Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications
Abstract
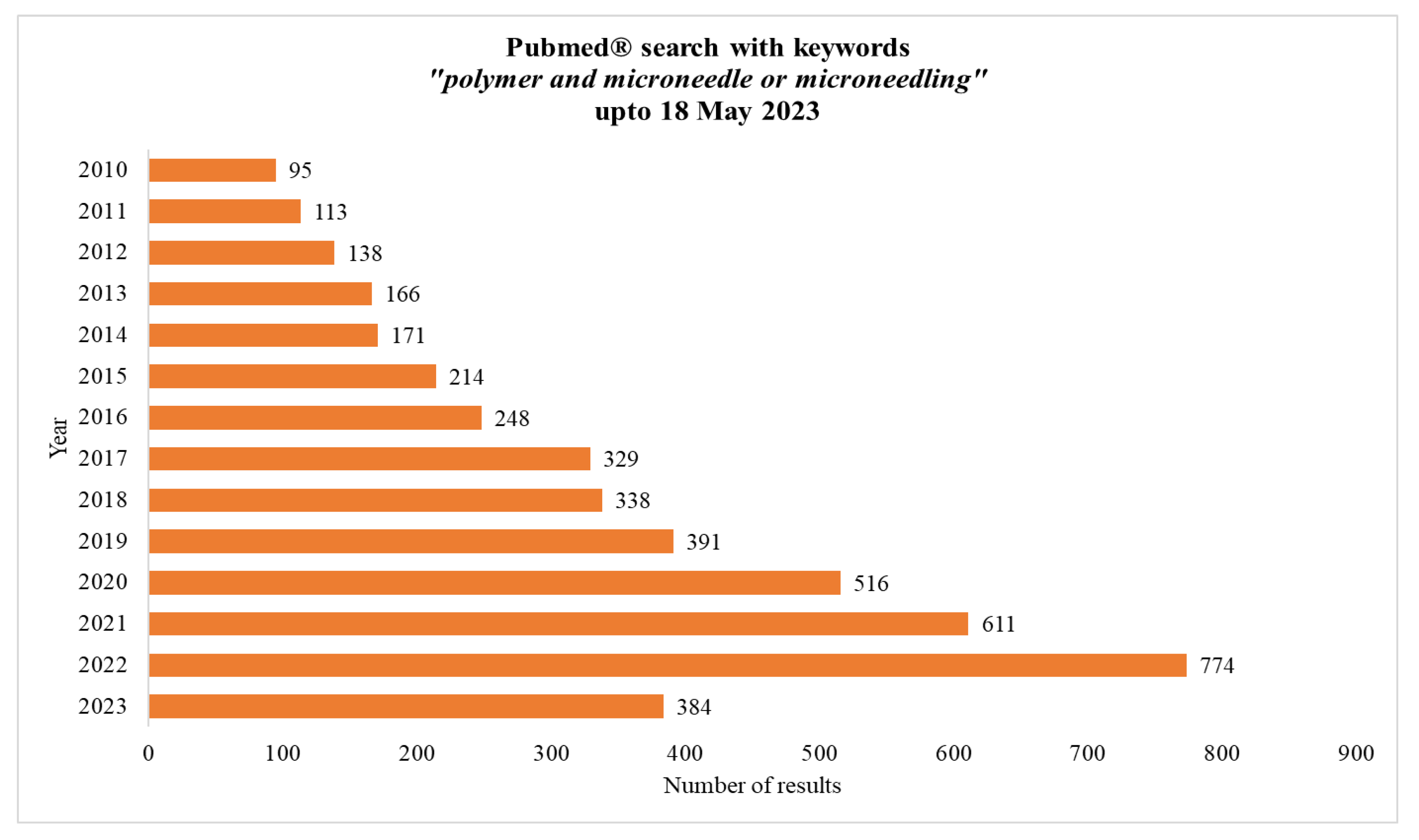
1. Introduction
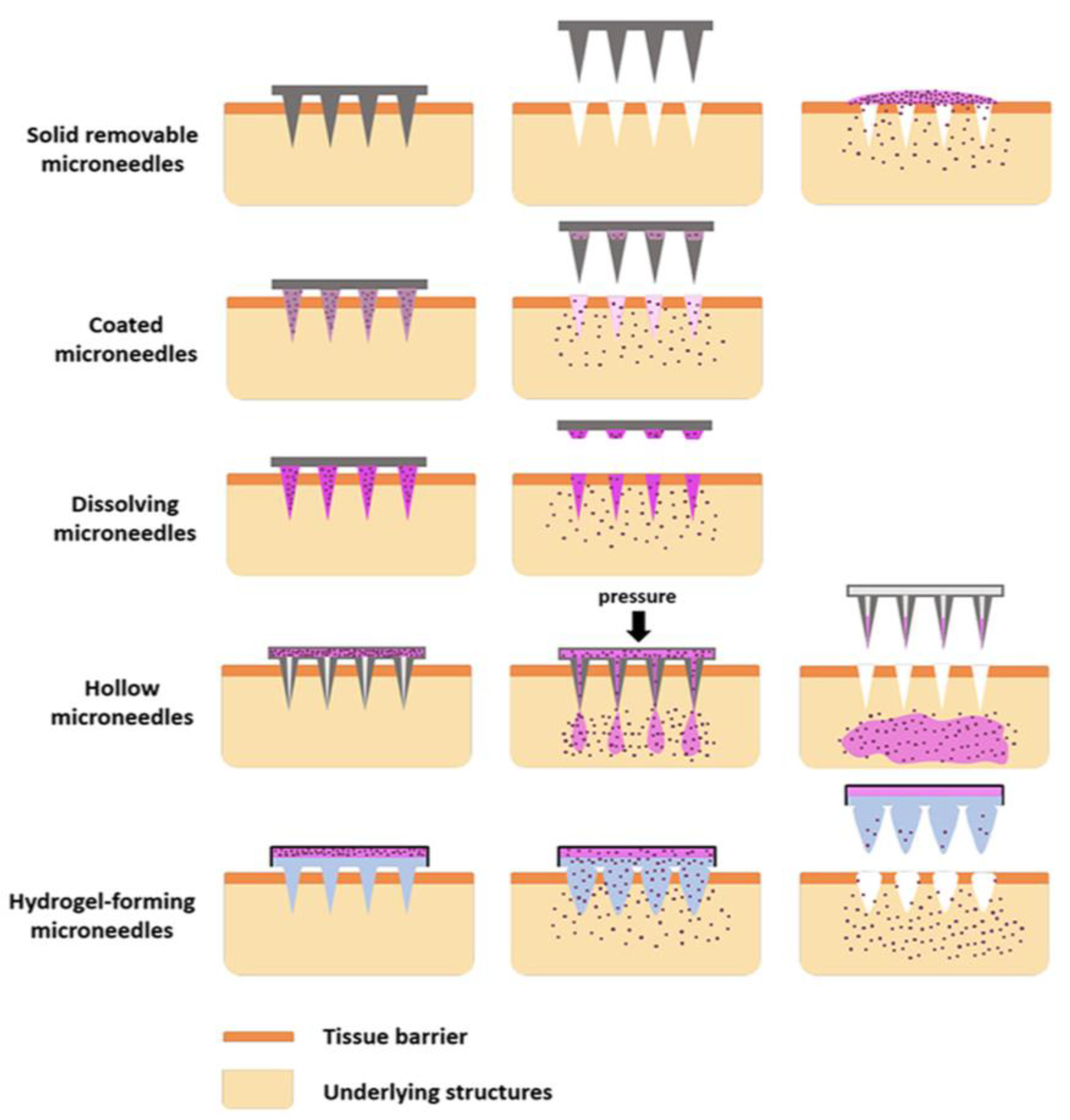
2. Types of Microneedles
2.1. Solid Microneedles
2.2. Hollow Microneedle
2.3. Coated Microneedles
2.4. Dissolving Microneedles
2.5. Hydrogel Microneedles
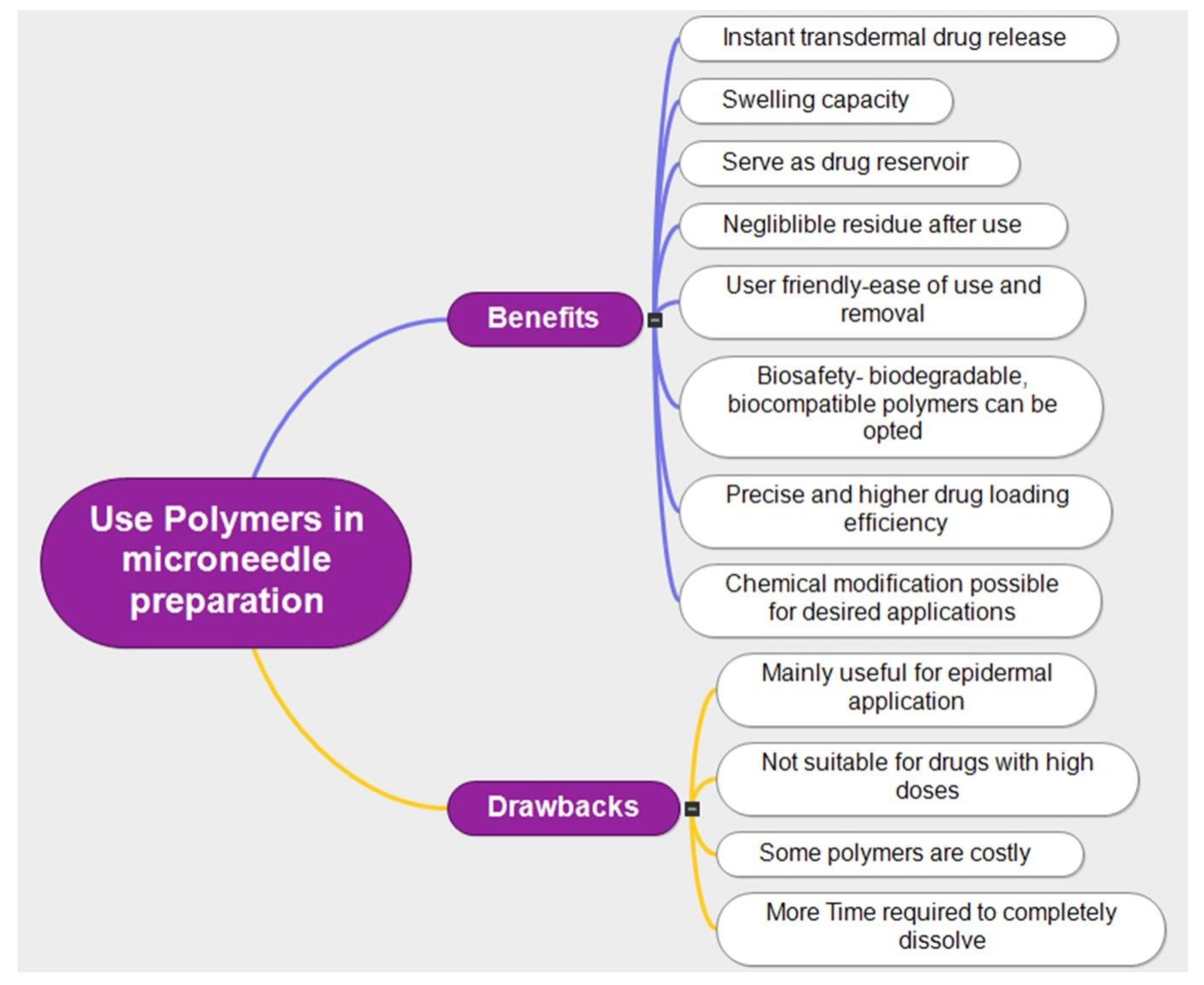
3. Polymeric Microneedle Technology
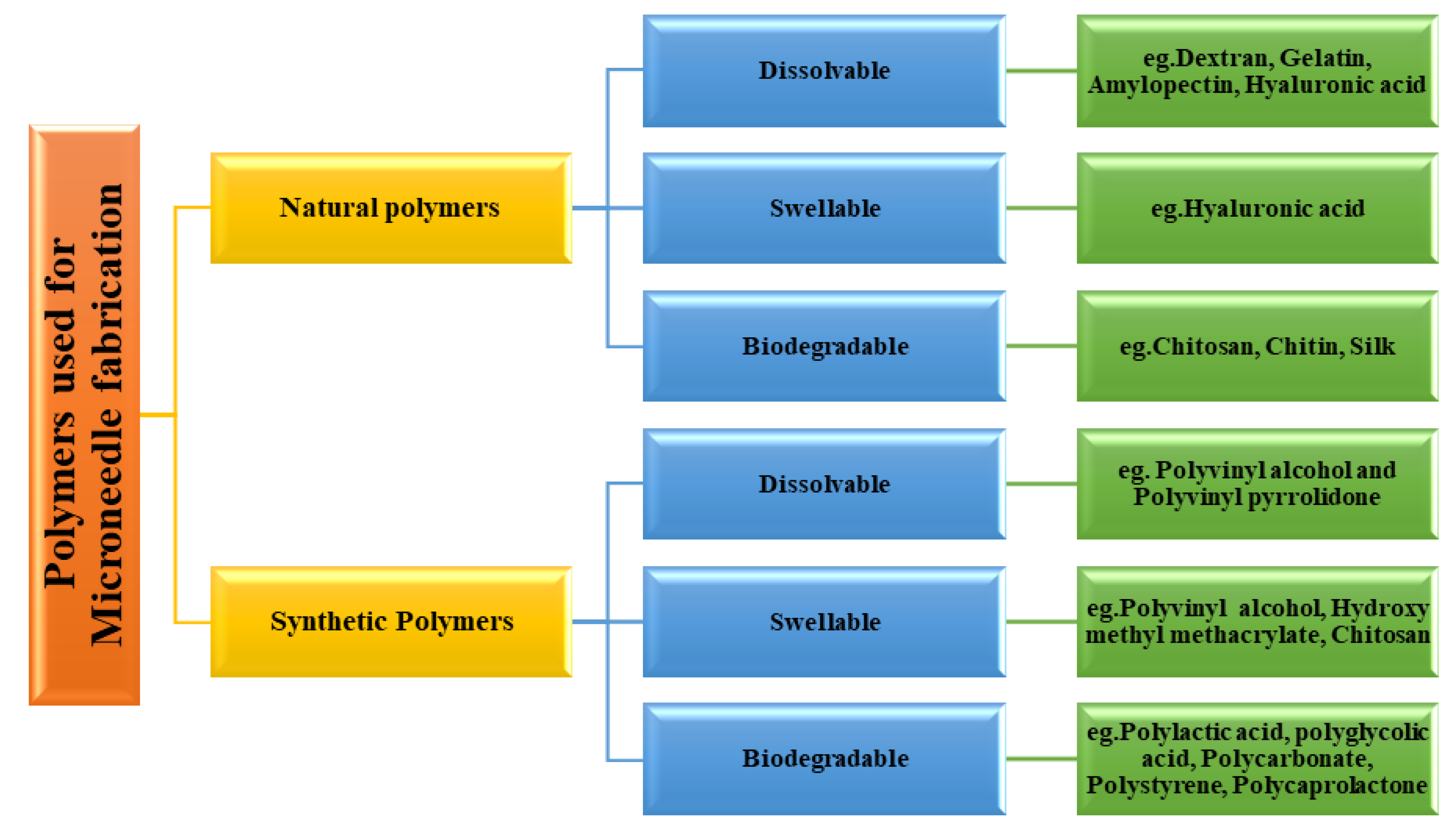
3.1. Classification of Polymer Used for Microneedle Fabrication
3.1.1. Dextran
3.1.2. Hyaluronic Acid
3.1.3. Chitin and Chitosan
3.1.4. Alginate
3.1.5. Gelatin
3.1.6. Gantrez
3.1.7. Hydroxypropyl Methylcellulose (HPMC)
3.1.8. Polycigycidyl Methacrylate
3.1.9. Polyvinyl Alcohol (PVA)
3.1.10. Polystyrene-Block-Poly-(Acrylic Acid) (PSPAA)
3.1.11. Polylactic Acid (PLA)
3.1.12. Polymethyl Methacrylate (PMMA)
3.1.13. Polystyrene
3.1.14. Polycaprolactone
4. Fabrication Techniques of Microneedles
4.1. Micromolding
4.2. Micromilling
4.3. Atomized Spraying to Fill Molds
4.4. 3D and 4D Printing
4.5. Laser Ablation
4.6. Photolithography
4.7. Printing Techniques
4.8. Etching
4.9. Electrospinning
4.10. Co-Extrusion
5. Biomedical Applications of Polymeric Microneedles
5.1. Therapeutic Applications
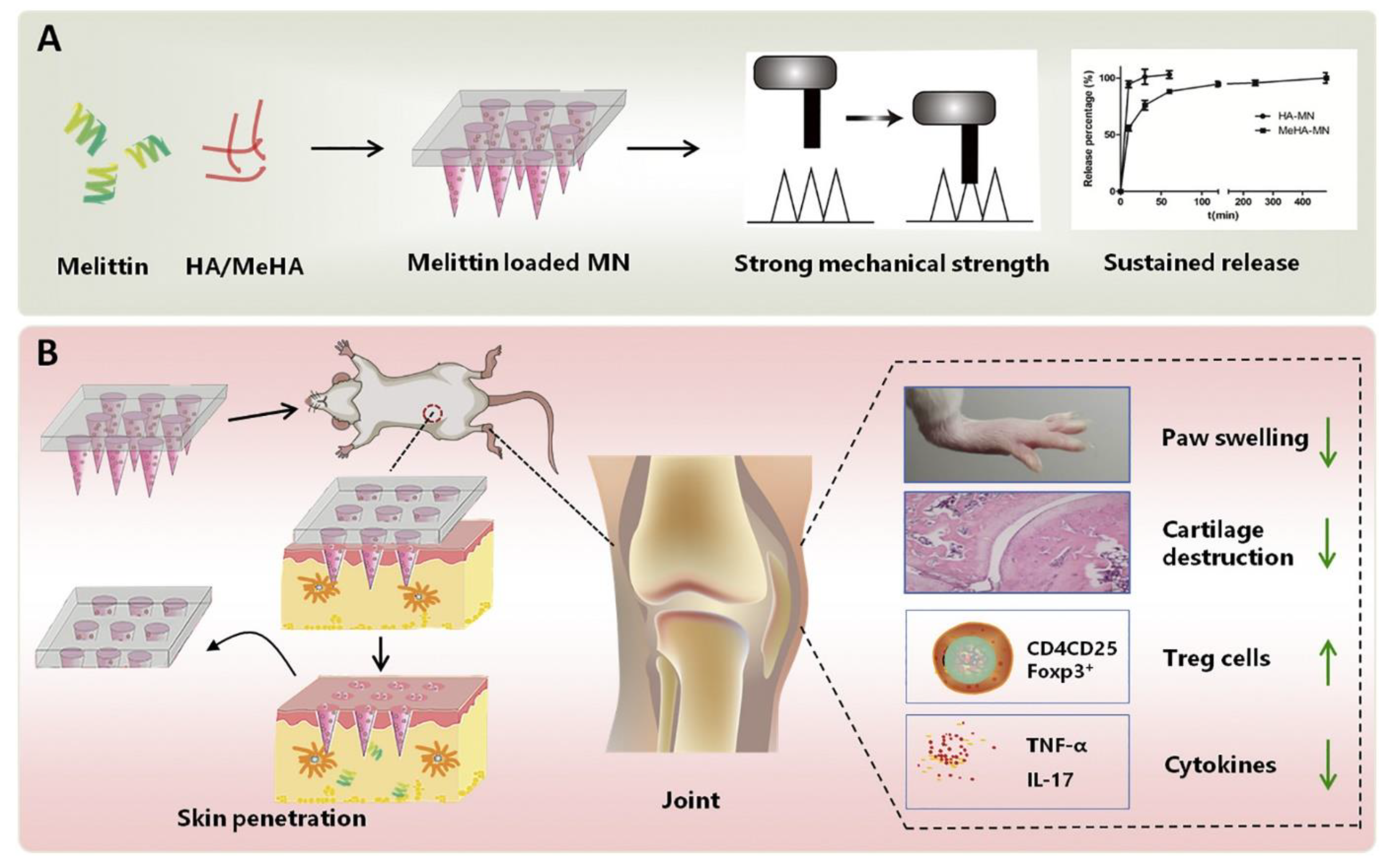
5.1.1. Rheumatoid Arthritis
5.1.2. Skin Diseases
Melasma
Psoriasis
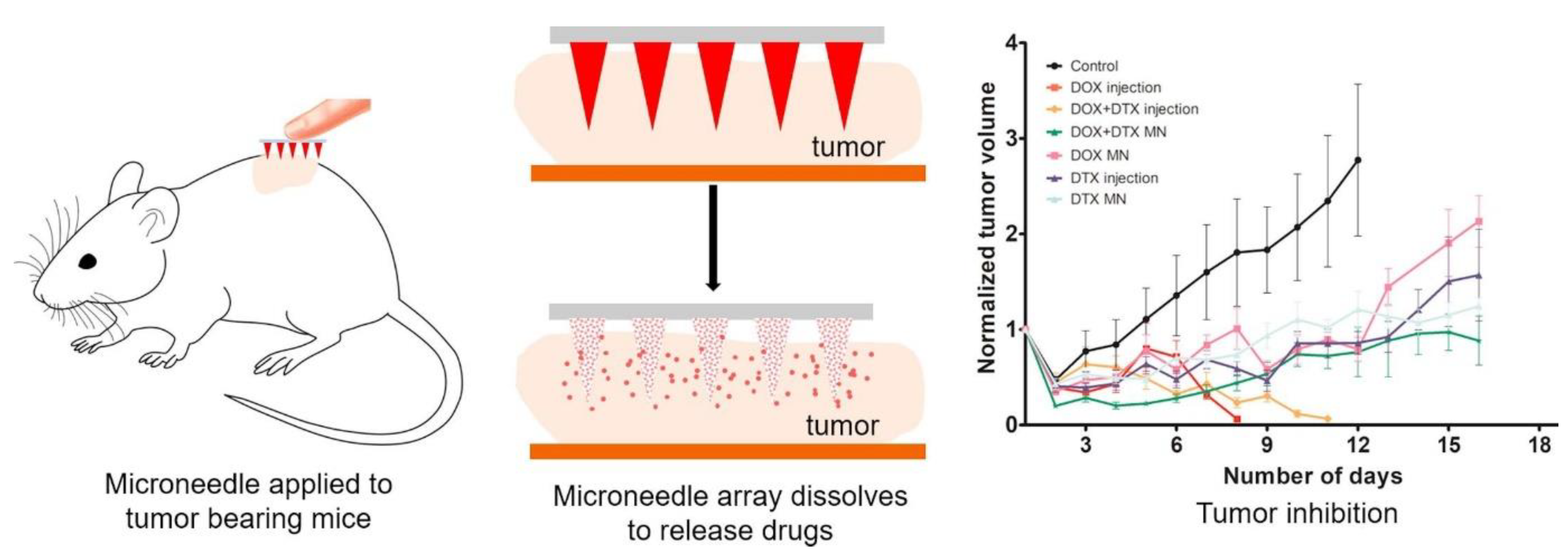
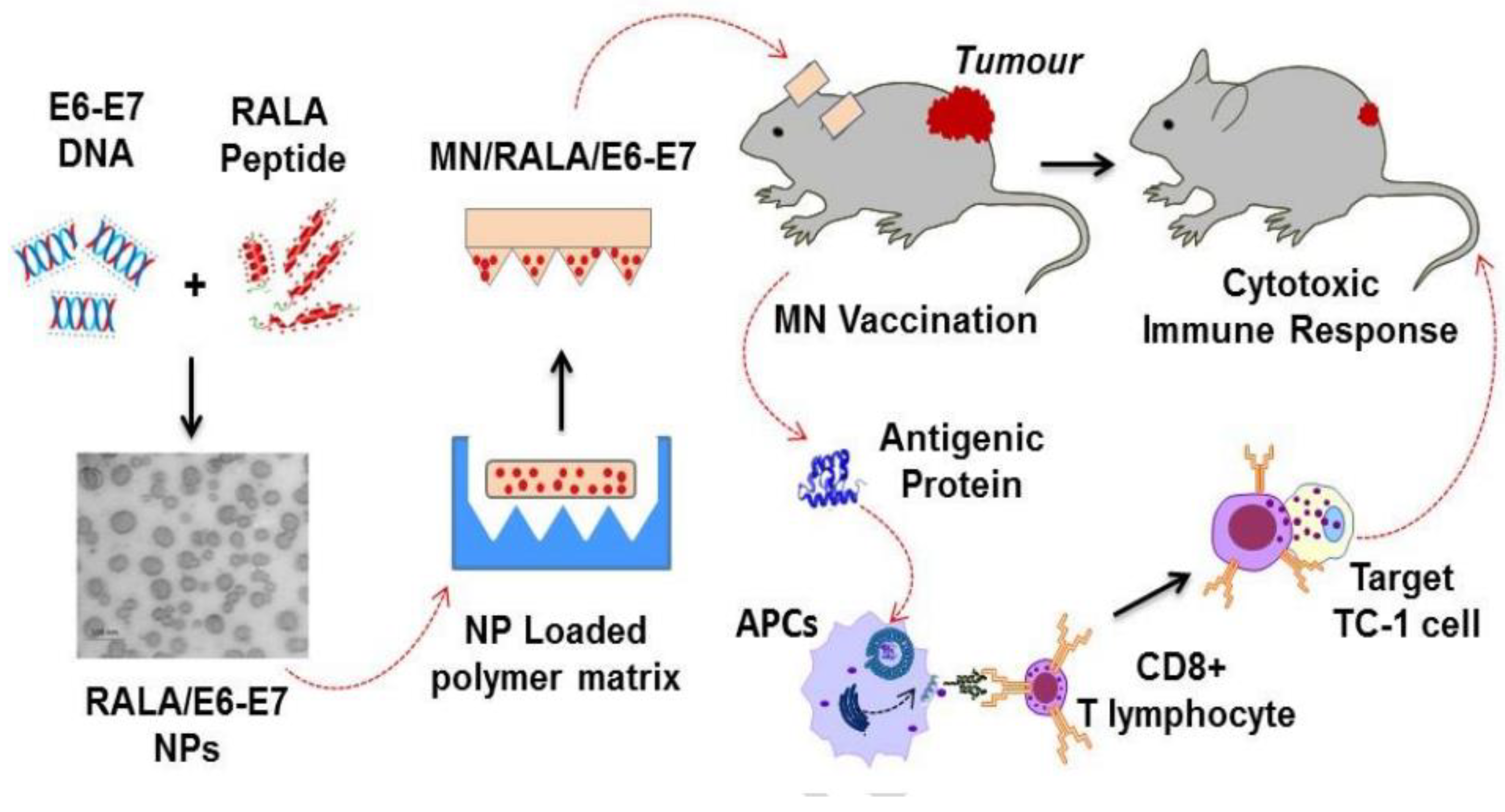
5.1.3. Cancer
5.1.4. Diabetes and Obesity
5.2. Drug Delivery
5.2.1. Transdermal
5.2.2. Intraocular
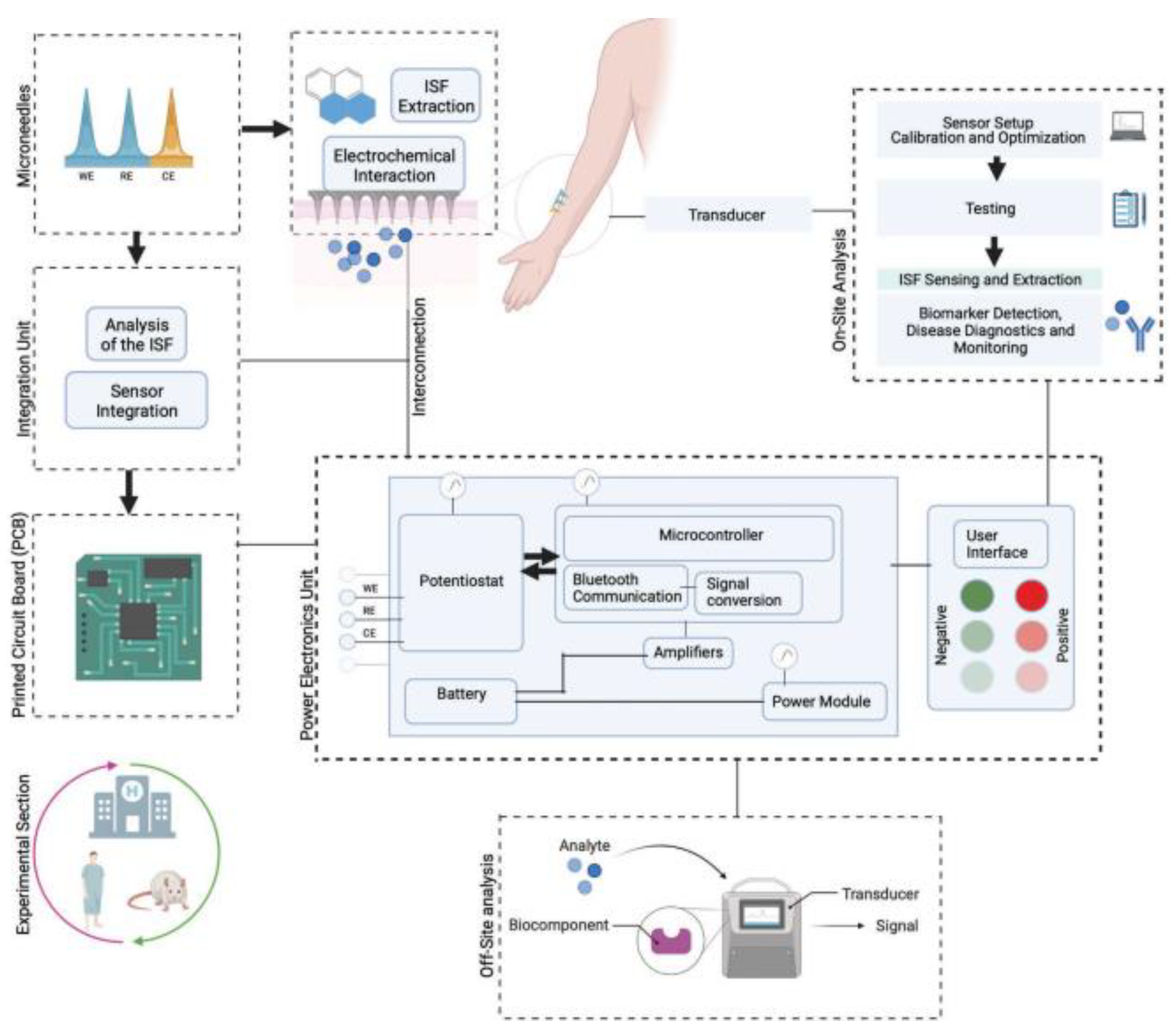
5.3. Diagnostic and Biosensing Applications
5.4. Vaccination
6. Regulatory Considerations and Patent Scenario
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-based delivery: An overview of current applications and trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Dugam, S.; Tade, R.; Dhole, R.; Nangare, S. Emerging era of microneedle array for pharmaceutical and biomedical applications: Recent advances and toxicological perspectives. Future J. Pharm. Sci. 2021, 7, 19. [Google Scholar] [CrossRef]
- Dawud, H.; Abu Ammar, A. Rapidly Dissolving Microneedles for the Delivery of Steroid-Loaded Nanoparticles Intended for the Treatment of Inflammatory Skin Diseases. Pharmaceutics 2023, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M. Chapter 18-Transdermal and Intravenous Nano Drug Delivery Systems: Present and Future. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–550. ISBN 978-0-12-814029-1. [Google Scholar]
- Gowda, B.H.J.; Ahmed, M.G.; Sahebkar, A.; Riadi, Y.; Shukla, R.; Kesharwani, P. Stimuli-Responsive Microneedles as a Transdermal Drug Delivery System: A Demand-Supply Strategy. Biomacromolecules 2022, 23, 1519–1544. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar]
- Gera, A.K.; Burra, R.K. The Rise of Polymeric Microneedles: Recent Developments, Advances, Challenges, and Applications with Regard to Transdermal Drug Delivery. J. Funct. Biomater. 2022, 13, 81. [Google Scholar] [CrossRef]
- Karim, Z.; Karwa, P.; Hiremath, S.R.R. Polymeric Microneedles for Transdermal Drug Delivery—A Review of Recent Studies. J. Drug Deliv. Sci. Technol. 2022, 77, 103760. [Google Scholar] [CrossRef]
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Siddaramaiah; Gowda, D.V.; Moin, A. Microneedles Drug Delivery Systems for Treatment of Cancer: A Recent Update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef]
- Mahato, R. Microneedles in drug delivery. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–353. [Google Scholar]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Damiri, F.; Rojekar, S.; Zehravi, M.; Ramproshad, S.; Dhoke, D.; Musale, S.; Mulani, A.A.; Modak, P.; Paradhi, R. Recent advancements in microneedle technology for multifaceted biomedical applications. Pharmaceutics 2022, 14, 1097. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: Two decades of research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, materials, production methods and commercial development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- Haj-Ahmad, R.; Khan, H.; Arshad, M.S.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.-W.; Ahmad, Z. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics 2015, 7, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Dissolving Microneedles for Transdermal Drug Delivery: Advances and Challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the Technique of Drug Delivery Enhancement in Diverse Organs and Tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-forming microneedles: Current advancements and future trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.C.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.-C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Lhernould, M.S.; Deleers, M.; Delchambre, A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015, 480, 152–157. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, L.; Xu, C. Recent Advances in the Design of Polymeric Microneedles for Transdermal Drug Delivery and Biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.; Deng, D.; Wu, J.; Miao, Y.; Li, Y. Research progress of advanced microneedle drug delivery system and its application in biomedicine. Colloids Surf. B Biointerfaces 2023, 226, 113302. [Google Scholar] [CrossRef]
