Abstract
Lamotrigine, widely used for managing epilepsy and bipolar disorder, carries potential side effects, including severe anticonvulsant hypersensitivity syndrome (AHS) or drug rash with eosinophilia and systemic symptoms (DRESS), which may lead to hepatotoxicity. Patients with Type 2 Diabetes (TD2) and Non-Alcoholic Fatty Liver Disease (NAFLD) are identified as more susceptible to these adverse reactions. This exploratory analysis aims to identify clinical parameters influencing lamotrigine pharmacokinetics across diverse populations, shedding light on toxicity and therapeutic drug monitoring (TDM) considerations. Starting with a retrospective analysis of 41 lamotrigine-treated patients at Hospital Santo António reveals changes or deviations from normal levels in various blood parameters and significant correlations between these parameters. Serum level changes, including creatinine, albumin, gamma-glutamyl transferase, total bilirubin, and Vitamin B12, are observed, with strong negative correlations between Vitamin B12 and creatinine. Then, we used GastroPlus and DILIsym to explore the impact of clinical parameters on lamotrigine for different patient populations. We constructed a Physiologically Based Pharmacokinetic (PBPK) model for lamotrigine in GastroPlus, based on ADMET predictions and data from the literature, to simulate the pharmacokinetic variability of lamotrigine in different populations, and we visualized the impact of increasing lamotrigine dose on its plasma concentration–time profiles (200 mg, 400 mg, 600 mg, 1200 mg) and reduced bioavailability. At higher doses, it is possible that the saturation of metabolic pathways leads to the formation of toxic metabolites or intermediates. These metabolites may exert inhibitory effects on drug-metabolizing enzymes or disrupt normal physiological processes, thereby impeding the drug’s clearance and potentially lowering its bioavailability. In DILIsym, we investigated lamotrigine’s DILI potential for individuals with diabetes and NAFLD. The results demonstrated an increased risk, emphasizing the need for careful monitoring. This study underscores the importance of understanding lamotrigine’s pharmacokinetics for tailored treatment decisions, improved outcomes, and minimized adverse reactions.
1. Introduction
The Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved lamotrigine for various central nervous system (CNS) conditions, including both generalized and focal seizures. The optimal dosage of lamotrigine is contingent upon the specific medical condition, as well as other factors such as the patient’s age and concomitant medication [1,2]. Recently, the FDA has issued a warning that lamotrigine can cause a rare but very serious reaction that excessively activates the body’s infection-fighting immune system [3]. The FDA has also added a safety warning related to patients with certain underlying cardiac disorders or arrhythmias to the prescribing information for lamotrigine. The warning states that lamotrigine could slow ventricular conduction (widen QRS) and induce proarrhythmia, including sudden death in people with structural heart disease or myocardial ischemia [4,5]. Lamotrigine is approved for various CNS indications and serves as a first-line treatment for several forms of epilepsy, encompassing generalized and focal seizures, commonly administered in combination with other anticonvulsant medications [6]. The EMA has not issued a similar warning about the potential cardiac effects of lamotrigine at this time. However, both regulatory agencies continue to monitor the safety and efficacy of lamotrigine and update their labeling and guidelines as new evidence becomes available.
Regarding other potential side effects, such as “anticonvulsant hypersensitivity syndrome” (AHS)/“drug rash with eosinophilia and systemic symptoms” (DRESS) syndrome, which may lead to hepatotoxicity, the FDA and EMA have not specifically mentioned these side effects in their warnings. However, both agencies emphasize the importance of monitoring patients for signs of serious side effects and recommend reporting any suspected adverse events [7,8]. While hepatotoxicity due to lamotrigine is rare, it can occur, and when it does, it can progress to drug-induced liver injury (DILI) [9,10]. DILI is a significant clinical problem [11,12] and has received increasing attention in recent decades due to its potential to cause serious health issues and being one of the most common and serious adverse drug reactions. In the management of DILI, the foremost step involves discontinuation of the implicated drug or medication in question. However, a comprehensive approach to treatment, incorporating both pharmacological interventions and supportive measures, is essential for optimal care. Diagnosing DILI can be challenging due to the lack of specific diagnostic markers and the complex pathogenesis [12]. Lamotrigine-induced hepatotoxicity has been associated with symptoms such as elevated transaminases, jaundice, and multisystem involvement, including disseminated intravascular coagulation and renal insufficiency [13,14,15]. Certain patient groups, particularly those with Type 2 Diabetes (TD2) and Non-Alcoholic Fatty Liver Disease (NAFLD), exhibit higher susceptibility to this adverse reaction [16,17,18]. The coexistence of TD2 and NAFLD influences the presentation and outcome of DILI [19], with NAFLD introducing changes in hepatic uptake, distribution, metabolism, and transport of xenobiotics, thereby increasing susceptibility to DILI [20]. Higher doses and rapid escalations of lamotrigine have been linked to an increased risk of hepatotoxicity [21].
In this study, we investigate the effects of excessive drug doses (doses outside the therapeutic window) to provide valuable insights into the mechanisms underlying DILI [22]. Overdosing amplifies the drug’s pharmacological effects, elucidating pathways involved in liver injury [23]. For example, an overdose may trigger an overactivation of the immune system, leading to a heightened inflammatory response that damages liver cells, aiding in the identification of key proteins and pathways involved in the drug’s hepatotoxicity [24,25,26]. Unlike certain drugs like carbamazepine, genetic investigations have not identified significant associations between specific variants and lamotrigine-induced liver injury [21,27]. The incomplete understanding of the mechanisms underlying lamotrigine’s action, which is crucial for its therapeutic effects, raises specific challenges, particularly in the interpretation of secondary effects such as DILI or cardiac problems (Figure 1). This nuanced understanding may not pose difficulties in dosage adjustments but can complicate the comprehensive assessment of potential adverse reactions [28,29]. Dosing protocols vary based on the illness, requiring careful monitoring by healthcare professionals [30].
Figure 1.
Overview of anticonvulsant lamotrigine, hepatotoxicity, and monitoring. Representation of the interplay between anticonvulsant lamotrigine, the potential risk of hepatotoxicity, and the crucial aspect of monitoring. The diagram encapsulates the dynamic relationship among these key concepts, offering a visual guide to enhance understanding and underscore the significance of vigilance in monitoring for optimal patient safety and therapeutic outcomes.
Lamotrigine is rapidly and completely absorbed after oral administration with negligible first-pass metabolism, and the absolute bioavailability is at 98%. Peak plasma concentrations occur anywhere from 1.4 to 4.8 h following drug administration. The plasma concentrations of lamotrigine increase in direct proportion to the dose administered over the range of 50–400 mg. The estimates of the mean apparent volume of distribution (Vd/F) of lamotrigine following oral administration ranged from 0.9 to 1.3 L/kg [31]. Lamotrigine is approximately 55% bound to human plasma proteins, and it is metabolized predominantly by glucuronic acid conjugation, with the major metabolite being an inactive 2-N-glucuronide conjugate [32]. The elimination half-life and apparent clearance of lamotrigine depend on whether the patient is receiving enzyme-inducing drugs or not. If stopping lamotrigine is necessary, it should ideally be administered gradually over two weeks [33]. Lamotrigine withdrawal seizures are possible but are less likely if the medication is weaned off gradually rather than abruptly. Lamotrigine is metabolized in the liver by glucuronidation, being mainly metabolized by UGT enzymes in the body and excreted by the kidneys, with CYP enzymes not participating in its metabolism [34].
While TDM is recommended to optimize lamotrigine dosage, especially when co-administered with specific drugs [35], the routine necessity of TDM is debated due to unclear concentration–response relationships [36,37]. This study seeks to explore the impact of clinical parameters on the pharmacokinetics of lamotrigine in different patient populations, shedding light on toxicity and therapeutic drug monitoring (TDM) considerations. A retrospective analysis of 41 lamotrigine-treated patients at Hospital Santo António revealed deviations in various blood parameters and significant correlations among them. Serum level changes, including creatinine, albumin, gamma-glutamyl transferase, total bilirubin, and Vitamin B12, were observed, with strong negative correlations between Vitamin B12 and creatinine. Using GastroPlus and DILIsym, we investigated the impact of clinical parameters on lamotrigine for various patient populations. In GastroPlus, a Physiologically Based Pharmacokinetic (PBPK) model simulated lamotrigine’s pharmacokinetic variability, revealing dose-dependent effects on plasma concentration and reduced bioavailability, possibly due to metabolic pathway saturation and toxic metabolite formation; in DILIsym, lamotrigine’s DILI risk in diabetes and NAFLD patients was highlighted, emphasizing the need for vigilant monitoring.
This study aims to elucidate how these pharmacokinetic aspects relate to liver function assessment and TDM, emphasizing the importance of understanding lamotrigine’s pharmacokinetics for tailored treatment decisions, improved outcomes, and adverse reaction mitigation.
2. Methods
2.1. Clinical Data Collection and Statistical Analysis
This study started with a retrospective collection and analysis of clinical data from 41 patients who underwent lamotrigine treatment at Hospital Santo António. The data utilized in this study were anonymized and obtained from hospital records. Ethical considerations were carefully addressed, and due to the retrospective nature of the study and the anonymization of the data, individual informed consent was not obtained. This patient cohort, aged 7 to 62 and comprising 29 females and 12 males, had their serum measurements meticulously gathered to monitor their therapeutic regimens. No serum lamotrigine data were available. We leveraged this dataset for a secondary, exploratory analysis, to investigate the relationships between various serum parameters, liver enzymes, and lamotrigine intake.
