation of macrophages and NK cells. Arch. Pharmacal Res. 2003, 26, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.K.; Kim, H.Y.; Park, S.M.; Lee, S.M. Immunomodulating effects of Korean mistletoe lectin in vitro and in vivo. Int. Immunopharmacol. 2009, 9, 1555–1561. [Google Scholar] [CrossRef]
- Kim, B.K.; Choi, M.J.; Park, K.Y.; Cho, E.J. Protective effects of Korean mistletoe lectin on radical-induced oxidative stress. Biol. Pharm. Bull. 2010, 33, 1152–1158. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Papoiu, A.D. What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep. 2008, 8, 306–311. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- McKenzie, C.V.; Colonne, C.K.; Yeo, J.H.; Fraser, S.T. Splenomegaly: Pathophysiological bases and therapeutic options. Int. J. Biochem. Cell Biol. 2018, 94, 40–43. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Huang, J.Y.; Li, S.P.; Gong, X.P.; Sun, J.B.; Mao, W.; Guo, S.N. Portulaca oleracea L. extracts alleviate 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Front. Nutr. 2022, 9, 986943. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Roy, S.; Sullivan, R.; Longley, B.J.; Schieke, S.M.; Ahmad, N. Protective effects of dietary grape against atopic dermatitis-like skin lesions in NC/NgaTndCrlj mice. Front. Immunol. 2022, 13, 1051472. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.M.D.; Madden, C.J. Neural control of the spleen as an effector of immune responses to inflammation: Mechanisms and treatments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R375–R384. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Peripheral itch sensitization in atopic dermatitis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2022, 71, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Peters, A.T. Atopic dermatitis. Allergy Asthma Proc. 2019, 40, 433–436. [Google Scholar] [CrossRef]
- Mack, M.R.; Kim, B.S. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innov. Ski. Sci. Mol. Popul. Health 2022, 2, 100131. [Google Scholar] [CrossRef]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.D.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Yamaguchi, H.L.; Yamaguchi, Y.; Peeva, E. Role of Innate Immunity in Allergic Contact Dermatitis: An Update. Int. J. Mol. Sci. 2023, 24, 12975. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Biology of human TH1 and TH2 cells. J. Clin. Immunol. 1995, 15, 121–129. [Google Scholar] [CrossRef] [PubMed]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M. Clinical and histological features of dry skin in atopic dermatitis. Acta Derm. Venereol. 1985, 114, 82–86. [Google Scholar] [CrossRef]
- Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Epidermal Hyaluronan in Barrier Alteration-Related Disease. Cells 2021, 10, 3096. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Kigasawa, K.; Kajimoto, K.; Hama, S.; Saito, A.; Kanamura, K.; Kogure, K. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int. J. Pharm. 2010, 383, 157–160. [Google Scholar] [CrossRef]
- Tong, J.; Li, Y.; Cai, X.; Lou, F.; Sun, Y.; Wang, Z.; Zheng, X.; Zhou, H.; Zhang, Z.; Fang, Z.; et al. CKBA suppresses mast cell activation via ERK signaling pathway in murine atopic dermatitis. Eur. J. Immunol. 2023, 53, e2350374. [Google Scholar] [CrossRef]
- Zeng, H.R.; Zhao, B.; Rui, X.; Jia, G.H.; Wu, Y.; Zhang, D.; Yu, H.N.; Zhang, B.R.; Yuan, Y. A TCM formula VYAC ameliorates DNCB-induced atopic dermatitis via blocking mast cell degranulation and suppressing NF-κB pathway. J. Ethnopharmacol. 2021, 280, 114454. [Google Scholar] [CrossRef]
- Lee, J.H.; Dong, L.; Noh, H.M.; Park, S.G.; Kim, S.H.; Jo, E.H.; Lee, D.S.; Park, M.C. Inhibitory Effects of Donkey Hide Gelatin on DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Front. Pharmacol. 2022, 13, 896450. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.K.L.; Tay, W.H.; Janela, B.; Yong, A.M.H.; Liew, T.H.; Madden, L.; Keogh, D.; Barkham, T.M.S.; Ginhoux, F.; Becker, D.L.; et al. Enterococcus faecalis Modulates Immune Activation and Slows Healing During Wound Infection. J. Infect. Dis. 2017, 216, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Hayes, D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: A General Biological Principle. Chem. Res. Toxicol. 2022, 35, 547–549. [Google Scholar] [CrossRef]




| Components | Part by Weight (%) |
|---|---|
| Korean mistletoe extracts | 1.00/2.00 |
| White petrolatum | 34.20 |
| White wax | 5.40 |
| Propylene glycol | 17.89 |
| Lecithin | 3.60 |
| Propyl paraben | 0.018 |
| Methyl paraben | 0.018 |
| Purified water | To 100 |
| Group | Spleen Weight (g) |
|---|---|
| Normal | 0.102 ± 0.012 |
| DNCB | 0.194 ± 0.026 |
| 1% ointment | 0.143 ± 0.003 * |
| 2% ointment | 0.122 ± 0.017 * |
| Group | Normal (μm) | DNCB (μm) | 1% Ointment (μm) | 2% Ointment (μm) |
|---|---|---|---|---|
| Epidermis | 15.5 ± 2.3 | 75.4 ± 13.2 | 57.0 ± 10.1 * | 46.9 ± 9.6 * |
| Dermis | 344.1 ± 49.4 | 573.8 ± 92.3 | 457.9 ± 64.1 * | 512.6 ± 87.7 |
| Group | Normal | DNCB | 1% Ointment | 2% Ointment |
|---|---|---|---|---|
| Number of mast cells | 17.0 ± 5.6 | 92.4 ± 49.3 | 30.6 ± 6.5 ** | 61.0 ± 19.0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.-E.; Lyu, S.-Y. Inhibitory Effect of Mistletoe Ointment on DNCB-Induced Atopic Dermatitis in BALB/c Mice. Sci. Pharm. 2024, 92, 3. https://doi.org/10.3390/scipharm92010003
Hong C-E, Lyu S-Y. Inhibitory Effect of Mistletoe Ointment on DNCB-Induced Atopic Dermatitis in BALB/c Mice. Scientia Pharmaceutica. 2024; 92(1):3. https://doi.org/10.3390/scipharm92010003
Chicago/Turabian StyleHong, Chang-Eui, and Su-Yun Lyu. 2024. "Inhibitory Effect of Mistletoe Ointment on DNCB-Induced Atopic Dermatitis in BALB/c Mice" Scientia Pharmaceutica 92, no. 1: 3. https://doi.org/10.3390/scipharm92010003
APA StyleHong, C.-E., & Lyu, S.-Y. (2024). Inhibitory Effect of Mistletoe Ointment on DNCB-Induced Atopic Dermatitis in BALB/c Mice. Scientia Pharmaceutica, 92(1), 3. https://doi.org/10.3390/scipharm92010003