- Abu-Much, A.; Darawshi, R.; Dawud, H.; Kasem, H.; Ammar, A.A. Preparation and characterization of flexible furosemide-loaded biodegradable microneedles for intradermal drug delivery. Biomater. Sci. 2022, 10, 6486–6499. [Google Scholar] [CrossRef]
- Chevala, N.T.; Jitta, S.R.; Marques, S.M.; Vaz, V.M.; Kumar, L. Polymeric Microneedles for Transdermal Delivery of Nanoparticles: Frontiers of Formulation, Sterility and Stability Aspects. J. Drug Deliv. Sci. Technol. 2021, 65, 102711. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, H.; Mei, L.; Zeng, X. Polymeric microneedle-mediated sustained release systems: Design strategies and promising applications for drug delivery. Asian J. Pharm. Sci. 2022, 17, 70–86. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Ishak, R.A.H.; Osman, R.; Awad, G.A.S. Dextran-based Nanocarriers for Delivery of Bioactives. Curr. Pharm. Des. 2016, 22, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, Y.; Li, C.; Li, Q.; Chen, P.; Li, W.; Liu, W.; Li, Z.; Liu, Y.; Zhang, S. Tofacitinib combined with melanocyte protector α-MSH to treat vitiligo through dextran based hydrogel microneedles. Carbohydr. Polym. 2023, 305, 120549. [Google Scholar] [CrossRef] [PubMed]
- Pitakjakpipop, H.; Rajan, R.; Tantisantisom, K.; Opaprakasit, P.; Nguyen, D.D.; Ho, V.A.; Matsumura, K.; Khanchaitit, P. Facile photolithographic fabrication of zwitterionic polymer microneedles with protein aggregation inhibition for transdermal drug delivery. Biomacromolecules 2021, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Chen, S.-X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef]
- Pitt, W.G.; Husseini, G.A.; Staples, B.J. Ultrasonic drug delivery–a general review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef]
- Feng, Y.H.; Zhang, X.P.; Li, W.X.; Guo, X.D. Stability and diffusion properties of insulin in dissolvable microneedles: A multiscale simulation study. Langmuir 2021, 37, 9244–9252. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Lv, J.; Ma, H.; Ye, G.; Jia, S.; He, J.; Jiaduo, W.; Ma, J.; Qu, Y.; Gou, K.; Zeng, R. Bilayer microneedles based on Bletilla striata polysaccharide containing asiaticoside effectively promote scarless wound healing. Mater. Des. 2023, 226, 111655. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Tiraton, T.; Suwantong, O.; Chuysinuan, P.; Ekabutr, P.; Niamlang, P.; Khampieng, T.; Supaphol, P. Biodegradable microneedle fabricated from sodium alginate-gelatin for transdermal delivery of clindamycin. Mater. Today Commun. 2022, 32, 104158. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 187–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, G.; Yu, W.; Liu, D.; Xu, B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater. Sci. Eng. C 2018, 85, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Du, W.; Sun, M.; Ling, G.; Zhang, P. Microneedle-mediated treatment for superficial tumors by combining multiple strategies. Drug Deliv. Transl. Res. 2023, 13, 1600–1620. [Google Scholar] [CrossRef]
- Demir, B.; Rosselle, L.; Voronova, A.; Pagneux, Q.; Quenon, A.; Gmyr, V.; Jary, D.; Hennuyer, N.; Staels, B.; Hubert, T. Innovative transdermal delivery of insulin using gelatin methacrylate-based microneedle patches in mice and mini-pigs. Nanoscale Horiz. 2022, 7, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.; Wang, C.; Jiang, X.; Qu, M.; Zhu, J.; Ren, L.; Vajhadin, F. Biodegradable β-cyclodextrin conjugated gelatin methacryloyl microneedle for delivery of water-insoluble drug. Adv. Healthc. Mater. 2020, 9, 2000527. [Google Scholar] [CrossRef]
- Volpe-Zanutto, F.; Vora, L.K.; Tekko, I.A.; McKenna, P.E.; Permana, A.D.; Sabri, A.H.; Anjani, Q.K.; McCarthy, H.O.; Paredes, A.J.; Donnelly, R.F. Hydrogel-forming microarray patches with cyclodextrin drug reservoirs for long-acting delivery of poorly soluble cabotegravir sodium for HIV Pre-Exposure Prophylaxis. J. Control. Release 2022, 348, 771–785. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Garland, M.J.; Alkilani, A.Z. Microneedle-iontophoresis combinations for enhanced transdermal drug delivery. Drug Deliv. Syst. 2014, 141, 121–132. [Google Scholar]
- Woolfson, A.D.; Morrow, D.I.J.; Morrissey, A.; Donnelly, R.F.; McCarron, P.A. Delivery Device and Method 2017. U.S. Patent 9549746B2, 24 January 2017. Available online: https://patents.google.com/patent/US9549746B2/en (accessed on 11 March 2023).