During the analysis of our dataset, we identified instances of missing values that required addressing before proceeding with further analysis. Given the inherent characteristics of our data, we hypothesized that these missing values followed a missing completely at random (MCAR) pattern. To assess this hypothesis, we conducted Little’s MCAR test, which scrutinizes the patterns of missing data against observed data patterns using a chi-square test [38]. A non-significant result (p-value > 0.05) from this test indicates that the data adheres to the MCAR assumption. In our specific analysis, the obtained p-value was 0.303, surpassing the conventional significance level of 0.05. Consequently, we lack substantial evidence to reject the null hypothesis that the data are MCAR. This suggests that the occurrence of missing data in our dataset is likely independent of both observed and unobserved variables. Thus, it is reasonable to assume that the missing data are indeed missing completely at random. Then, we proceeded with the removal of the identified missing values, ensuring they did not introduce bias or interfere with subsequent analyses.
Central and dispersion indices, frequency descriptive statistics (%), and other methods were utilized for data classification and analysis. Spearman’s rank correlation coefficient was used to assess the strength and direction of the relationship between the two variables. R studio software (version 2023.06.0+421) was used to analyze the data. p values of less than 0.05 were considered statistically significant.
2.2. In Silico Prediction of Pharmacokinetic and Physicochemical Properties of Lamotrigine and Physiologically Based Pharmacokinetic (PBPK) Modeling Development
The chemical structure of lamotrigine was drawn in MedChem Designer (Version 5.5; Simulation Plus Inc., Lancaster, CA, USA) and then imported into ADMET Predictor® in a MOL file format. Lamotrigine was described according to its physicochemical and PK properties using ADMET Predictor® (Version 10.4; Simulation Plus Inc., Lancaster, CA, USA), a software tool that accurately predicts several features of compounds, including physicochemical and PK properties. The literature was searched for information on parameters including Log P, molecular weight, solubility, human jejunum effective permeability (Peff), diffusion coefficient (Diff. Coeff.), CYP-mediated metabolism and transport, and blood–brain barrier (BBB) permeability.
The PBPK model for lamotrigine was developed using GastroPlus software (Version 9.8.3; Simulation Plus Inc., Lancaster, CA, USA). The chemical structure of lamotrigine and all the physicochemical and PK parameters previously computed by the ADMET Predictor® were imported into this software. We did not construct a new PBPK model for lamotrigine, we based our model on literature findings [39,40]. Then, PK parameters of lamotrigine were simulated for different doses: 200, 400, 600, and 1200 mg administered orally for 24 h. Lamotrigine metabolites were assumed not to contribute to liver toxicity. The drug disposition-based parameters were determined in a PBPK model in a virtual 30-year-old healthy American male patient. The simulation provided quantitative and visual (plots) outputs of the PK features for the different doses.
Moreover, we used the DILIsym simulation mode in GastroPlus, a specialized implementation of the population simulator mode. It is designed for running population simulations for virtual subjects, aligning with SimPops™ and SimCohorts™ modules in the DILIsym software. Parameters not derived from body weight are treated as standard population simulation parameters, incorporating random variability. This mode runs a series of simulations that simulate subjects from specific populations defined in DILIsym software. The results were saved in a format (.xlsx file) that is recognized by DILIsym software and was used as input for DILIsym predictions. The “DILIsym” simulation mode in v9.6 allowed us to select a mapping of GastroPlus outputs.
2.3. DILIsym Simulation Mode within GastroPlus
The DILIsym simulation mode only works with the PBPK module of GastroPlus. In order for the body weight-related parameters in GastroPlus to reasonably match the corresponding simulated individual in DILIsym, the body weights for each individual were imported into the program. By doing this, mismatches were prevented if a subject’s PBPK is predicted in GastroPlus based on a relatively high or low body weight but the DILIsym SimPops individual was on the other end of the body weight spectrum. Then, we picked the compound scaffold (Compound W) that matched the desired framework in DILIsym.
Simulation platform DILIsym version 8A was used to conduct the simulations. DILIsym is a mathematical representation of DILI. Briefly, DILIsym consists of several smaller submodels that are mathematically integrated to simulate an organism-level response. This work utilized submodels representing drug distribution, mitochondrial dysfunction and toxicity, bile acid physiology and pathophysiology, hepatocyte life cycle, and liver injury biomarkers.
A PBPK representation of lamotrigine was constructed within DILIsym, based on GatroPlus, to describe liver exposure upon conventional oral tablet administration. The DILIsym PBPK model framework used for lamotrigine consists of compartments for the liver, blood, muscle, gut, and other tissues. The tissue distribution of lamotrigine was assumed to be permeability-limited [41]. Parameters used in the PBPK submodel for lamotrigine are shown in Table 1.

Table 1.
Input data used in GastroPlus™ and DILIsym to simulate plasma concentrations and simulate different populations. These values were retrieved from the literature [40].
DILIsym allows the use of SimPop, a rather large population of more than 100 simulated individuals. SimPops are created by adding variability to a number of parameters, the ranges for which values were found using information from the available literature. We selected human SimPops with healthy individuals and TD2-related traits like hyperglycemia, and NAFLD are also part of DILIsym NAFLD. The TD2 SimPops also considers the variety of each DILI toxicity mechanism and may be used for any hepatotoxic mechanism, including the production of reactive nitrogen species and reactive oxygen species (RNS-ROS), the direct disruption of mitochondrial activity, and/or the blockage of bile acid transport. SimPops are generated for simulating responses in NAFLD individuals and for compounds that cause injury through RNS-ROS generation, direct mitochondrial function disruption, bile acid transport inhibition, and/or lipotoxicity, as well as disease-related variability in body mass, plasma glucose, plasma FFA, liver GSH, mitochondria function, lipogenesis, liver TG synthesis, plasma TG, liver bile acid uptake transporters, and lipotoxicity and variability in basolateral and canalicular BA transporters, apoptosis, necrosis, and regeneration. It is important to note that pharmacokinetic variability is not inherently built into the SimPops. This is because pharmacokinetic variability tends to be highly drug-specific and is better incorporated as part of the PBPK model inputs (this being the main reason we used a PBPK model). The variation in susceptibility within our simulated population is intentionally designed to exceed what one might encounter in a significantly larger population of real patients. This approach allows us to explore various potential scenarios and outcomes, shedding light on the complex interplay between lamotrigine, individual patient characteristics, and the risk of drug-induced liver injury.
For this study, we used SimPops to simulate a 2-week treatment of 200 mg of lamotrigine for individuals with different diseases, so we could compare clinical parameters between the simulated healthy volunteers, NAFLD, and TD2 patients. A brief description of the SimPops selected in the software can be seen in the Supplementary Materials (Table S1).
3. Results
3.1. Hospital Santo António Data Analysis
We gathered demographic characteristics and clinical parameter data from 41 epilepsy patients undergoing lamotrigine therapy at Hospital Santo António. The mean age and standard deviation of this patient group was 30.1 +/− 18.58 years.
The demographic and clinical characteristics are presented in Table 2 after addressing missing values. To enhance the clarity of our data presentation, we opted to categorize patients into age and dose groups, providing a clearer depiction of the distribution of age and dosage among those undergoing lamotrigine treatments. Regarding lamotrigine dose groups, seven patients (21.21%) were in the “Very Low—1” group (doses < 100 mg) with a minimum dose of 20 mg, nine patients (27.27%) fell into the “Low—2” group (doses between 100 and 200 mg), sixteen patients (48.5%) were in the “Average—3” group (doses between 200 and 400 mg), and only one patient (3.03%) was in the “High—4” group (doses > 400 mg) with a maximum dose of 600 mg. Age can influence the PK parameters of lamotrigine. Research has shown that while the bioavailability of lamotrigine is not significantly affected by age, the drug’s clearance is lower in elderly patients compared to young patients of comparable body weight. Specifically, a study found that lamotrigine clearance was 27.2% lower in elderly patients, indicating that lower dosages may be needed in this population [45]. This suggests that age-related changes in drug clearance may necessitate dosage adjustments to ensure optimal and safe therapeutic levels of lamotrigine in elderly individuals. This distribution is as expected as it seems to follow the guidelines and is similar to other cases found in the literature. Interestingly, the average age for the “Very Low” group is approximately 40 years, and younger ages are grouped with higher doses (Figure S1). The table also includes information about reported drug combination therapy. Among the 37 patients, 62.16% (±0.49) were reported as receiving combination therapy, while 37.84% (±0.49) of the patients did not have any recorded information about taking other drugs as part of their treatment regimen. The drugs most used as complementary to therapy were valproic acid (e.g., from 100 to 15,000 mg), followed by lithium, levetiracetam, phenytoin, carbamazepine, rufinamide, and clonazepam.

Table 2.
Demographic characteristics and clinical information of the patients from Hospital Santo António. Interquartile range (IQR) for a categorical variable has no standard interpretation. NA means no valid or available data for that particular observation or variable exists.
Then, we proceeded to assess how many individuals had altered serum level parameters by calculating the number of individuals with out-of-range parameter values. The normal range varies based on each parameter and patient characteristics. For instance, the normal range of clearance differs between children and adults. Table 3 summarizes the results of that analysis. Serum creatinine levels were altered in 43.34% of the measured patients. Following creatinine, the parameters most frequently affected were albumin (37.83%), gamma-glutamyl transferase (24.32%), total bilirubin (21.62%), and Vitamin B12 (18.92%). Interestingly, we did find that all the individuals who had values out of range for Vitamin B12 also had out-of-range parameter values for creatinine. However, not all patients whose serum levels were registered had their Vitamin B12 values measured.

Table 3.
Summary of individuals with out-of-range serum levels. These parameters were measured for therapeutic drug monitoring of lamotrigine of the patients from Hospital Santo Antônio.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phos-phatase, and γ-glutamyl transferase (GGT) are four enzymes of diagnostic importance in liver disease. Interestingly, no patients showed altered values for ALT.
The results presented in Table 4 show Spearman’s rank correlation analysis between various pairs of variables. Spearman’s correlation evaluates monotonic relationships, which can be nonlinear. Spearman’s correlation coefficient can range from −1 to 1, just like Pearson’s correlation. A positive coefficient indicates a positive monotonic relationship (both variables increase together or decrease together), while a negative coefficient implies a negative monotonic relationship (as one variable increases, the other decreases). A coefficient of 0 means no monotonic relationship. The p-value indicates the statistical strength and significance of the correlation. Overall, the correlation analysis suggests several significant correlations between different variables with different strengths: weak correlation (absolute value of rho < 0.3), low to moderate correlation (0.3 ≤ |rho| < 0.5), moderate correlation (0.5 ≤ |rho| < 0.7), strong correlation (0.7 ≤ |rho| < 0.9), and very strong correlation (0.9 ≤ |rho| ≤ 1.0).

Table 4.
Summary of Spearman’s correlation analysis.
The correlation analysis uncovers intriguing patterns and associations that hold promise for advancing our understanding of lamotrigine use in epileptic patients. Notable correlations include: (a) moderate association—variables like “Dose” exhibit moderate negative or positive correlations with markers such as “ASAT”, “VLDL cholesterol”, “Trough concentration”, and “Total cholesterol”. The trough concentration refers to the level of a drug present in the bloodstream just before the subsequent dose is administered, although it may not necessarily represent the lowest concentration observed during the dosing interval (b) low to moderate influences—variables like “Dose” display low to moderate positive correlations with “Gamma-glutamyl transferase” and “Chlorates”, indicating a mild impact of lamotrigine dosage on these markers; (c) strong correlations—some strong associations are evident, such as the robust positive correlations between “Vitamin B12” and variables like “Dose”, “Trough concentration”, “Combination”, and “Creatinine”. This suggests that these factors substantially influence Vitamin B12 levels in epileptic patients. Additionally, “Trough concentration” exhibits a strong positive correlation with “Chlorates”, implying a significant influence of lamotrigine concentration on blood chemistry measures related to “Chlorates”. Notably, there is a very strong negative correlation between “Vitamin B12” and “Creatinine”, indicating an inversely proportional relationship. These identified correlations serve as a valuable foundation for further investigations to probe causal links. For example, the strong correlation between lamotrigine dosage and Vitamin B12 levels may prompt in-depth research into how lamotrigine affects Vitamin B12 metabolism or absorption. The correlations involving blood chemistry measures and specific variables may suggest these markers as potential indicators of lamotrigine’s effects in epileptic patients.
After understanding that Vitamin B12 is highly correlated with several variables we assess the correlation of this parameter with other variables, Table 5. The analysis reinforces the correlation seen previously, for dose, trough concentration, and creatinine the correlation is the same in both directions. However, it does not inform on the direction of the relationship. We are not able to determine whether changes in “Vitamin B12” are causing changes in “Dose LTG”, or vice versa, just from the correlation result. To establish a causal relationship or understand the direction of the association, there is a need to perform experiments, conduct longitudinal studies, or use other statistical methods. Spearman’s correlation, like other correlation methods, is primarily used to identify associations or dependencies between variables, not to establish causation or determine the specific direction of the relationship.

Table 5.
Spearman’s rank correlation analysis of Vitamin B12 with the remaining parameters.
3.2. Lamotrigine PBPK Model and Population Simulation Results
In this phase of our study, we harnessed the power of a PBPK model in GastroPlus to simulate a spectrum of doses, spanning from therapeutic levels to overdose scenarios, in a population. Using DILIsym population simulation mode, which provided valuable insights into compound behaviors under diverse conditions. This specialized mode executed a series of simulations with distinct virtual subjects, each characterized by a random sample of physiological and pharmacokinetic parameters, closely resembling a heterogeneous population. The robust capability of the simulation allowed for the assessment of combined effects arising from variations in population physiology and formulation variables, considering not precise values but distributions. These variables included transit times, pH levels in various compartments, pharmacokinetic parameters, plasma protein binding, blood–plasma concentration ratio, renal clearance, and dose. Distributions for these variables were defined as means with coefficients of variation in absolute or log space, offering flexibility with normal, log-normal, or uniform distributions.
The model accurately predicted the distribution and elimination of lamotrigine. The calculated steady state volume of distribution (Vss) was 1.84 L/kg, which agreed with the values reported in the literature [40,46]. As the kidneys play a crucial role in drug elimination, renal clearance is influenced by a combination of filtration, reabsorption, secretion, and metabolism. Using the GastroPlus PBPK kidney model recommended by the software, the systemic clearance was estimated at 3.248 L/h, calculated as the product of the fraction of drug unbound in the plasma (fup) and the glomerular filtration rate (GFR). The half-life (t1/2) of lamotrigine is reported to range from 13.5 to 15 h. The t1/2 was estimated to be 27.58 h. This indicates that in the setting of an overdose, the half-life of lamotrigine is prolonged compared to its half-life in therapeutic doses.
The results of the DILIsym population simulation are succinctly summarized in Table 6. This table focuses on average pharmacokinetic parameters for population-wide concentrations at doses of 200, 400, 600, and 1200 mg. While observed data were collected for 200 mg, other concentrations representative of overdose scenarios lacked observed data from the literature [40]. The bioavailability of lamotrigine, estimated at 98%, indicates rapid and complete absorption with minimal first-pass metabolism effects. Bioavailability (F%) exhibited a decreasing trend with increasing doses. Peak plasma concentration (Cmax) increased with higher doses, potentially impacting drug efficacy and side effects. Time to reach peak concentration (Tmax) did not exhibit a consistent trend with increasing doses, indicating potential external factors influencing Tmax. Despite obtaining similar results for Tmax and Cmax at 200 mg, overall bioavailability was lower due to introduced variability in the DILIsym simulation mode. The decrease in bioavailability observed at higher doses may stem from the saturation of metabolic pathways, particularly those involving glucuronic acid conjugation. This saturation could potentially trigger the production of toxic metabolites or intermediates. These harmful byproducts might then exert inhibitory effects on drug-metabolizing enzymes or interfere with regular physiological processes, consequently hindering the drug’s clearance. Such interference could lead to a reduced apparent bioavailability of the drug. This scenario could lead to decreased drug availability, potentially impacting toxic effects. The increase in the area under the concentration–time curve (AUC0–inf and AUC0–t) with higher doses signifies elevated overall drug exposure. Notably, our AUC values were lower compared to literature data, possibly due to variations in populations or study conditions. Our study encapsulates the DILIsym population simulation, and differences in dosing regimens between our simulation and literature data may impact AUC. It is essential to highlight that our simulation does not incorporate potential drug interactions that may influence drug exposure, a consideration commonly addressed in the existing literature. These findings hold significant implications for dosing considerations and potential toxicity, underscoring the critical need for meticulous monitoring in lamotrigine administration.

Table 6.
Results of the average PK parameters simulated: F%, Cmax, Tmax, AUC0–inf, AUC0–t, and 90% confidence interval for the different concentrations of lamotrigine. Each dose is presented with the values for the single simulation and a DILIsym population simulation. For 200 mg, a known therapeutic dose, we examined values from the literature for comparison [40]. The simulation length was 24 h for 176 selected subjects. Only the values for 200 mg doses were included, as all the other concentrations represent an overdose.
3.3. DILI Potential of Lamotrigine for Different Individuals
Then, the simulated blood, gut, liver, and sinusoidal liver concentration–time profiles were exported from GastroPlus for DILIsym using the Specify Data function. We simulated a 2-week regimen with these doses. In Figure 2, we can observe that with increasing drug concentration, a noticeable trend emerges in the simulated subjects’ plasma concentration, revealing an expected rise in the maximum values. The curves corresponding to different concentrations distinctly deviate from each other at the peak points. Furthermore, the shapes of these peaks vary across the three concentrations (400 mg, 600 mg, and 1200 mg). This is expected because higher doses can imply larger plasmatic concentrations. In 2 weeks, we can see the accumulation of the drug, hence the increasing increase in plasmatic concentration during time.
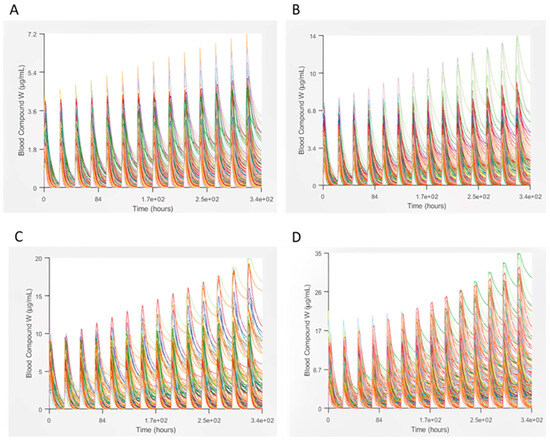
Figure 2.
Plasma concentration profile of Lamotrigine in a 2-week regimen for different doses: (A)—200, (B)—400, (C)—600, and (D)—1200 mg.
The most specific indicator of a drug-induced liver injury in clinical trials is believed to be the occurrence of subjects experiencing drug-associated elevations in both serum ALT/AST and serum total bilirubin (TB) without a significant elevation in serum alkaline phosphatase. The eDISH (evaluation of drug-induced serious hepatotoxicity) is a well-described method/tool that visually ALT/AST and total bilirubin in the form of a graph by displaying peak levels for each subject.