- Pamornpathomkul, B.; Ngawhirunpat, T.; Tekko, I.A.; Vora, L.; McCarthy, H.O.; Donnelly, R.F. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur. J. Pharm. Sci. 2018, 121, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Khiste, R.; Bhapkar, N.; Kulkarni, N. A Review on Applications of Hydroxy Propyl Methyl Cellulose and Natural polymers for the development of modified release drug delivery systems. Res. J. Pharm. Technol. 2021, 14, 1163–1170. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of Enhancing Skin Permeability of Acyclovir through Sterile Topical Lyophilized Wafer on Self-Dissolving Microneedle-Treated Skin. Dose-Response 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Poddar, D.; Singh, A.; Bansal, S.; Thakur, S.; Jain, P. Direct synthesis of Poly (Ԑ-Caprolactone)-block-poly (glycidyl methacrylate) copolymer and its usage as a potential nano micelles carrier for hydrophobic drugs. J. Indian Chem. Soc. 2022, 99, 100537. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; He, J.; Zhang, M.; Li, Z.; Ni, P. Fabrication of aminated poly (glycidyl methacrylate)-based polymers for co-delivery of anticancer drugs and the p53 gene. J. Mater. Chem. B 2020, 8, 9555–9565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Wang, B.B.; Li, W.X.; Fei, W.M.; Cui, Y.; Guo, X.D. In vivo safety assessment, biodistribution and toxicology of polyvinyl alcohol microneedles with 160-day uninterruptedly applications in mice. Eur. J. Pharm. Biopharm. 2021, 160, 1–8. [Google Scholar] [CrossRef]
- Sillankorva, S.; Pires, L.; Pastrana, L.M.; Bañobre-López, M. Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles. Viruses 2022, 14, 964. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Seo, M.-S.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef]
- Seeni, R.Z.; Yu, X.; Chang, H.; Chen, P.; Liu, L.; Xu, C. Iron oxide nanoparticle-powered micro-optical coherence tomography for in situ imaging the penetration and swelling of polymeric microneedles in the skin. ACS Appl. Mater. Interfaces 2017, 9, 20340–20347. [Google Scholar] [CrossRef]
- Wu, L.; Shrestha, P.; Iapichino, M.; Cai, Y.; Kim, B.; Stoeber, B. Characterization method for calculating diffusion coefficient of drug from polylactic acid (PLA) microneedles into the skin. J. Drug Deliv. Sci. Technol. 2021, 61, 102192. [Google Scholar] [CrossRef]
- Wu, L.; Park, J.; Kamaki, Y.; Kim, B. Optimization of the fused deposition modeling-based fabrication process for polylactic acid microneedles. Microsyst. Nanoeng. 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.-G.; Kim, M.; Han, M.; Huh, H.W.; Kim, J.-S.; Kim, J.C.; Park, J.-H.; Lee, H.-K.; Cho, C.-W. Characterization of hepatitis B surface antigen loaded polylactic acid-based microneedle and its dermal safety profile. Pharmaceutics 2020, 12, 531. [Google Scholar] [CrossRef]
- Ju, J.; Hsieh, C.-M.; Tian, Y.; Kang, J.; Chia, R.; Chang, H.; Bai, Y.; Xu, C.; Wang, X.; Liu, Q. Surface enhanced Raman spectroscopy based biosensor with a microneedle array for minimally invasive in vivo glucose measurements. ACS Sens. 2020, 5, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Luo, Y.; Chen, H.; Xu, S.; Zhang, D.; Sang, H.; Xu, C.; Zhang, M. Comparison of the efficacy of seven types of microneedles for treating rabbit hypertrophic scar model. Nanoscale Adv. 2023, 5, 927–933. [Google Scholar] [CrossRef]
- Bocchino, A.; Teixeira, S.R.; Iadanza, S.; Melnik, E.; Kurzhals, S.; Mutinati, G.C.; O’Mahony, C. Development and Characterization of Passivation Methods for Microneedle-based Biosensors. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11–15 July 2022; pp. 1275–1278. [Google Scholar]
- Kanaki, Z.; Chandrinou, C.; Orfanou, I.-M.; Kryou, C.; Ziesmer, J.; Sotiriou, G.A.; Klinakis, A.; Tamvakopoulos, C.; Zergioti, I. Laser-Induced Forward Transfer Printing on Microneedles for Transdermal Delivery of Gemcitabine. Int. J. Bioprint. 2022, 8, 554. [Google Scholar] [CrossRef]
- Hegarty, C.; McConville, A.; McGlynn, R.J.; Mariotti, D.; Davis, J. Design of composite microneedle sensor systems for the measurement of transdermal pH. Mater. Chem. Phys. 2019, 227, 340–346. [Google Scholar] [CrossRef]
- Eum, J.; Kim, Y.; Um, D.J.; Shin, J.; Yang, H.; Jung, H. Solvent-free polycaprolactone dissolving microneedles generated via the thermal melting method for the sustained release of capsaicin. Micromachines 2021, 12, 167. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Functionalization of carbon nanomaterials for biomedical applications. C 2019, 5, 72. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Eskens, O.; Amin, S. Challenges and effective routes for formulating and delivery of epidermal growth factors in skin care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-Malky, H.S.; Nasrullah, M.Z. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Thakur, R.R.S.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Guangzhi, G.; Yanni, W.; Fengsen, M. Drug delivery with dissolving microneedles: Skin puncture, its influencing factors and improvement strategies. J. Drug Deliv. Sci. Technol. 2022, 76, 103653. [Google Scholar]
- Angel, D.; Carrillo, J.; Gomerz, E.; Antonia, D.; Prieto, M.; Maria, J.; Gomez, J.R. The effect of focused MW is on the reaction of ethyl N-trichloroethyls ledene carbamatic with pyrazole derivatives. Cannizaro reaction. Synlett 1998, 10, 10. [Google Scholar]
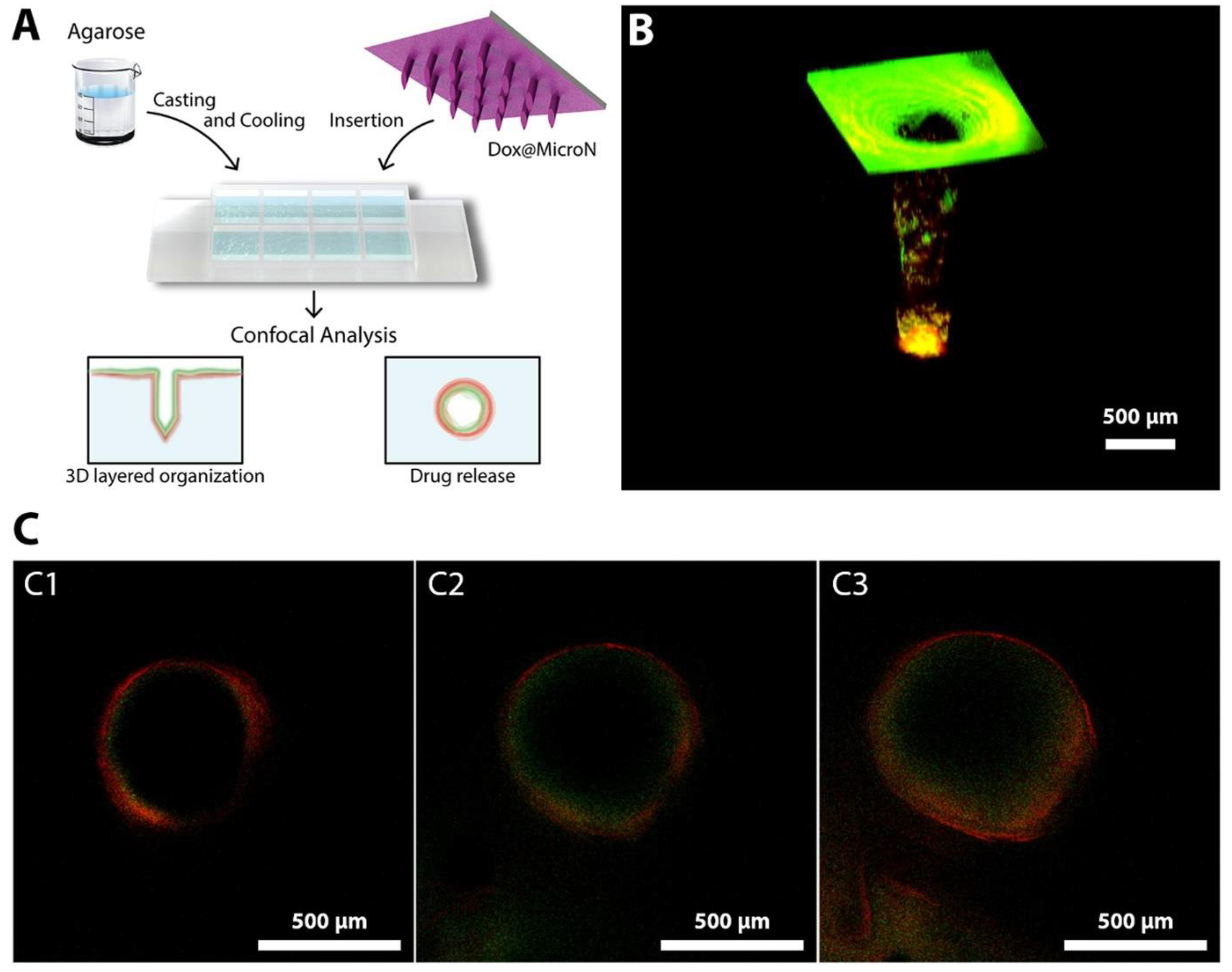
- Zhuang, J.; Rao, F.; Wu, D.; Huang, Y.; Xu, H.; Gao, W.; Zhang, J.; Sun, J. Study on the Fabrication and Characterization of Tip-Loaded Dissolving Microneedles for Transdermal Drug Delivery. Eur. J. Pharm. Biopharm. 2020, 157, 66–73. [Google Scholar] [CrossRef]
- McElvany, K.D. FDA requirements for preclinical studies. In Clinical Trials in the Neurosciences; Karger Publishers: Basel, Switzerland, 2009; Volume 25, pp. 46–49. [Google Scholar]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. High-resolution two-photon polymerization: The most versatile technique for the fabrication of microneedle arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Celis, P.; Vazquez, E.; Soria-Hernandez, C.G.; Bargnani, D.; Rodriguez, C.A.; Ceretti, E.; García-López, E. Evaluation of ball end micromilling for Ti6Al4V ELI microneedles using a nanoadditive under MQL condition. Int. J. Precis. Eng. Manuf. Green Technol. 2022, 9, 1231–1246. [Google Scholar] [CrossRef]
- O’Toole, L.; Kang, C.-W.; Fang, F.-Z. Precision micro-milling process: State of the art. Adv. Manuf. 2021, 9, 173–205. [Google Scholar] [CrossRef]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.; Sarabi, M.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion. Adv. Funct. Mater. 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, O.-C.; Jo, W.; Lee, H.J.; Moon, M.-W. 4D Printing Technology: A Review. 3D Print. Addit. Manuf. 2015, 2, 159–167. [Google Scholar] [CrossRef]
- Wan, X.; Luo, L.; Liu, Y.; Leng, J. Direct Ink Writing Based 4D Printing of Materials and Their Applications. Adv. Sci. 2020, 7, 2001000. [Google Scholar] [CrossRef]
- Papadimitriou, P.; Andriotis, E.G.; Fatouros, D.; Tzetzis, D. Design and Prototype Fabrication of a Cost-Effective Microneedle Drug Delivery Apparatus Using Fused Filament Fabrication, Liquid Crystal Display and Semi-Solid Extrusion 3D Printing Technologies. Micromachines 2022, 13, 1319. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, K. Stereolithography 3D Bioprinting. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 93–108. ISBN 978-1-07-160520-2. [Google Scholar]
- Zhao, Z.; Tian, X.; Song, X. Engineering materials with light: Recent progress in digital light processing based 3D printing. J. Mater. Chem. C 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From oral formulations to drug-eluting implants: Using 3D and 4D printing to develop drug delivery systems and personalized medicine. Bio-Des. Manuf. 2022, 5, 85–106. [Google Scholar] [CrossRef]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef]
- Tran, K.T.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Ross, S.; Scoutarios, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Drug Delivery Transl. Res 2015, 5, 451. [Google Scholar]
- Lechuga, Y.; Kandel, G.; Miguel, J.A.; Martinez, M. Development of an Automated Design Tool for FEM-Based Characterization of Solid and Hollow Microneedles. Micromachines 2023, 14, 133. [Google Scholar] [CrossRef]
- Howells, O.; Blayney, G.J.; Gualeni, B.; Birchall, J.C.; Eng, P.F.; Ashraf, H.; Sharma, S.; Guy, O.J. Design, fabrication, and characterisation of a silicon microneedle array for transdermal therapeutic delivery using a single step wet etch process. Eur. J. Pharm. Biopharm. 2022, 171, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hardick, O.; Stevens, B.; Bracewell, D.G. Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency. J. Mater. Sci. 2011, 46, 3890–3898. [Google Scholar] [CrossRef]
- Ali, R.; Mehta, P.; Monou, P.K.; Arshad, M.S.; Panteris, E.; Rasekh, M.; Singh, N.; Qutachi, O.; Wilson, P.; Tzetzis, D. Electrospinning/electrospraying coatings for metal microneedles: A design of experiments (DOE) and quality by design (QbD) approach. Eur. J. Pharm. Biopharm. 2020, 156, 20–39. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, Y.; Leu, M.; Bourell, D. Additive manufacturing-enabled design, manufacturing, and lifecycle performance. Addit. Manuf. 2020, 36, 101646. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, G.; Aharodnikau, U.E.; Yunusov, K.; Sun, Y.; Liu, T.; Solomevich, S.O. Recent Advances in Polymer Microneedles for Drug Transdermal Delivery: Design Strategies and Applications. Macromol. Rapid Commun. 2022, 43, 2200037. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, J.; Li, W.; Yang, L.; Dong, H.; Zhang, X. Programmable Polymeric Microneedles for Combined Chemotherapy and Antioxidative Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 55559–55568. [Google Scholar] [CrossRef]
- Yao, W.; Tao, C.; Zou, J.; Zheng, H.; Zhu, J.; Zhu, Z.; Zhu, J.; Liu, L.; Li, F.; Song, X. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: A biocomfortable attempt to treat Rheumatoid Arthritis. Int. J. Pharm. 2019, 563, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larrañeta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and characterisation of novel poly (Vinyl Alcohol)/poly (Vinyl Pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef]
- Du, G.; He, P.; Zhao, J.; He, C.; Jiang, M.; Zhang, Z.; Zhang, Z.; Sun, X. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J. Control. Release 2021, 336, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An Up-to-Date Comprehensive Review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Machekposhti, S.A.; Soltani, M.; Najafizadeh, P.; Ebrahimi, S.A.; Chen, P. Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. J. Control. Release 2017, 261, 87–92. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Hao, Y.Y.; Yu, R.X.; Zhang, C.; Chen, B.Z.; Cui, Y.; Guo, X.D. Hydroquinone cream-based polymer microneedle roller for the combined treatment of large-area chloasma. Eur. J. Pharm. Biopharm. 2023, 185, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Shravanth, S.H.; Osmani, R.A.M.; L, J.S.; Anupama, V.P.; Rahamathulla, M.; Gangadharappa, H.V. Microneedles-based drug delivery for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2021, 64, 102668. [Google Scholar] [CrossRef]