If Figure 3, we can see the graphics resulting from the simulation of healthy individuals, TD2 individuals, and NAFLD individuals. In the case of healthy individuals, we can see that all of them fall within the normal range. However, when we evaluate the eDISH plot for TD2 patients and NAFLD, we obtain different results, as expected from what we see in the literature. Some of the values are in the normal range while some are in Temple’s corollary range, meaning that there are patients at risk of drug-induced liver injury. In particular, for TD2 patients, we can see that some of the individuals simulated occupy the middle upper zone of the eDISH plot.
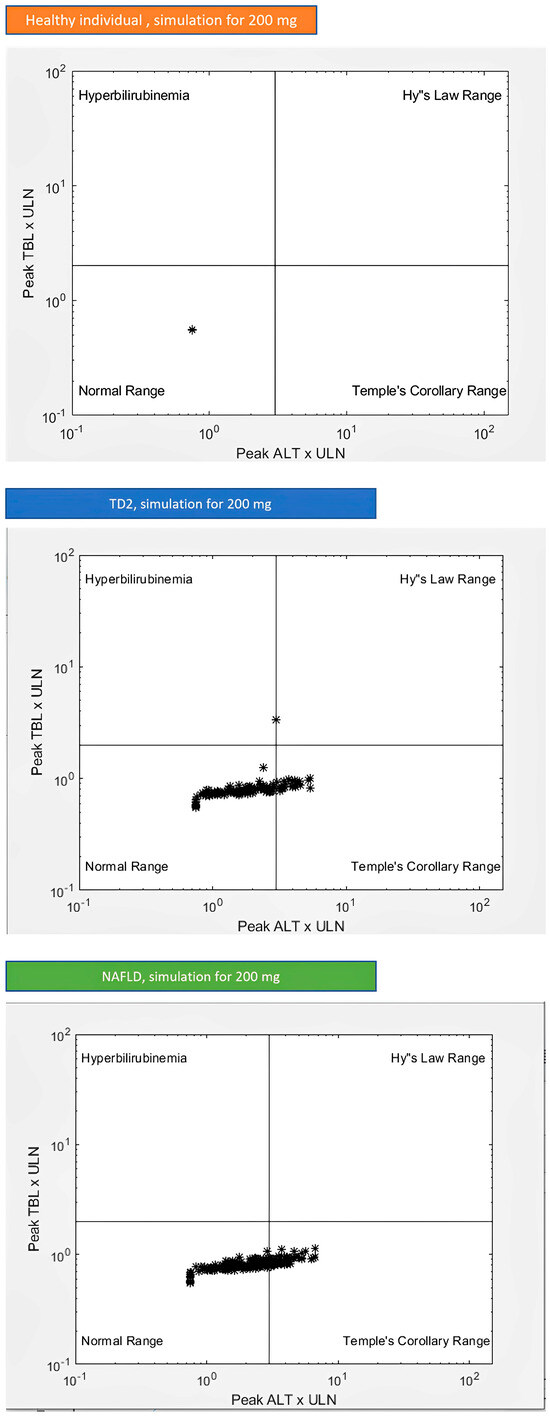
Figure 3.
Graphical representation of the in silico results, to assess a drug’s liver safety profile (eDISH plot) for healthy individuals and individuals with TD2 and NAFLD.
Lamotrigine is recognized for its potential to cause rare but well-known idiosyncratic liver injury, which can be severe and, in some cases, fatal. This liver injury can be unpredictable and may occur within the first few weeks of starting therapy, particularly in the context of concomitant use of other anticonvulsant medications and hypersensitivity syndrome. This liver injury can sometimes be severe and may even lead to fatal outcomes. AST, ALT, alkaline phosphatase, and GGT are four enzymes of diagnostic importance in liver disease. The criteria for drug-induced liver disease diagnosis include either: ALT ≥ 5 ×upper limit of normal (ULN); ALP ≥ 2 × ULN; or ALT ≥ 3 × ULN and total bilirubin ≥ 2 × ULN (this last criterion is also known as Hy’s law). In Table 7, we can see the number of simulated individuals with parameters altered for each subgroup. These results show increased values for the outcome variables for the TD2 and NAFLD individuals. This was expected since these individuals have metabolic changes that interfere with the ADMET properties of drugs. These differences can be seen in the literature; therefore, they were expected. NAFLD individuals only showed the ALT at or over 3 × ULN parameter altered with 64 of the simulated individuals possessing this alteration. None of the healthy, TD2, or NAFLD individuals had cases of Hy’s law, which proves the safe profile of lamotrigine.

Table 7.
The number of simulated individuals with parameters altered is suggestive of DILI for the different populations: healthy, TD2, and NAFLD individuals.
4. Discussion
4.1. Interplay of Lamotrigine, Vitamin B12, Creatinine, and Lipid Metabolism: Implications for Renal and Hepatic Systems
This study provides key insights into the interplay of lamotrigine dosing, patient demographic changes, or deviations from normal levels in various blood parameters. As expected, younger patients are associated with higher lamotrigine doses (Figure S1), suggesting age-related variations in pharmacokinetics [39,47]. Interpreting altered creatinine levels in 43.24% of patients as indicative of potential renal impairment underscores the importance of vigilant monitoring, especially for epilepsy patients undergoing lamotrigine therapy [48]. This finding highlights the need for ongoing assessment and close attention to renal function to ensure the early detection and management of any potential complications.
A significant finding is the negative correlation between Vitamin B12 and creatinine levels. These results are supported by the literature. Higher creatinine levels may impact Vitamin B12 clearance, raising the risk of deficiency [49,50]. Evidence suggests the potentially complex relationship between altered creatinine and Vitamin B12 levels in patients with kidney disease [51]. A deficiency of Vitamin B12 and impaired creatinine clearance, as well as high levels of both, can occur in individuals with certain health conditions, including kidney disease. Moreover, chronic kidney disease can affect Vitamin B12 metabolism and lead to lower levels of the vitamin in the body. Reduced kidney function may impact nutrient absorption, including Vitamin B12 [49,52]. Notably, some patients lacked Vitamin B12 measurements, potentially affecting the analysis. People at risk of developing Vitamin B12 deficiencies while taking lamotrigine should monitor their vitamin levels to ensure optimal health because anticonvulsants have been associated with Vitamin B12 deficiency [53]. Furthermore, elevated Vitamin B12 levels can indicate liver problems [52,54].
For the sake of exploration, one could consider two hypothetical mechanisms. The first being renal transporter interaction, lamotrigine may hypothetically influence renal transporters involved in the clearance of both creatinine and Vitamin B12. The drug could potentially alter the activity of transporters responsible for reabsorbing creatinine in the renal tubules, leading to decreased serum creatinine levels. Concurrently, this alteration in renal transporters might influence the absorption or metabolism of Vitamin B12, resulting in decreased levels. Secondly, lamotrigine can be responsible for inflammatory response and Vitamin B12 metabolism. Lamotrigine might induce an inflammatory response that, in turn, could affect the metabolism of Vitamin B12. Inflammation has been associated with alterations in Vitamin B12 absorption and transport [55,56]. If lamotrigine induces such an inflammatory response, it could hypothetically lead to decreased Vitamin B12 levels alongside changes in serum creatinine.
The existing literature on the association between Vitamin B12, antiseizure drugs and liver damage yields varied findings, resulting in uncertainty about the connection between elevated Vitamin B12 levels in epilepsy treatment and liver dysfunction. Hauser et al. found that valproate therapy increased Vitamin B12 levels and impaired liver synthetic function in children with epilepsy [57]. However, Jacobsen et al. found no signs of permanent liver damage in epileptics under anticonvulsant treatment [58], and Elkhatib et al. found that elevated serum Vitamin B12 concentration in patients receiving chronic parenteral nutrition was not an indicator of hepatic pathology [59]. Rao et al. found that changes in serum amino acid profiles in epileptic patients treated with anticonvulsant drugs occurred in patients with alteration of serum liver enzymes, but it is uncertain whether this implies a causal relation. Overall, the papers suggest that there may be some association between anticonvulsant treatment and liver dysfunction, but the relationship between heightened Vitamin B12 levels and liver dysfunction is not clear. Additionally, when assessing liver function, elevated Vitamin B12 levels, which may be a proxy for poor liver function, are not a significant predictor of death following ICU admission [51,54].
While our study does not establish a definitive cause-and-effect relationship between creatinine and Vitamin B12 levels concerning overall health, it underscores the significance of further clinical investigations. Exploring potential relationships and interactions among these factors is crucial, urging the need for additional studies to provide a more comprehensive understanding of these associations. We need to recognize that the relationship between anticonvulsants and Vitamin B12 levels can be complex and may vary depending on individual responses, the specific antiseizure drug used, and the duration of treatment.
In our study, all the patients who exhibited altered values of Vitamin B12 had elevated serum levels of Vitamin B12, and not a single patient showed values lower than the reference range. We thoroughly assessed the patients receiving polytherapy and found that only three out of the seven individuals were on valproate treatment. Valproate treatment is typically associated with higher Vitamin B12 levels [57]. Hence, the elevated levels of Vitamin B12 observed in these patients could be attributed to medications other than lamotrigine that were included in their combination therapy. Additionally, we examined the patient’s medical records to determine whether they had altered Vitamin B12 values during earlier hospital visits, and interestingly, all of them had previously shown altered levels of this parameter in the different visits we accessed. A noteworthy observation is that patients on a 150 mg dosage of lamotrigine did not receive a concurrent valproate prescription, whereas those on a 400 mg dosage underwent valproate therapy. The complexity of polytherapy could potentially impact the outcomes. Once again, larger controlled studies are needed to clarify the relationship between lamotrigine, Vitamin B12 levels, creatinine, and other medications’ effects. No definitive association between Vitamin B12 and lamotrigine exists.