- Gowda, B.H.J.; Ahmed, M.G.; Hani, U.; Kesharwani, P.; Wahab, S.; Paul, K. Microneedles as a momentous platform for psoriasis therapy and diagnosis: A state-of-the-art review. Int. J. Pharm. 2023, 632, 122591. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Shi, Y. Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020, 10, 14040–14049. [Google Scholar] [CrossRef]
- Tekko, I.A.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.O.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.B.; Pires, L.R. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int. J. Pharm. 2020, 591, 119942. [Google Scholar] [CrossRef]
- Demartis, S.; Anjani, Q.K.; Volpe-Zanutto, F.; Paredes, A.J.; Jahan, S.A.; Vora, L.K.; Donnelly, R.F.; Gavini, E. Trilayer dissolving polymeric microneedle array loading Rose Bengal transfersomes as a novel adjuvant in early-stage cutaneous melanoma management. Int. J. Pharm. 2022, 627, 122217. [Google Scholar] [CrossRef]
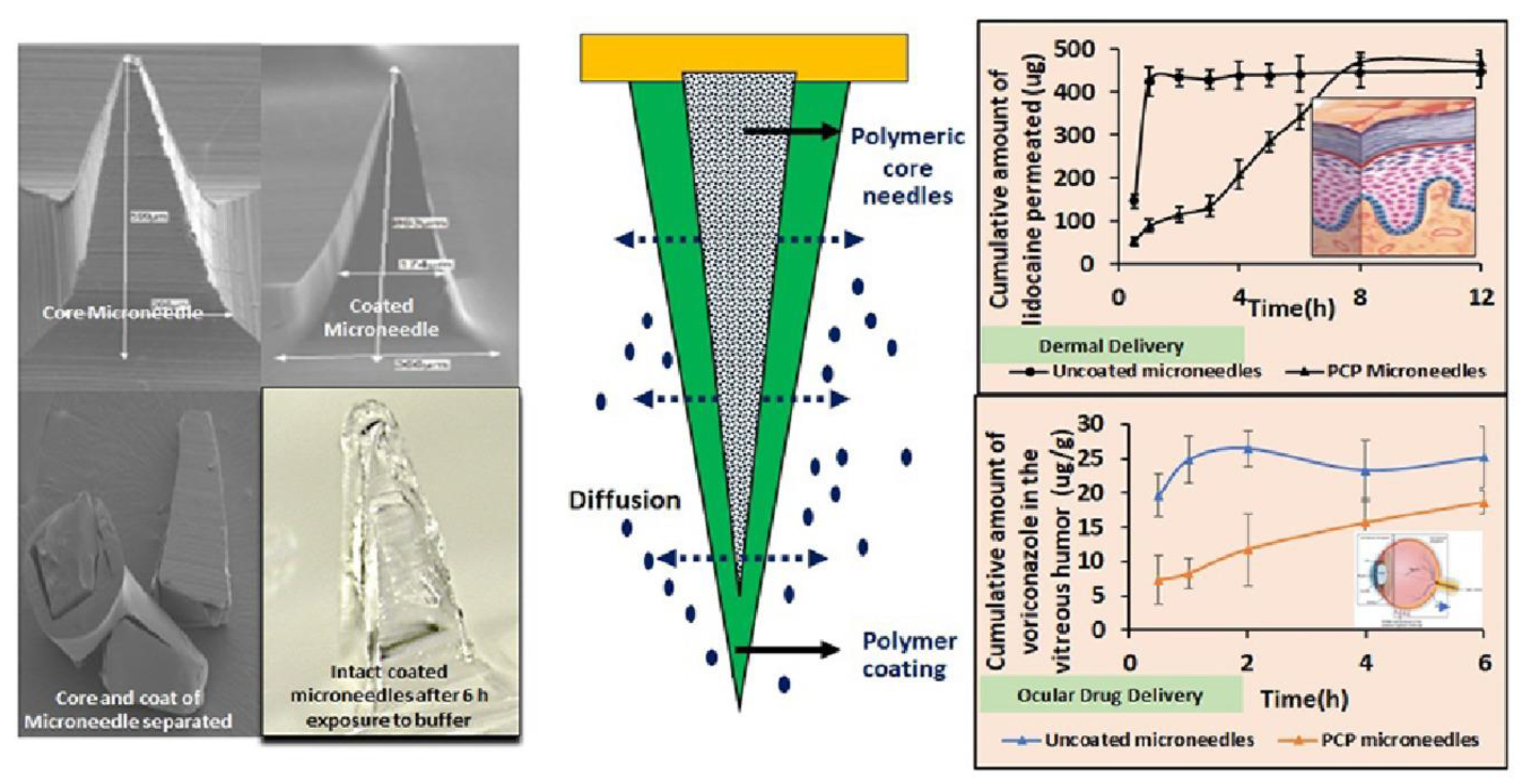
- Matadh, A.V.; Jakka, D.; Pragathi, S.G.; Rangappa, S.; Shivakumar, H.N.; Maibach, H.; Reena, N.M.; Murthy, S.N. Polymer-Coated Polymeric (PCP) Microneedles for Controlled Dermal Delivery of 5-Fluorouracil. AAPS PharmSciTech 2022, 24, 9. [Google Scholar] [CrossRef]
- Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Alamri, A.; Unnisa, A.; Awadelkareem, A.M.; Elkhalifa, A.O.; et al. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials 2022, 15, 7693. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Bankar, N.G.; Kulkarni, M.V.; Venuganti, V.V.K. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int. J. Pharm. 2019, 556, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (Vinyl Alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Liang, K.; Xu, S.; Sun, L.; Duan, H.; Xi, F.; Xu, C.; Chen, P. Transdermal Delivery of Anti-Obesity Compounds to Subcutaneous Adipose Tissue with Polymeric Microneedle Patches. Small Methods 2017, 1, 1700269. [Google Scholar] [CrossRef]
- Rabiei, M.; Kashanian, S.; Bahrami, G.; Derakhshankhah, H.; Barzegari, E.; Samavati, S.S.; McInnes, S.J.P. Dissolving microneedle-assisted long-acting Liraglutide delivery to control type 2 diabetes and obesity. Eur. J. Pharm. Sci. 2021, 167, 106040. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, R.; Lin, Y.; Wang, C.; Tan, Y.; Xie, W.; Sun, S. Improved Therapeutic Efficiency against Obesity through Transdermal Drug Delivery Using Microneedle Arrays. Pharmaceutics 2021, 13, 827. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Fakhraei Lahiji, S.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Xian, Y.; Singh, P.; Feng, J.; Cui, S.; Carrier, A.; Oakes, K.; Luan, T.; Zhang, X. Multifunctional Graphene-Oxide-Reinforced Dissolvable Polymeric Microneedles for Transdermal Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Z.; Ashfaq, M.; Zhang, X.P.; Zhang, J.N.; Guo, X.D. In vitro and in vivo assessment of polymer microneedles for controlled transdermal drug delivery. J. Drug Target. 2018, 26, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Caffarel–Salvador, E.; Migalska, K.; Woolfson, A.D.; Donnelly, R.F. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release 2012, 159, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Drug Delivery to the Eye Enabled by Microneedles. In Drug Product Development for the Back of the Eye; Kompella, U.B., Edelhauser, H.F., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Boston, MA, USA, 2011; pp. 331–360. ISBN 978-1-4419-9920-7. [Google Scholar]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef]
- Jakka, D.; Matadh, A.V.; Shankar, V.K.; Shivakumar, H.N.; Murthy, S.N. Polymer Coated Polymeric (PCP) Microneedles for Controlled Delivery of Drugs (Dermal and Intravitreal). JPharmSci 2022, 111, 2867–2878. [Google Scholar] [CrossRef]
- Barrett, C.; Dawson, K.; O’Mahony, C.; O’Riordan, A. Development of Low Cost Rapid Fabrication of Sharp Polymer Microneedles for In Vivo Glucose Biosensing Applications. ECS J. Solid State Sci. Technol. 2015, 4, S3053. [Google Scholar] [CrossRef]
- Joshi, P.; Riley, P.R.; Mishra, R.; Azizi Machekposhti, S.; Narayan, R. Transdermal Polymeric Microneedle Sensing Platform for Fentanyl Detection in Biofluid. Biosensors 2022, 12, 198. [Google Scholar] [CrossRef]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens. Actuators B Chem. 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Keirouz, A.; Mustafa, Y.L.; Turner, J.G.; Lay, E.; Jungwirth, U.; Marken, F.; Leese, H.S. Conductive Polymer-Coated 3D Printed Microneedles: Biocompatible Platforms for Minimally Invasive Biosensing Interfaces. Small 2023, 19, 2206301. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, K.M.; Faraji Rad, Z. Sampling Dermal Interstitial Fluid Using Microneedles: A Review of Recent Developments in Sampling Methods and Microneedle-Based Biosensors. Adv. Mater. Interfaces 2023, 10, 2201763. [Google Scholar] [CrossRef]