Moreover, we also observed significant correlations between Vitamin B12 and lipid parameters (total cholesterol, triglycerides, non-HDL cholesterol, LDL cholesterol, and VDL cholesterol). These findings indicate that there might be some level of interdependence or mutual influence between Vitamin B12 levels and lipid metabolism, which can be confirmed by the literature [60,61]. Vitamin B12 is essential for various biochemical processes, including converting homocysteine to methionine, a critical step in lipid metabolism. Methionine is a precursor for synthesizing phospholipids, major components of cell membranes and lipoproteins. Adequate Vitamin B12 levels support healthy lipid metabolism and profiles. Vitamin B12 plays a role in regulating enzymes affecting lipid metabolism [62,63]. Deficiency may alter enzyme activity and disrupt lipid profiles. However, observed correlations may result from factors influencing both Vitamin B12 and lipids independently. As correlation does not imply causation, further research is necessary. According to the literature, anticonvulsant drugs can impact cholesterol levels, with variations among different medications [64]. Regarding lamotrigine, the evidence about its effects on cholesterol levels is somewhat conflicting. Some studies have reported increases in total cholesterol and LDL cholesterol levels in patients taking lamotrigine, similar to the effects seen with other anticonvulsants. On the other hand, other studies have not found significant changes in cholesterol levels associated with lamotrigine use [65,66]. The impact of lamotrigine on lipid metabolism varies among individuals and can be influenced by factors like dose, treatment duration, and individual characteristics. Effects on lipid metabolism may also differ between epilepsy and bipolar disorder patients [67].
While evidence supporting the unequivocal benefits of TDM for lamotrigine concentrations is inconclusive, its value persists in specific scenarios. These include evaluating treatment response, ensuring adherence, investigating side effects, considering coadministration effects, and accounting for individual metabolic differences [36,37]. Despite our research offering valuable insights, we acknowledge certain limitations. The study’s retrospective nature relies on existing medical records, potentially introducing inaccuracies or missing data. Additionally, the small sample size and the specificity of the study location may limit generalizability, with the study not controlling for potential confounding variables.
The purpose of incorporating clinical data was to assess which clinical parameters were altered and how they could influence the pharmacokinetics of lamotrigine. This allowed us to highlight population groups in which the pharmacokinetics of lamotrigine could be influenced. Moreover, it enabled us to formulate hypotheses for each group, explaining how their metabolism might be altered or saturated, potentially leading to hepatotoxicity and DILI.
4.2. Pharmacokinetic Evaluation of Lamotrigine and Implications for Liver Function
After the analysis of clinical parameter, using the PBPK model and population simulation of lamotrigine, we gained valuable insights into its pharmacokinetics at different doses and its potential implications for liver function assessment and therapeutic drug monitoring in different populations. Firstly, the bioavailability of lamotrigine decreases as the dose increases, indicating that a smaller proportion of the administered dose reaches systemic circulation. The saturation of metabolic pathways could be a contributing factor to this observation. Lamotrigine is metabolized predominantly by glucuronic acid conjugation. At higher doses, the enzymes responsible for this metabolism might become saturated leading to potentially leading to a lower apparent bioavailability. This could result in less of the drug being available to cause toxic effects. Lamotrigine is known to exhibit linear pharmacokinetics over the therapeutic dose range. This means that the drug’s concentration in the body will increase proportionally with an increase in the dose. The bioavailability of lamotrigine is usually estimated at 98%, indicating that almost all of the administered dose is available in the blood for distribution to the tissue. However, it is important to note that while the pharmacokinetics of lamotrigine are generally linear, individual variations can occur. Factors such as the genetic polymorphisms of the major metabolizing enzymes (UGT1A4, UGT2B7) can influence the pharmacokinetics of lamotrigine [68]. At very high doses like the ones we have been simulating, it is possible that the absorption or metabolic pathways could become saturated, leading to a decrease in bioavailability. However, this is generally not observed within the therapeutic dose range for lamotrigine [69]. Secondly, the peak Cmax of lamotrigine increases with higher doses. This finding is expected, as higher doses result in higher maximum drug concentrations in the plasma. On the contrary, lamotrigine’s Tmax does not follow a consistent trend with increasing doses. This suggests that lamotrigine Tmax may be influenced by other factors beyond the dose, such as individual patient characteristics or drug interactions, which require further investigation.
Furthermore, the AUC0–inf and the AUC0–t both increase with higher doses of lamotrigine. This indicates higher overall drug exposure with increased doses, which may have implications for dosing considerations, potential toxicity, and the need for therapeutic drug monitoring. Our findings underscore the importance of therapeutic drug monitoring in patients receiving lamotrigine, especially at higher doses, to ensure optimal drug levels and minimize the risk of adverse effects.
These observed differences emphasize the importance of therapeutic monitoring. For instance, higher Cmax levels may indicate potential drug accumulation and an increased risk of side effects, which means that a 600 mg dose might be excessive for certain individuals. The simulation of a 1200 mg dose allowed us to evaluate the implications of lamotrigine overdose [70,71]. This finding underscores the importance of avoiding such high doses and highlights the potential risks associated with exceeding recommended dosages. Overall, this in silico analysis supports the need for cautious and personalized prescribing practices. Therapeutic monitoring is essential to ensure optimal drug exposure, minimize side effects, and prevent potential overdose situations [72,73]. We guided our simulations by comparing our results for lamotrigine at the dose 200 mg with values available in the literature.
Our in silico study, though valuable, has limitations. The PBPK model is a simplified representation of drug metabolism and does not capture real-world complexities. It relies on simulated data, not clinical data. The model used a population sample, specifically representing a standard American male weighing 70 kg. The selection of weight and height for this standard subject was determined based on user-defined ranges of Body Mass Index (BMI) and body weights, utilizing built-in bivariate distribution functions relevant to the corresponding population group. Although the model is based on existing knowledge and research, real-world patient responses to lamotrigine may vary, and factors not accounted for in the model could influence drug pharmacokinetics. While efforts were made to use accurate data and parameters, uncertainties in input data and model assumptions could impact the study’s outcomes. Further studies that combine PK data with clinical observations are needed to bridge the gap between in silico analysis and real-world outcomes.
4.3. Overall Liver Function Assessment
In our study, we employed DILIsym simulations and SimPops to investigate the pharmacokinetics of lamotrigine across diverse patient populations, including individuals with comorbidities such as T2D and NAFLD [74,75]. The SimPops allowed for the analysis of lamotrigine concentration–time profiles in plasma for different doses, shedding light on potential interactions and metabolic changes in patients with epilepsy who have common comorbidities [74,75,76]. Our typical simulated population consisted of roughly 176 individuals, intentionally designed to exceed variation found in a larger real patient population. The use of SimPops is crucial to introduce population variability, particularly in predicting low-frequency events like DILI, as not all individuals are equally susceptible. This variation in personal traits is essential to understanding DILI susceptibility [77,78]. From the results, it is evident that as the simulated lamotrigine doses increase, there is a corresponding rise in plasma concentration, with distinct deviations at peak points among different concentrations. The simulated 2-week regimen highlights the accumulation of the drug over time.
Diagnosing DILI is often challenging, as no single objective confirmatory lab test is available [79]. DILI diagnosis is primarily based on exclusion criteria, ruling out other potential causes of liver injury. It is crucial to recognize that approximately 10% of individuals experiencing hepatocellular jaundice attributed to drug use may progress to liver failure, making the prompt identification and monitoring of DILI cases essential for patient safety [80]. Therefore, more research is needed to develop reliable biomarkers and diagnostic tools to improve DILI’s early detection and management, ultimately enhancing patient outcomes. Until then, clinicians must remain vigilant and consider DILI as a potential cause when evaluating patients with drug-induced liver dysfunction. The assessment of our virtual population using the eDISH plot, a tool for evaluating drug-induced serious hepatotoxicity, reveals that healthy individuals fall within the normal range, while individuals with TD2 and NAFLD exhibit variations, placing some at risk of drug-induced liver injury. Notably, TD2 patients occupy the middle upper zone of the eDISH plot. Table 7 summarizes the number of simulated individuals with altered parameters suggestive of DILI for different populations. TD2 and NAFLD individuals show increased values for outcome variables, aligning with expected metabolic changes affecting drug ADMET properties. Importantly, no individuals, whether healthy, TD2, or NAFLD, exhibited Hy’s law cases, emphasizing the overall safe profile of lamotrigine. These findings underscore the relevance of in silico simulations in assessing the liver safety profile of drugs across diverse patient populations [77,78]. The regulatory definition of a “Hy’s Law Case” involves specific criteria, including ALT levels greater than three times the ULN and total bilirubin levels greater than two times the ULN, along with evidence of hepatocellular injury (R values > 0.5), drug-induced ALT elevations exceeding three times the ULN (known as “Temple’s Corollary”), and the absence of other more likely causes for the injury [80]. Risk management strategies for DILI are generally ineffective, and mild elevations in ALT may not always reflect liver injury but could be attributed to other factors, such as muscle-related issues. Some drugs can cause ALT elevations due to hepatocyte death or leakage, but they are rarely associated with clinically significant liver injury, like statins, heparins, and tacrine [81,82,83].
In the literature, there has been a reported case of progressive hepatic necrosis with a fatality linked to lamotrigine use [84]. Additionally, previous research indicates that individuals receiving lamotrigine as an additional medication experience adverse response more frequently compared to those taking the medication alone, suggesting the importance of understanding potential drug interactions [85].