- Amarnani, R.; Shende, P. Microneedles in diagnostic, treatment and theranostics: An advancement in minimally-invasive delivery system. Biomed Microdevices 2021, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Ahmed, M.G.; Sanjana, A. Can Microneedles Replace Hypodermic Needles? Reson 2022, 27, 63–85. [Google Scholar] [CrossRef]
- Liu, G.-S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.-R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Arshad, M.S.; Fatima, S.; Nazari, K.; Ali, R.; Farhan, M.; Muhammad, S.A.; Abbas, N.; Hussain, A.; Kucuk, I.; Chang, M.-W.; et al. Engineering and characterisation of BCG-loaded polymeric microneedles. J. Drug Target. 2020, 28, 525–532. [Google Scholar] [CrossRef]
- Xue, P.; Zhang, X.; Chuah, Y.J.; Wu, Y.; Kang, Y. Flexible PEGDA-based microneedle patches with detachable PVP–CD arrowheads for transdermal drug delivery. RSC Adv. 2015, 5, 75204–75209. [Google Scholar] [CrossRef]
- Dabholkar, N.; Gorantla, S.; Waghule, T.; Rapalli, V.K.; Kothuru, A.; Goel, S.; Singhvi, G. Biodegradable microneedles fabricated with carbohydrates and proteins: Revolutionary approach for transdermal drug delivery. Int. J. Biol. Macromol. 2021, 170, 602–621. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Bonilla-Martínez, D.; Angélica, M.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vázquez, A.L. Microneedles: A valuable physical enhancer to increase transdermal drug delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef]
- Matt, C.; Hess, T.; Benlian, A. Digital transformation strategies. Bus. Inf. Syst. Eng. 2015, 57, 339–343. [Google Scholar] [CrossRef]
- Lu, S.; Cao, G.; Zheng, H.; Li, D.; Shi, M.; Qi, J. Simulation and experiment on droplet formation and separation for needle-type micro-liquid jetting dispenser. Micromachines 2018, 9, 330. [Google Scholar] [CrossRef]
- Won, Y.-W.; Patel, A.N.; Bull, D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 2014, 35, 5627–5635. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Hyun, D.; Park, H.; Lee, S.; Kim, C.; Kim, C. A novel fabrication process for out-of-plane microneedle sheets of biocompatible polymer. Micromech. Microeng. 2007, 17, 1184. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef]
- Dewia, H.A.; Fangben Mengb, B.S.; Guoa, C.; Norlingc, B.; Chenb, X.; Lima, S. Investigation of electron transfer from isolated spinach thylakoids to indium tin oxide. RSC Adv. 2014, 4, 48815–48820. [Google Scholar]
- Yang, S.; Feng, Y.; Zhang, L.; Chen, N.; Yuan, W.; Jin, T. A scalable fabrication process of polymer microneedles. Int. J. Nanomed. 2012, 7, 1415–1422. [Google Scholar]
- Sabbagh, F.; Kim, B.-S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Monteiro-Riviere, N.A.; Lusk, J.; Morel, P.; Minghetti, P.; Lenardi, C.; Chichkov, B.N.; Narayan, R.J. Fabrication of polymer microneedles using a two-photon polymerization and micromolding process. J. Diabetes Sci. Technol. 2009, 3, 304–311. [Google Scholar] [CrossRef]
- JEmer, J.; Waldorf, H. Nonhyaluronic Acid. Aesthetic Dermatol. Curr. Perspect. 2018, 29. [Google Scholar]
- Ito, Y.; Kobuchi, S.; Inoue, G.; Kakumu, E.; Aoki, M.; Sakaeda, T.; Takada, K. Dissolving microneedles for enhanced local delivery of capsaicin to rat skin tissue. J. Drug Target. 2017, 25, 420–424. [Google Scholar] [CrossRef]
- Liu, Y.; Eng, P.F.; Guy, O.J.; Roberts, K.; Ashraf, H.; Knight, N. Advanced deep reactive-ion etching technology for hollow microneedles for transdermal blood sampling and drug delivery. IET Nanobiotechnol. 2013, 7, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Ling, M.-H.; Lai, K.-Y.; Pramudityo, E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Lee, M.-T.; Lee, I.-C.; Tsai, S.-W.; Chen, C.-H.; Wu, M.-H.; Juang, Y.-J. Spin coating of polymer solution on polydimethylsiloxane mold for fabrication of microneedle patch. J. Taiwan Inst. Chem. Eng. 2017, 70, 42–48. [Google Scholar] [CrossRef]
- Kim, D.; Cao, Y.; Mariappan, D.; Bono, M.S., Jr.; Hart, A.J.; Marelli, B. A microneedle technology for sampling and sensing bacteria in the food supply chain. Adv. Funct. Mater. 2021, 31, 2005370. [Google Scholar] [CrossRef]
- Juster, H.; van der Aar, B.; de Brouwer, H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Transdermal delivery of therapeutics through dissolvable gelatin/sucrose films coated on PEGDA microneedle arrays with improved skin permeability. J. Mater. Chem. B 2019, 7, 7515–7524. [Google Scholar] [CrossRef]
- Jin, J.; Reese, V.; Coler, R.; Carter, D.; Rolandi, M. Chitin Microneedles for an Easy-to-Use Tuberculosis Skin Test. Adv. Healthc. Mater. 2014, 3, 349–353. [Google Scholar] [CrossRef]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Available online: https://www.google.co.in/search?q=https%3A%2F%2Fwww.fda.gov%2Fmedia%2F107708&sxsrf=AJOqlzW0n0UDrfJpR3-O2EM2kSubcn348g%3A1678378634552&source=hp&ei=igYKZLb-HpaYseMP7tme4AM&iflsig=AK50M_UAAAAAZAoUmkCCY0_nlhfFzTSYHEJjGlT-_S9p&ved=0ahUKEwi267vSn8_9AhUWTGwGHe6sBzwQ4dUDCAg&uact=5&oq=https%3A%2F%2Fwww.fda.gov%2Fmedia%2F107708&gs_lcp=Cgdnd3Mtd2l6EAM6BwgjEOoCECdQpgZYpgZgqhBoAXAAeACAAakBiAGpAZIBAzAuMZgBAKABAqABAbABCg&sclient=gws-wiz (accessed on 9 March 2023).
- U.S. Food & Drug Adeministration. 510 (k) Devices Cleared in 2022. FDA 2023. Available online: https://www.fda.gov/medical-devices/510k-clearances/510k-devices-cleared-2023 (accessed on 1 April 2023).
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, M.J.; Das, D.B. Translation of polymeric microneedles for treatment of human diseases: Recent trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef]
- Pulse Biosciences, Inc. Prospective, Open Label, Multi-Center, Non-Significant Risk Study of Nano-Pulse StimulationTM (NPSTM) Technology in Healthy Adults with Sebaceous Hyperplasia. 2020. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Yonsei University. A Study on the Effectiveness and Safety Evaluation of Combination Therapy with 1927 nm Thulium Laser and Fractional Microneedle Radiofrequency Equipment for Improvement of Skin Aging. 2020. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Zosano Pharma Corporation. Randomized, Double-Blind, Multi-Center, Parallel-Group, Dose-Ranging Comparison of the Safety and Efficacy of the ZP-Zolmitriptan Intracutaneous Microneedle Systems to Placebo for the Acute Treatment of Migraine. 2018. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- University of California, Davis. The Use of Microneedles With Topical Botulinum Toxin for Treatment of Palmar Hyperhidrosis. 2017. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Prausnitz, M. A Phase I Study of the Safety, Reactogenicity, Acceptability and Immunogenicity of Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle. 2019. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Radius Health, Inc. A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study of BA058 Administered Via a Coated Transdermal Microarray Delivery System (BA058 Transdermal) in Healthy Postmenopausal Women With Osteoporosis. 2020. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- University of British Columbia. Controlled Comparison in Canadian Seniors of Seasonal Influenza Vaccines for 2011–2012. 2015. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Felner, E. Insulin Delivery Using Microneedles in Type 1 Diabetes. 2013. Available online: clinicaltrials.gov (accessed on 21 February 2021).
- Available online: https://www.google.co.in/search?q=https%3A%2F%2Fmembers.wto.org%2Fcrnattachments%2F2018%2FTBT%2FUSA%2F18&sxsrf=AJOqlzX9CZBzGBT7QS6wz2is2N1zZTlmdg%3A1678379222786&source=hp&ei=1ggKZO2jLZfr-QaWu5joCw&iflsig=AK50M_UAAAAAZAoW5q2YHj6V_wBM8woXfxGBuLbcRHoi&ved=0ahUKEwjt5vrqoc_9AhWXdd4KHZYdBr0Q4dUDCAg&uact=5&oq=https%3A%2F%2Fmembers.wto.org%2Fcrnattachments%2F2018%2FTBT%2FUSA%2F18&gs_lcp=Cgdnd3Mtd2l6EAMyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECcyBwgjEOoCECdQ7wpY7wpg_gpoAXAAeACAAQCIAQCSAQCYAQCgAQKgAQGwAQo&sclient=gws-wiz (accessed on 9 March 2023).
- Chapter 1-Scope of the Regulations. Available online: https://www.gov.uk/government/consultations/consultation-on-the-future-regulation-of-medical-devices-in-the-united-kingdom/outcome/chapter-1-scope-of-the-regulations (accessed on 9 March 2023).
- Falo, J.L.D.; Erdos, G. Bioactive Components Conjugated to Dissolvable Substrates of Microneedle Arrays. U.S. Patent 10737083B2, 11 August 2020. Available online: https://patents.google.com/patent/US10737083B2/en?oq=US10737083 (accessed on 9 March 2023).
- Kaspar, R.L.; Speaker, T. Microneedle Arrays Formed from Polymer Films. U.S. Patent 10377062B2, 13 August 2019. Available online: https://patents.google.com/patent/US10377062B2/en?oq=US10377062 (accessed on 9 March 2023).
- Chiou, J.-C.; Hung, C.-C.; Chang, C.-W. Method for Fabricating Microneedle Array and Method for Fabricating Embossing Mold of Microneedle Array. U.S. Patent 7429333B2, 30 September 2008. Available online: https://patents.google.com/patent/US7429333B2/en?oq=US7429333 (accessed on 9 March 2023).
- Jin, T. Fabrication Process of Phase-Transition Microneedle Patch. U.S. Patent 10195410B2, 5 February 2019. Available online: https://patents.google.com/patent/US10195410B2/en?oq=US10195410 (accessed on 9 March 2023).
- Ghartey-Tagoe, E.; Wendorf, J.; Williams, S.; Singh, P.; Worsham, R.W.; Trautman, J.C.; Bayramov, D.; Bowers, D.L.; Klemm, A.R.; Klemm, S.R.; et al. Method of Vaccine Delivery via Microneedle Arrays. U.S. Patent 9498524B2, 22 November 2016. Available online: https://patents.google.com/patent/US9498524B2/en?oq=US9498524 (accessed on 9 March 2023).
- Park, J.-H.; Prausnitz, M.R. Microneedle Devices and Production Thereof. U.S. Patent 9302903B2, 5 April 2016. Available online: https://patents.google.com/patent/US9302903B2/en?oq=US9302903 (accessed on 9 March 2023).
- Allen, M.G.; Prausnitz, M.R.; McAllister, D.V.; Cros, F.P.M. Microneedle Devices and Methods of Manufacture and Use Thereof. U.S. Patent 8708966B2, 29 April 2014. Available online: https://patents.google.com/patent/US8708966B2/en?oq=US8708966 (accessed on 9 March 2023).
- Falo, L.D., Jr.; Erdos, G.; Ozdoganlar, O.B. Dissolvable Microneedle Arrays for Transdermal Delivery to Human Skin. U.S. Patent 8834423B2, 16 September 2014. Available online: https://patents.google.com/patent/US8834423B2/en?oq=US8834423 (accessed on 9 March 2023).
- Kato, H. Microneedle and Method for Manufacturing Microneedle. U.S. Patent 10682504B2, 16 June 2020. Available online: https://patents.google.com/patent/US10682504B2/en?oq=US10682504 (accessed on 9 March 2023).