Despite valuable insights, this study has limitations, including reliance on in silico simulations and the absence of real-world patient data for validation. Genetic variability, drug–drug interactions, and long-term effects of lamotrigine were not fully explored. Additionally, the study’s simulations cover a 2-week regimen, but the long-term effects of lamotrigine on liver function and the risk of DILI over extended treatment periods are not fully addressed. The PBPK model relies on certain assumptions and parameters that may not fully reflect real-world scenarios. Variability in drug metabolism and individual response to lamotrigine could be influenced by factors not considered in the model. The eDISH plots used to assess liver safety are based on indirect markers and not on direct measures of liver function or injury. As such, they may not provide a comprehensive assessment of DILI risk. All in silico studies involve some level of uncertainty due to model assumptions and input data. While efforts were made to use accurate data, uncertainties could still impact the study’s outcomes. Not all individuals will experience DILI even with drugs that have DILI liabilities, and simulations based on an “average” or “median” human or animal may not accurately predict DILI for every individual [69]. Therefore, population variability plays a crucial role in predicting low-frequency events like DILI. Considering the variability in individual characteristics that contribute to DILI, susceptibility is crucial to identifying potential individual risk factors. Therefore, the relationship between TD2 and NAFLD for DILI is noteworthy. Both TD2 and NAFLD can independently affect liver function, making individuals with these conditions potentially more susceptible to DILI when taking medications like lamotrigine. Individuals with pre-existing liver conditions like NAFLD may have reduced liver function and impaired drug metabolism, increasing their vulnerability to DILI. Similarly, individuals with TD2 may experience altered drug metabolism due to insulin resistance [86] and changes in liver enzyme activity, further elevating the risk of DILI [79,80]. The eDISH plots suggested an increased risk in T2D and NAFLD patients, prompting the importance of vigilance in these patients [80].
In subsequent studies, we aim to delve into a comprehensive examination of these patients to unravel the development and mechanisms that lead to hepatotoxicity and, in severe cases, DILI. The close monitoring of liver enzymes and overall liver health is crucial when prescribing lamotrigine to patients with these underlying conditions to ensure the early detection of any adverse drug reactions and prevent serious liver injury. Further research, including clinical studies and validation, is necessary to better understand the real-world implications and ensure the safe and effective use of lamotrigine in clinical practice.
5. Conclusions
The retrospective analysis of patients treated with lamotrigine at Hospital Santo António unveiled deviations from normal levels in various blood parameters, establishing significant correlations among them. Observing changes in serum levels, including creatinine, albumin, gamma-glutamyl transferase, total bilirubin, and Vitamin B12, we identified strong negative correlations between Vitamin B12 and creatinine. Subsequently, we employed GastroPlus and DILIsym to investigate the impact of clinical parameters on lamotrigine across different patient populations. Constructing a PBPK model in GastroPlus, grounded in ADMET predictions and literature data, we simulated the pharmacokinetic variability of lamotrigine in diverse populations. Our simulations visualized the effects of increasing doses of lamotrigine on its plasma concentration–time profiles (200 mg, 400 mg, 600 mg, 1200 mg) and indicated reduced bioavailability at higher doses. The potential saturation of enzymes responsible for metabolism at elevated doses might result in a lower apparent bioavailability. Utilizing DILIsym, we delved into lamotrigine’s DILI potential for individuals with diabetes and NAFLD. The outcomes revealed an increased risk, underscoring the necessity for vigilant monitoring. This study accentuates the criticality of comprehending lamotrigine’s pharmacokinetics for tailored treatment decisions, enhanced outcomes, and minimized adverse reactions.
Notable discoveries include the observed negative correlation between Vitamin B12 and creatinine levels, along with significant correlations between Vitamin B12 and lipid parameters and the decrease in bioavailability in higher doses. The development of PBPK models has provided valuable insights into lamotrigine’s behavior at different doses, emphasizing the significance of therapeutic drug monitoring, particularly at higher doses. The study identifies potential implications for liver function, emphasizing the importance of prudent and personalized prescribing practices. The comprehensive liver function assessment, conducted through DILIsym simulations and SimPops, offers a broader perspective on dosing, therapeutic monitoring, and treatment outcomes across diverse patient populations. The study acknowledges the challenges in diagnosing DILI and underscores the importance of vigilance in assessing patients with potential liver dysfunction. This research contributes valuable insights into the intricate interplay between lamotrigine pharmacokinetics, serum parameters, and liver function. The findings underscore the significance of personalized medicine, careful monitoring, and ongoing research to ensure the safe and effective use of lamotrigine in diverse patient populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92010015/s1, Table S1. Summary of the SimPops simulated, including population sample size and the number of parameters varied; Figure S1. Distribution of the average age according to the different dose groups. The categories in-clude: “Very Low—1” group (doses < 100 mg) with a minimum dose of 20 mg, “Low—2” group (doses between 100 and 200 mg), “Average—3” group (doses between 200 and 400 mg “High—4” group (doses > 400 mg).
Author Contributions
Conceptualization, N.V. and B.C.; methodology B.C., I.S., J.C.O., H.R. and N.V.; formal analysis, B.C. and N.V.; investigation, B.C., I.S., J.C.O., H.R. and N.V.; resources, N.V.; writing—original draft preparation, B.C.; writing—review and editing, B.C. and N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional through the COMPETE 2020—Operational Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia, in the framework of the projects in CINTESIS, R&D Unit (reference UIDB/4255/2020), and within the scope of the project “RISE–LA/P/0053/2020”. This work was also supported by FCT and FEDER (European Union), award number IF/00092/2014/CP1255/CT0004 and the Chair of Onco-Innovation at FMUP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
B.C. thanks the Chair of Onco-Innovation for her PhD grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Medicines Agency. Lamictal—Referral. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/lamictal (accessed on 9 January 2024).
- FDA. Lamictal Label—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020241s045s051lbl.pdf (accessed on 9 January 2024).
- FDA. FDA Drug Safety Communication: FDA Warns of Serious Immune System Reaction with Seizure and Mental Health Medicine Lamotrigine (Lamictal). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-serious-immune-system-reaction-seizure-and-mental-health (accessed on 9 January 2024).
- French, J.A.; Perucca, E.; Sander, J.W.; Bergfeldt, L.; Baulac, M.; Auerbach, D.S.; Keezer, M.; Thijs, R.D.; Devinsky, O.; Vossler, D.G.; et al. FDA Safety Warning on the Cardiac Effects of Lamotrigine: An Advisory from the Ad Hoc ILAE/AES Task Force. Epilepsia Open 2021, 6, 45–48. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kotake, K.; Watanabe, N.; Fujiwara, T.; Sakamoto, S. Lamotrigine in the Maintenance Treatment of Bipolar Disorder. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Chouchana, M.; Smati, J.; Bloch, V.; Fontan, J.-E.; Etain, B.; Delage, C. Lamotrigine in Mood Disorders: Flash Survey on Prescribing Habits and Blood Tests Practices. Encephale 2023, 49, 640–644. [Google Scholar] [CrossRef]
- Avancini, J.; Maragno, L.; Santi, C.G.; Criado, P.R. Drug Reaction with Eosinophilia and Systemic Symptoms/Drug-Induced Hypersensitivity Syndrome: Clinical Features of 27 Patients. Clin. Exp. Dermatol. 2015, 40, 851–859. [Google Scholar] [CrossRef]
- Tempark, T.; John, S.; Rerknimitr, P.; Satapornpong, P.; Sukasem, C. Drug-Induced Severe Cutaneous Adverse Reactions: Insights into Clinical Presentation, Immunopathogenesis, Diagnostic Methods, Treatment, and Pharmacogenomics. Front. Pharmacol. 2022, 13, 832048. [Google Scholar] [CrossRef]