| Factor Affecting MN Fabrication | Practical Parameters | Practical Considerations | References |
|---|---|---|---|
| Material properties | Mechanical properties (stiffness, strength, and toughness). | High stiffness and strength may cause materials to be more difficult to be molded or etched, while materials that are too brittle may be prone to breakage. | [74] |
| Chemical properties (reactivity, solubility, and stability). | Highly reactive or unstable materials may require specialized handling or storage conditions. | [75] | |
| Biocompatibility (non-toxic, non-immunogenic, and non-inflammatory). | Material should be biocompatible for clinical or medical use. | [76] | |
| Compatibility with drug formulations (physical, chemical, and biological interactions). | The material should not adsorb or absorb the drug to avoid any loss of drug efficacy. | [77] | |
| Device design | Needle length and diameter. | The length and diameter of the MNs should be optimized for the intended application, taking into account factors such as skin thickness and drug delivery requirements. | [78] |
| Needle shape and geometry. | The shape and geometry of the MNs can affect their mechanical properties and ability to penetrate the skin. | [79] | |
| Delivery target. | Design considerations shall take into account the target site (depth of penetration topical or deeper penetration), and whether it is a local or systemic action/sensing. | ||
| Quality control | Inspection and testing. | Inspected and tested to ensure they meet quality standards and specifications (device dimensions, insertion force, insertion depth, failure force, skin irritation, skin permeation, payload release, lubrication, flexibility, shelf life, etc.) | [9] |
| Precision and accuracy of alignment. | Alignment and registration of MNs are important for ensuring consistent performance and drug delivery. | ||
| Cost | Materials and manufacturing costs. Ease of fabrication. | Materials should be economic. The material should be easy to fabricate using the chosen manufacturing technique. | [80] |
| Regulatory requirements | FDA and other regulatory requirements. | MNs may be subject to regulatory requirements, such as safety and efficacy testing, before they can be approved for use. | [81] |
| Method of MN Fabrication | Precision of Fabrication | Scalability | Ability to Control Shape and Size | Ability to Fabricate Complex Structures and Patterns | Resolution | Cost |
|---|---|---|---|---|---|---|
| Micromolding | ✓ | ✓ | ✓ | ✓ | ✓ | Low |
| Micromilling | ✓ | ✓ | ✓ | ✓ | ✓ | High |
| Atomized Spraying to Fill Molds | ✓ | ✓ | ✘ | ✘ | ✓ | Low |
| 3D Printing | ✓ | ✓ | ✓ | ✓ | ✓ | Low |
| Laser Ablation | ✓ | ✘ | ✓ | ✓ | ✓ | High |
| Photolithography | ✓ | ✘ | ✓ | ✓ | ✓ | High |
| Printing Techniques | ✓ | ✓ | ✓ | ✘ | ✓ | Low |
| Etching | ✓ | ✓ | ✓ | ✓ | ✓ | High |
| Electrospinning | ✓ | ✓ | ✘ | ✘ | ✓ | High |
| Co-Extrusion | ✓ | ✓ | ✓ | ✘ | ✓ | Low |
| Additive Fabrication Methods | Materials Used | Type of Microneedles | Layer Resolution | Potential Source | Application | References |
|---|---|---|---|---|---|---|
| Direct-ink writing (DIW) | Shape memory polymer (SMP) | Solid, hollow | 20–50 µm | Laser beam | Tissue engineering | [90] |
| Fused filament fabrication (FFF) | Shape memory hydrogel (SMH) | Coated, hydrogel | 40–70 µm | Laser beam | Prototyping in product development | [91] |
| Stereolithography (SLA) | Liquid polymer | Solid, hollow, coated | 50–100 µm | UV light | Anticancer drug delivery | [92] |
| Digital light processing (DLP) | Shape memory composite (SMC) | Biodegradable, coated, hydrogel | 25–150 µm | UV light | Transdermal drug delivery | [93] |
| Selective laser sintering (SLS) | Liquid crystal elastomer | Solid, biodegradable, hydrogel | 80–90 µm | Laser beam | Disease delivery with medical device | [94] |
| Inject method | Shape memory alloy | Biodegradable, solid | 70–100 µm | - | Topical application | [95] |
| Polymers | Fabrication Method | Application | References |
|---|---|---|---|
| Amylopectin | Photolithography | Drug delivery of cosmetics and nutrients | [144] |
| Chondroitin sulphate | 2-Photon polymerization (2PP) | Decompressing loaded sodium chondroitin sulphate | [145] |
| Carboxy methyl cellulose | 2PP, droplet-born air blowing (DAB) method | To enhance local skin health and rats’ immune function, hair regrowth | [146] |
| Dextran | Photon polymerization | Treatment of skin cancer | [16] |
| GA lactose | Atomized spraying process | Protein delivery | [147] |
| Trehalose | Micromolding | To facilitate peptide delivery | [148] |
| Maltose | Atomized spraying process | Drug carrier for anti-cancer agent | [149] |
| Fructose | Micromolding | Biosensing | [150] |
| Raffinose | Atomized spraying process | Delivery of doxorubicin | [151] |
| Thermoplastic starch | Electro-discharge process | Insulin delivery in diabetics | [152] |
| Poly-lactic-acid | Fused deposition modelling (FDM), micromolding | Immunization, biosensing | [153] |
| Poly-lactic-co-glycolic acid | 2PP, micromolding | Vaccine delivery | [10] |
| Polycarbonate PMVEs/MA copolymer | UV lithography, electroforming, laser-based method for micromolding, micromolding | Treatment of poisoning | [154] |
| Poly-vinyl-alcohol | Micromolding | Delivery of Nicotinamide Mononucleotide | [155] |
| Poly-vinyl-pyrrolidine | 2PP, atomized spraying process | Intradermal drug delivery system, to facilitate peptide delivery | [156] |
| Polyglycolic acid | Fused deposition modelling | Vaccination | [14] |
| Hyaluronic acid | Micromolding | Treatment of diabetes mellitus, diagnosis | [157] |
| Sodium chondroitin | Solvent casting | In topical formulation and local analgesic action | [158] |
| Polyethylene glycol | Inject printing | GAP 26 gap junction blocker, biosensor | [159] |
| Chitosan | Electrospraying, micromolding | Immunotherapy, provide stability to the antigen used in MN vaccination | [160] |
| Polycaprolactone | Printed scaffolds | Cancer therapy | [151] |
| Hydroxy propyl methyl cellulose | Atomized spraying to fill molds | Treatment of Alzheimer’s disease | [18] |
| Cellulose | --- | Stabilizer and film forming | [159] |
| Polysorbate 80 | Inject printing | Used in cardiac disease | [161] |
| Polydimethylsiloxane | Mold casting | Cosmetic | [162] |
| Polydiacetylenes | Phase inversion | Diagnostic | [163] |
| Polycarbonate | Gas pulling | --- | [164] |
| Sucrose | --- | Protein stabilizer | [165] |
| Chitin | Electrospraying, micromolding | Diagostic tool for tuberculosis | [166] |
| Pullulan | Electro-discharge machining process | Deliver protein and peptide-like FITC-BSA | [167] |
| Identifier | Starting Year | Clinical Condition | Description | Clinical Trial Phase | References |
|---|---|---|---|---|---|
| NCT04253418 | 2019 | Sebaceous hyperplasia; skin abnormalities; and skin lesion | Nano-pulse stimulation (NPS) in sebaceous hyperplasia optimization study | N/A | [171] |
| NCT04249115 | 2019 | Lesion skin; seborrheic keratosis; skin lesion; and benign skin tumor | Nano-pulse stimulation (NPS) in seborrheic keratosis optimization study | N/A | [171] |
| NCT03739398 | 2018 | Wrinkle | A study on the effectiveness and safety evaluation of combination therapy with 1927 nm thulium laser and fractional MN radiofrequency equipment for improvement of skin aging | N/A | [172] |
| NCT02745392 | 2016 | Acute migraine | Safety and efficacy of ZP-zolmitriptan intracutaneous MN system for acute treatment of migraine (Zotrip) | Phase 2 Phase 3 | [173] |
| NCT03203174 | 2015 | Hyperhidrosis | The use of MN with topical botulinum toxin for treatment of palmer hyperhidrosis | Phase 1 | [174] |
| NCT02438423 | 2015 | Influenza | Inactivated influenza vaccine delivered by MN patch or by hypodermic needle | Phase 1 | [175] |
| NCT01674621 | 2012 | Post-menopausal osteoporosis | Phase 2 study of BA058 (abaloparatide) TD delivery in postmenopausal women with osteoporosis | Phase 2 | [176] |
| NCT01368796 | 2011 | Influenza vaccines | Comparison of 4 influenza vaccines in seniors (PCIRNRT09) | Phase 4 | [177] |
| NCT00837512 | 2008 | Type 1 diabetes mellitus | Insulin delivery using MN in type 1 diabetes | Phase 2 Phase 3 | [178] |
| US Patent Number | Title | References |
|---|---|---|
| US10737083B2 | Bioactive components conjugated to dissolvable substrates of MN arrays | [181] |
| US10377062B2 | MN arrays formed from polymer films | [182] |
| US7429333B2 | Method for fabricating MN array and method for fabricating embossing mold of MN array | [183] |
| US10195410B2 | Fabrication process of phase-transition MN patch | [184] |
| US9498524B2 | Method of vaccine delivery via MN arrays | [185] |
| US9302903B2 | MN devices and production thereof | [186] |
| US8708966B2 | MN devices and methods of manufacture and use thereof | [187] |
| US8834423B2 | Dissolvable MN arrays for TD delivery to human skin | [188] |
| US10682504B2 | MN and method for manufacturing MN | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, D.; Gadade, D.; Chapaitkar, N.; Shelke, S.; Pekamwar, S.; Aher, R.; Ahire, A.; Avhale, M.; Badgule, R.; Bansode, R.; et al. Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications. Sci. Pharm. 2023, 91, 27. https://doi.org/10.3390/scipharm91020027
Kulkarni D, Gadade D, Chapaitkar N, Shelke S, Pekamwar S, Aher R, Ahire A, Avhale M, Badgule R, Bansode R, et al. Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications. Scientia Pharmaceutica. 2023; 91(2):27. https://doi.org/10.3390/scipharm91020027
Chicago/Turabian StyleKulkarni, Deepak, Dipak Gadade, Nutan Chapaitkar, Santosh Shelke, Sanjay Pekamwar, Rushikesh Aher, Ankita Ahire, Manjusha Avhale, Rupali Badgule, Radhika Bansode, and et al. 2023. "Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications" Scientia Pharmaceutica 91, no. 2: 27. https://doi.org/10.3390/scipharm91020027
APA StyleKulkarni, D., Gadade, D., Chapaitkar, N., Shelke, S., Pekamwar, S., Aher, R., Ahire, A., Avhale, M., Badgule, R., Bansode, R., & Bobade, B. (2023). Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications. Scientia Pharmaceutica, 91(2), 27. https://doi.org/10.3390/scipharm91020027