- Lens, S.; Crespo, G.; Carrión, J.A.; Miquel, R.; Navasa, M. Severe Acute Hepatitis in the Dress Syndrome: Report of Two Cases. Ann. Hepatol. 2010, 9, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cabriales, S.A.; Shear, N.H.; Gonzalez-Moreno, E.I. Liver Involvement in the Drug Reaction, Eosinophilia, and Systemic Symptoms Syndrome. World J. Clin. Cases 2019, 7, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Checa, J.C.; Bagnaninchi, P.; Ye, H.; Sancho-Bru, P.; Falcon-Perez, J.M.; Royo, F.; Garcia-Ruiz, C.; Konu, O.; Miranda, J.; Lunov, O.; et al. Advanced Preclinical Models for Evaluation of Drug-Induced Liver Injury—Consensus Statement by the European Drug-Induced Liver Injury Network [PRO-EURO-DILI-NET]. J. Hepatol. 2021, 75, 935–959. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Gerbes, A.L. Challenges and Future of Drug-Induced Liver Injury Research—Laboratory Tests. Int. J. Mol. Sci. 2022, 23, 6049. [Google Scholar] [CrossRef]
- Im, S.G.; Yoo, S.H.; Park, Y.M.; Lee, S.J.; Jang, S.K.; Jeon, D.O.; Cho, H.J.; Oh, M.J. Liver Dysfunction Induced by Systemic Hypersensitivity Reaction to Lamotrigine: Case Report. Clin. Mol. Hepatol. 2015, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Iriki, H.; Ouchi, T.; Ito, H.; Sawada, M.; Mukai, M.; Nomura, H.; Baba, Y.; Adachi, T.; Funakoshi, T.; Amagai, M.; et al. Case of Lamotrigine-induced Drug Adverse Reaction under Tocilizumab Treatment with Clinical and Virological Features of Drug-induced Hypersensitivity Syndrome. J. Dermatol. 2018, 45, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Azukizawa, H.; Asada, H.; Niihara, H.; Morita, E.; Yamauchi, T.; Mizukawa, Y.; Kusakabe, Y.; Numazawa, S.; Izumi, M.; et al. Drug-induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms Due to Lamotrigine Differs from That Due to Other Drugs. J. Dermatol. 2019, 46, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Marie, S.; Frost, K.L.; Hau, R.K.; Martinez-Guerrero, L.; Izu, J.M.; Myers, C.M.; Wright, S.H.; Cherrington, N.J. Predicting Disruptions to Drug Pharmacokinetics and the Risk of Adverse Drug Reactions in Non-Alcoholic Steatohepatitis Patients. Acta Pharm. Sin. B 2023, 13, 1–28. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of Nonalcoholic Fatty Liver Disease as Risk Factor for Drug-Induced Hepatotoxicity. J. Clin. Transl. Res. 2017, 3, 212–232. [Google Scholar] [CrossRef]
- Lee, C.; Lui, D.T.; Lam, K.S. Non-alcoholic Fatty Liver Disease and Type 2 Diabetes: An Update. J. Diabetes Investig. 2022, 13, 930–940. [Google Scholar] [CrossRef]
- Kosmalski, M.; Ziółkowska, S.; Czarny, P.; Szemraj, J.; Pietras, T. The Coexistence of Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. J. Clin. Med. 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.G.; Rau, M.; Jahn, D.; Geier, A. Changes in Drug Transport and Metabolism and Their Clinical Implications in Non-Alcoholic Fatty Liver Disease. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 625–640. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Olmos, L.; De Mena, F.; Pieczanski, P.; Moncalvo, J.R. Bioequivalence of lamotrigine 50-mg tablets in healthy male volunteers: A randomized, single-dose, 2-period, 2-sequence crossover study. Arzneimittelforschung 2012, 62, 470–476. [Google Scholar] [CrossRef]
- Jee, A.; Sernoskie, S.C.; Uetrecht, J. Idiosyncratic Drug-Induced Liver Injury: Mechanistic and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2954. [Google Scholar] [CrossRef]
- Karaoulanis, S.E.; Syngelakis, M.; Fokas, K. Rhabdomyolysis after Lamotrigine Overdose: A Case Report and Review of the Literature. Ann. Gen. Psychiatry 2016, 15, 6. [Google Scholar] [CrossRef]
- Fontana, R.J. Pathogenesis of Idiosyncratic Drug-Induced Liver Injury and Clinical Perspectives. Gastroenterology 2014, 146, 914–928.e1. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Biomarkers of Drug-Induced Liver Injury: Progress and Utility in Research, Medicine, and Regulation. Expert. Rev. Mol. Diagn. 2018, 18, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular Biomarkers in Drug-Induced Liver Injury: Challenges and Future Perspectives. Front. Pharmacol. 2020, 10, 1667. [Google Scholar] [CrossRef]
- Nicoletti, P.; Barrett, S.; McEvoy, L.; Daly, A.K.; Aithal, G.; Lucena, M.I.; Andrade, R.J.; Wadelius, M.; Hallberg, P.; Stephens, C.; et al. Shared Genetic Risk Factors Across Carbamazepine-Induced Hypersensitivity Reactions. Clin. Pharmacol. Ther. 2019, 106, 1028–1036. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Understanding Lamotrigine’s Role in the CNS and Possible Future Evolution. Int. J. Mol. Sci. 2023, 24, 6050. [Google Scholar] [CrossRef]
- Matsuo, F.; Gay, P.; Madsen, J.; Tolman, K.G.; Rollins, D.E.; Risner, M.E.; Lai, A.A. Lamotrigine High-Dose Tolerability and Safety in Patients with Epilepsy: A Double-Blind, Placebo-Controlled, Eleven-Week Study. Epilepsia 1996, 37, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic Drugs—Best Practice Guidelines for Therapeutic Drug Monitoring: A Position Paper by the Subcommission on Therapeutic Drug Monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Garnett, W.R. Lamotrigine: Pharmacokinetics. J. Child. Neurol. 1997, 12, S10–S15. [Google Scholar] [CrossRef]
- Milosheska, D.; Lorber, B.; Vovk, T.; Kastelic, M.; Dolžan, V.; Grabnar, I. Pharmacokinetics of Lamotrigine and Its Metabolite N-2-Glucuronide: Influence of Polymorphism of UDP-Glucuronosyltransferases and Drug Transporters. Br. J. Clin. Pharmacol. 2016, 82, 399–411. [Google Scholar] [CrossRef]
- Douglas-Hall, P.; Dzahini, O.; Gaughran, F.; Bile, A.; Taylor, D. Variation in Dose and Plasma Level of Lamotrigine in Patients Discharged from a Mental Health Trust. Ther. Adv. Psychopharmacol. 2017, 7, 17–24. [Google Scholar] [CrossRef]
- Mitra-Ghosh, T.; Callisto, S.P.; Lamba, J.K.; Remmel, R.P.; Birnbaum, A.K.; Barbarino, J.M.; Klein, T.E.; Altman, R.B. PharmGKB Summary: Lamotrigine Pathway, Pharmacokinetics and Pharmacodynamics. Pharmacogenet Genom. 2020, 30, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, F.; Brasso, C.; Villari, V.; Rocca, P. Current Status of Therapeutic Drug Monitoring in Mental Health Treatment: A Review. Pharmaceutics 2022, 14, 2674. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Grover, S.; Rao, G.P. Clinical Practice Guidelines for Management of Bipolar Disorder. Indian. J. Psychiatry 2017, 59, S51–S66. [Google Scholar] [CrossRef]
- St Louis, E.K.; Rosenfeld, W.E.; Bramley, T. Antiepileptic Drug Monotherapy: The Initial Approach in Epilepsy Management. Curr. Neuropharmacol. 2009, 7, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Little’s Test of Missing Completely at Random. Stata J. Promot. Commun. Stat. Stata 2013, 13, 795–809. [Google Scholar] [CrossRef]
- van Dijkman, S.C.; de Jager, N.C.B.; Rauwé, W.M.; Danhof, M.; Della Pasqua, O. Effect of Age-Related Factors on the Pharmacokinetics of Lamotrigine and Potential Implications for Maintenance Dose Optimisation in Future Clinical Trials. Clin. Pharmacokinet. 2018, 57, 1039–1053. [Google Scholar] [CrossRef]
- Conner, T.M.; Reed, R.C.; Zhang, T. A Physiologically Based Pharmacokinetic Model for Optimally Profiling Lamotrigine Disposition and Drug–Drug Interactions. Eur. J. Drug Metab. Pharmacokinet. 2018, 44, 389–408. [Google Scholar] [CrossRef]
- Porat, D.; Azran, C.; Kais, H.; Dahan, A. Managing the Unpredictable: Mechanistic Analysis and Clinical Recommendations for Lamotrigine Treatment after Bariatric Surgery. J. Clin. Med. 2021, 10, 5627. [Google Scholar] [CrossRef]
- Rambeck, B.; Wolf, P. Lamotrigine Clinical Pharmacokinetics. Clin. Pharmacokinet. 1993, 25, 433–443. [Google Scholar] [CrossRef]
- Agabeyoglu, I.; Incecayir, T. Pharmacokinetic Modelling of Lamotrigine from Plasma Concentrations in Healthy Volunteers. J. Bioanal. Biomed. 2009, 1, 41–45. [Google Scholar] [CrossRef]
- Argikar, U.A.; Remmel, R.P. Variation in Glucuronidation of Lamotrigine in Human Liver Microsomes. Xenobiotica 2009, 39, 355–363. [Google Scholar] [CrossRef]
- Polepally, A.R.; Brundage, R.C.; Remmel, R.P.; Leppik, I.E.; Pennell, P.B.; White, J.R.; Ramsay, R.E.; Kistner, B.M.; Birnbaum, A.K. Lamotrigine Pharmacokinetics following Oral and Stable-labeled Intravenous Administration in Young and Elderly Adult Epilepsy Patients: Effect of Age. Epilepsia 2018, 59, 1718–1726. [Google Scholar] [CrossRef]
- Cohen, A.F.; Land, G.S.; Breimer, D.D.; Yuen, W.C.; Winton, C.; Peck, A.W. Lamotrigine, a New Anticonvulsant: Pharmacokinetics in Normal Humans. Clin. Pharmacol. Ther. 1987, 42, 535–541. [Google Scholar] [CrossRef]
- Battino, D.; Estienne, M.; Avanzini, G. Clinical Pharmacokinetics of Antiepileptic Drugs in Paediatric Patients. Clin. Pharmacokinet. 1995, 29, 341–369. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Zhou, X.Y.; Ahmed, S.N. Managing the Patient with Epilepsy and Renal Impairment. Seizure 2020, 76, 143–152. [Google Scholar] [CrossRef]
- McMahon, G.M.; Hwang, S.-J.; Tanner, R.M.; Jacques, P.F.; Selhub, J.; Muntner, P.; Fox, C.S. The Association between Vitamin B12, Albuminuria and Reduced Kidney Function: An Observational Cohort Study. BMC Nephrol. 2015, 16, 7. [Google Scholar] [CrossRef]
- Linnebank, M.; Moskau, S.; Semmler, A.; Widman, G.; Stoffel-Wagner, B.; Weller, M.; Elger, C.E. Antiepileptic Drugs Interact with Folate and Vitamin B12 Serum Levels. Ann. Neurol. 2011, 69, 352–359. [Google Scholar] [CrossRef]
- Sugihara, T.; Koda, M.; Matono, T.; Okamoto, K.; Murawaki, Y.; Isomoto, H.; Tokunaga, S. Risk Assessment of Hepatocellular Carcinoma in General Population by Liver Stiffness in Combination with Controlled Attenuation Parameter Using Transient Elastography: A Cross Sectional Study. Yonago Acta Med. 2017, 60, 106–112. [Google Scholar] [CrossRef][Green Version]
- Wu, H.H.L.; Wang, A.Y.-M. Vitamin B12 and Chronic Kidney Disease. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2022; pp. 325–353. [Google Scholar]
- Dinç, D.; Schulte, P.F.J. The Use of Anticonvulsants and the Levels of Folate, Vitamin B12 and Homocysteine. Tijdschr. Psychiatr. 2018, 60, 20–28. [Google Scholar]
- Callaghan, F.M.; Leishear, K.; Abhyankar, S.; Demner-Fushman, D.; McDonald, C.J. High Vitamin B12 Levels Are Not Associated with Increased Mortality Risk for ICU Patients after Adjusting for Liver Function: A Cohort Study. ESPEN J. 2014, 9, e76–e83. [Google Scholar] [CrossRef]
- Romain, M.; Sviri, S.; Linton, D.M.; Stav, I.; van Heerden, P.V. The Role of Vitamin B12 in the Critically Ill—A Review. Anaesth. Intensive Care 2016, 44, 447–452. [Google Scholar] [CrossRef]
- Tal, S.; Shavit, Y.; Stern, F.; Malnick, S. Association Between Vitamin B12 Levels and Mortality in Hospitalized Older Adults. J. Am. Geriatr. Soc. 2010, 58, 523–526. [Google Scholar] [CrossRef]
- Hauser, E.; Seidl, R.; Freilinger, M.; Male, C.; Herkner, K. Hematologic Manifestations and Impaired Liver Synthetic Function during Valproate Monotherapy. Brain Dev. 1996, 18, 105–109. [Google Scholar] [CrossRef]
- Jacobsen, N.O.; Mosekilde, L.; Myhre-Jensen, O.; Pedersen, E.; Wildenhoff, K.E. Liver Biopsies in Epileptics during Anticonvulsant Therapy. Acta Med. Scand. 1976, 199, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, I.; Cao, W.; Rao, S.; Fryer, J.; Buchman, A.L. Serum B12 Concentration Is Elevated in Patients Receiving Chronic Parenteral Nutrition, But Is Not a Marker of Intestinal Failure-Associated Liver Disease. J. Clin. Gastroenterol. 2010, 44, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S.; Aljuraiban, G.S.; Danish Hussain, S.; Alnaami, A.M.; Saravanan, P.; Al-Daghri, N. Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients 2020, 12, 2395. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Jayashri, R.; Sukumar, N.; Venkataraman, H.; Pradeepa, R.; Gokulakrishnan, K.; Anjana, R.M.; McTernan, P.G.; Tripathi, G.; Patel, V.; et al. Vitamin B12 Deficiency Is Associated with Adverse Lipid Profile in Europeans and Indians with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, H.W.; Redfield, B.G.; Bieri, J.G.; Weissbach, H. Studies on the role of vitamin b12 for the synthesis of methionine in liver. Ann. N. Y Acad. Sci. 1964, 112, 791–798. [Google Scholar] [CrossRef]
- Ge, Y.; Zadeh, M.; Mohamadzadeh, M. Vitamin B12 Regulates the Transcriptional, Metabolic, and Epigenetic Programing in Human Ileal Epithelial Cells. Nutrients 2022, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.V.; Davidson, B.A.; Escalaya, L.; Costella, J.; Saposnik, G.; Burneo, J.G. Antiepileptic Drug Use for Treatment of Epilepsy and Dyslipidemia: Systematic Review. Epilepsy Res. 2015, 113, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S.; Skidmore, C.T.; Abidin, C.J.; Morales, M.C.; Chervoneva, I.; Capuzzi, D.M.; Sperling, M.R. Effects of Antiepileptic Drugs on Lipids, Homocysteine, and C-reactive Protein. Ann. Neurol. 2009, 65, 448–456. [Google Scholar] [CrossRef]
- Mintzer, S.; Trinka, E.; Kraemer, G.; Chervoneva, I.; Werhahn, K.J. Impact of Carbamazepine, Lamotrigine, and Levetiracetam on Vascular Risk Markers and Lipid-lowering Agents in the Elderly. Epilepsia 2018, 59, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, K.M.; Moreira, C.L.R.L.; Lafer, B. Metabolic Syndrome and Bipolar Disorder: What Should Psychiatrists Know? CNS Neurosci. Ther. 2012, 18, 160–166. [Google Scholar] [CrossRef]
- Suzuki, T.; Mihara, K.; Nagai, G.; Kagawa, S.; Nakamura, A.; Nemoto, K.; Kondo, T. Relationship Between UGT1A4 and UGT2B7 Polymorphisms and the Steady-State Plasma Concentrations of Lamotrigine in Patients With Treatment-Resistant Depressive Disorder Receiving Lamotrigine as Augmentation Therapy. Ther. Drug Monit. 2019, 41, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, G.D. Variability in the Human Drug Response. Thromb. Res. 1983, 29, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Alabi, A.; Todd, A.; Husband, A.; Reilly, J. Safety Profile of Lamotrigine in Overdose. Ther. Adv. Psychopharmacol. 2016, 6, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Alyahya, B.; Friesen, M.; Nauche, B.; Laliberté, M. Acute Lamotrigine Overdose: A Systematic Review of Published Adult and Pediatric Cases. Clin. Toxicol. 2017, 56, 81–89. [Google Scholar] [CrossRef]
- Bondareva, I. Individualizing Antiepileptic Therapy for Patients. In Individualized Drug Therapy for Patients; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–371. [Google Scholar]
- Chong, E.; Dupuis, L.L. Therapeutic Drug Monitoring of Lamotrigine. Ann. Pharmacother. 2002, 36, 917–920. [Google Scholar] [CrossRef]
- Tien, N.; Wu, T.-Y.; Lin, C.-L.; Chu, F.-Y.; Wang, C.C.N.; Hsu, C.Y.; Tsai, F.-J.; Fang, Y.-J.; Lim, Y.-P. Association of Epilepsy, Anti-Epileptic Drugs (AEDs), and Type 2 Diabetes Mellitus (T2DM): A Population-Based Cohort Retrospective Study, Impact of AEDs on T2DM-Related Molecular Pathway, and via Peroxisome Proliferator-Activated Receptor γ Transactivation. Front. Endocrinol. 2023, 14, 1156952. [Google Scholar] [CrossRef]
- Luef, G.; Rauchenzauner, M.; Waldmann, M.; Sturm, W.; Sandhofer, A.; Seppi, K.; Trinka, E.; Unterberger, I.; Ebenbichler, C.F.; Joannidis, M.; et al. Non-Alcoholic Fatty Liver Disease (NAFLD), Insulin Resistance and Lipid Profile in Antiepileptic Drug Treatment. Epilepsy Res. 2009, 86, 42–47. [Google Scholar] [CrossRef]
- Shlobin, N.A.; Sander, J.W. Drivers for the Comorbidity of Type 2 Diabetes Mellitus and Epilepsy: A Scoping Review. Epilepsy Behav. 2020, 106, 107043. [Google Scholar] [CrossRef]
- Howell, B.A.; Yang, Y.; Kumar, R.; Woodhead, J.L.; Harrill, A.H.; Clewell, H.J.; Andersen, M.E.; Siler, S.Q.; Watkins, P.B. In Vitro to in Vivo Extrapolation and Species Response Comparisons for Drug-Induced Liver Injury (DILI) Using DILIsymTM: A Mechanistic, Mathematical Model of DILI. J. Pharmacokinet. Pharmacodyn. 2012, 39, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, G.; Yang, K.; Gebremichael, Y.; Howell, B.A.; Murray, F.J.; Jacobson-Kram, D.; Jaeschke, H.; Kuffner, E.; Gelotte, C.K.; Lai, J.C.K.; et al. Application of the DILIsym® Quantitative Systems Toxicology Drug-Induced Liver Injury Model to Evaluate the Carcinogenic Hazard Potential of Acetaminophen. Regul. Toxicol. Pharmacol. 2020, 118, 104788. [Google Scholar] [CrossRef]
- Brennan, P.N.; Cartlidge, P.; Manship, T.; Dillon, J.F. Guideline Review: EASL Clinical Practice Guidelines: Drug-Induced Liver Injury (DILI). Frontline Gastroenterol. 2021, 13, 332–336. [Google Scholar] [CrossRef]
- Chopra, S.; Saxena, R. Drug-Induced Liver Injury—Perspectives from Pathology. Curr. Pharmacol. Rep. 2018, 4, 182–192. [Google Scholar] [CrossRef]
- Teschke, R.; Danan, G. Advances in Idiosyncratic Drug-Induced Liver Injury Issues: New Clinical and Mechanistic Analysis Due to Roussel Uclaf Causality Assessment Method Use. Int. J. Mol. Sci. 2023, 24, 10855. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H. Drug-Induced Liver Injury Throughout the Drug Development Life Cycle: Where We Have Been, Where We Are Now, and Where We Are Headed. Perspectives of a Clinical Hepatologist. Pharmaceut Med. 2013, 27, 165–191. [Google Scholar] [CrossRef]
- Church, R.J.; Watkins, P.B. In Silico Modeling to Optimize Interpretation of Liver Safety Biomarkers in Clinical Trials. Exp. Biol. Med. 2018, 243, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, K.; Costanza, C.; Behling, C.; Hassanin, T.; Masliah, E. Fatal Progressive Hepatic Necrosis Associated with Lamotrigine Treatment: A Case Report and Literature Review. Dig. Dis. Sci. 2002, 47, 1921–1925. [Google Scholar] [CrossRef]
- Bresnahan, R.; Panebianco, M.; Marson, A.G. Lamotrigine Add-on Therapy for Drug-Resistant Generalised Tonic-Clonic Seizures. Cochrane Database Syst. Rev. 2020, 2020, CD007783. [Google Scholar] [CrossRef]
- Davidson, M.D.; Ballinger, K.R.; Khetani, S.R. Long-Term Exposure to Abnormal Glucose Levels Alters Drug Metabolism Pathways and Insulin Sensitivity in Primary Human Hepatocytes. Sci. Rep. 2016, 6, 28178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).