In Vitro and In Silico Screening Analysis of Artabotrys sumatranus Leaf and Twig Extracts for α-Glucosidase Inhibition Activity and Its Relationship with Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Artabotrys sumatranus
2.3. α-Glucosidase Inhibition Assay
2.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
2.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Gas Chromatography—Mass Spectrometry (GC-MS) Analysis
2.9. Liquid Chromatography—Mass Spectrometry (LC-MS-MS) Analysis
2.10. Docking Study
3. Results and Discussion
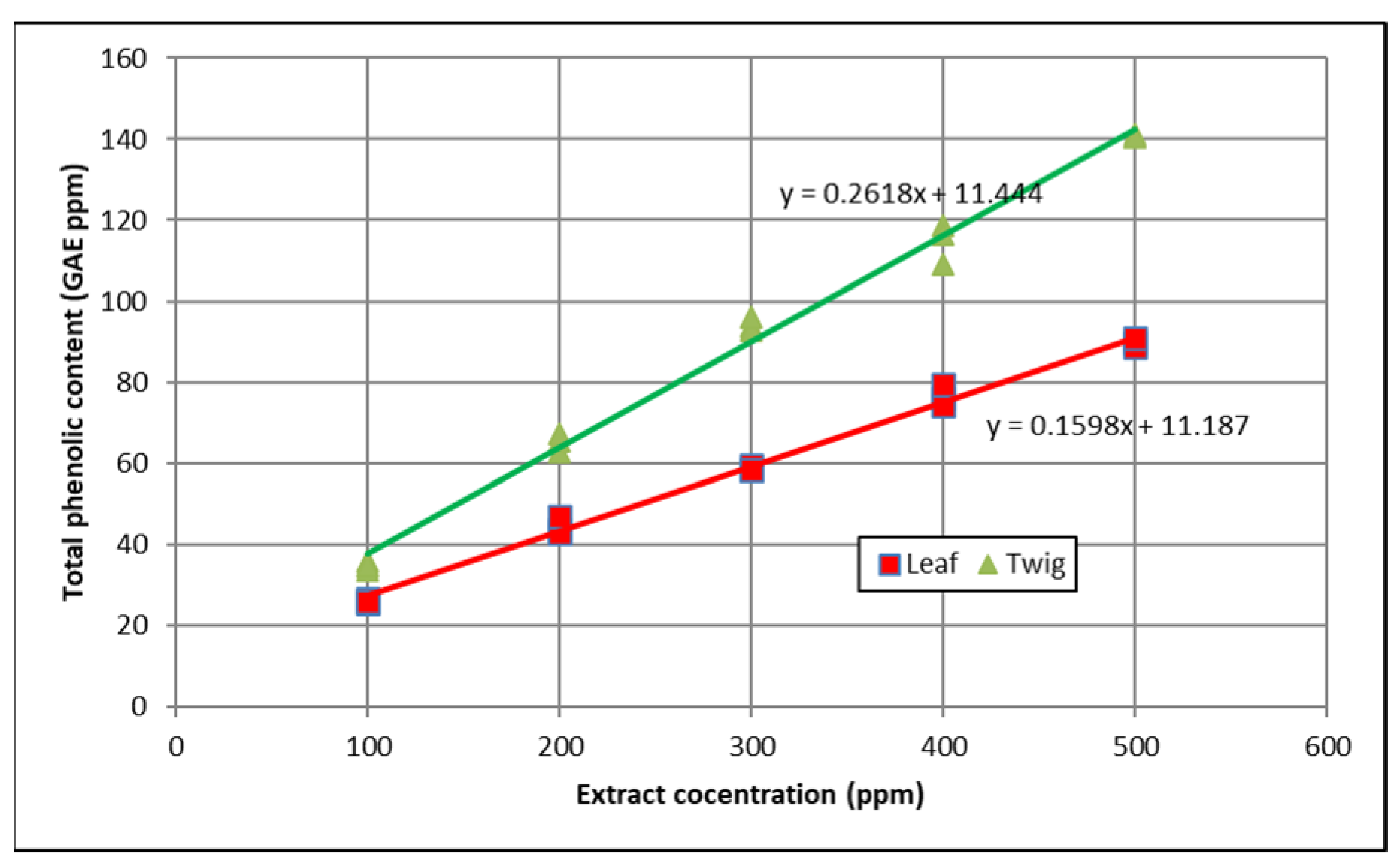
3.1. Total Phenolic Content
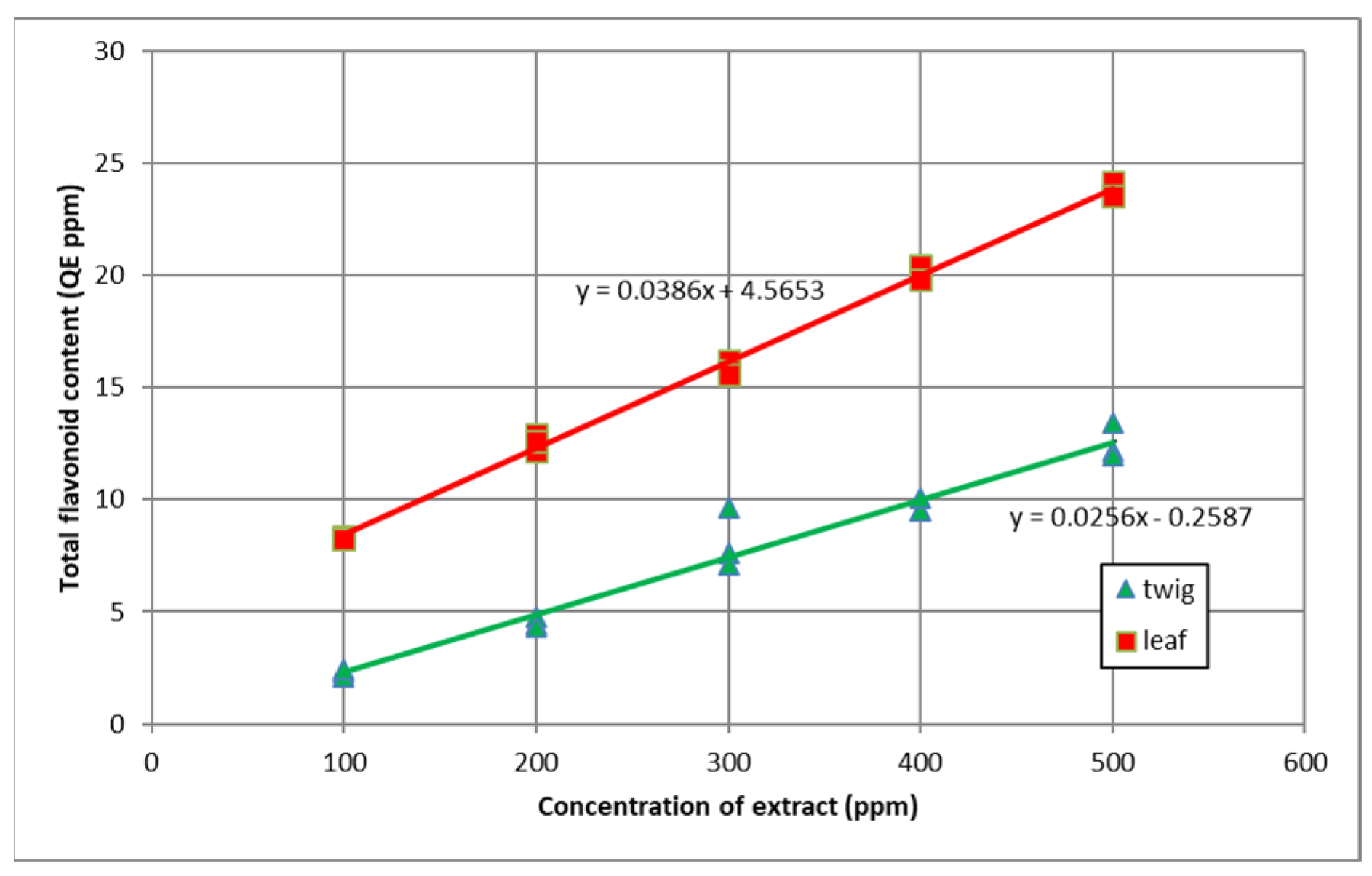
3.2. Total Flavonoid Content
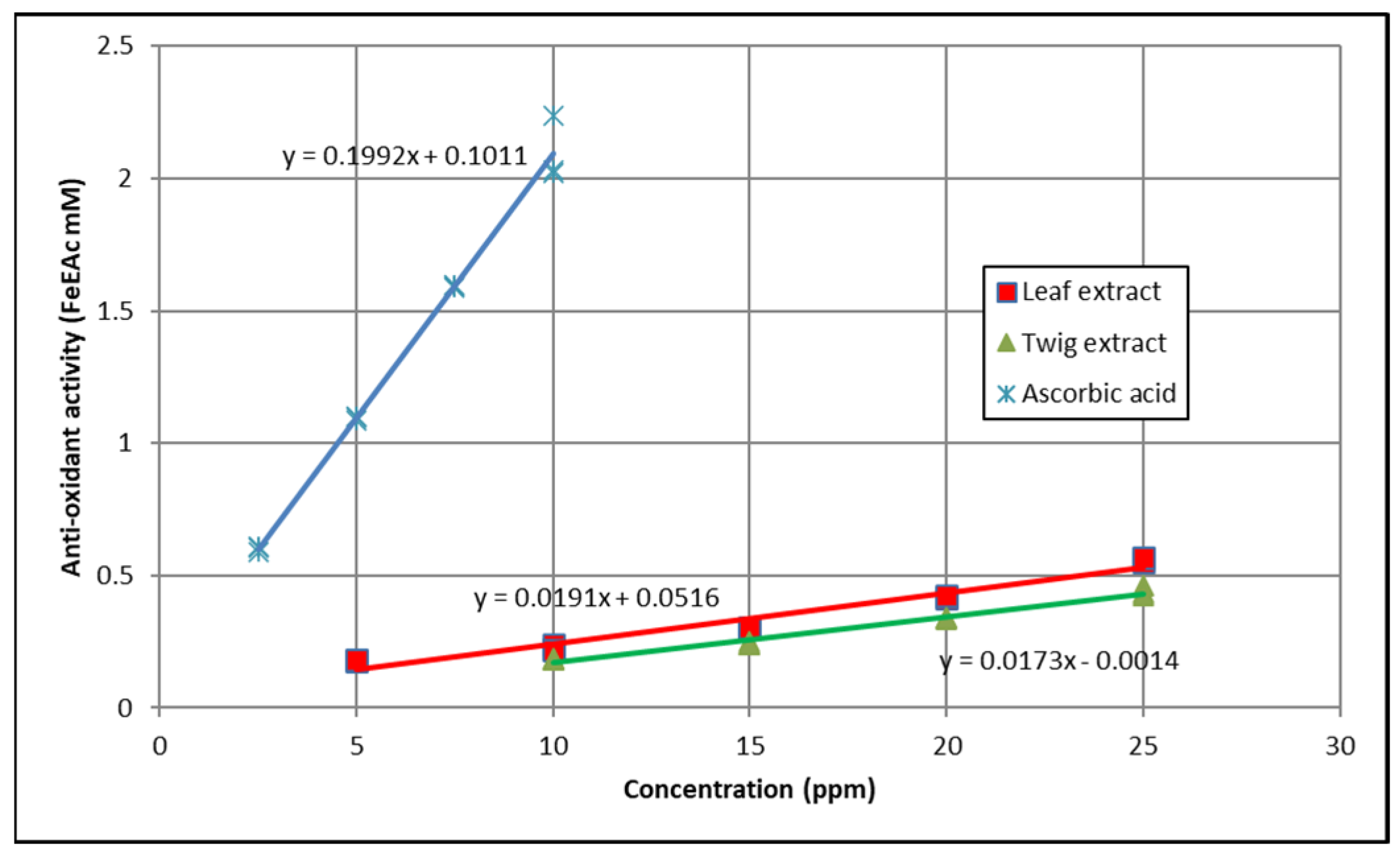
3.3. Ferric Reducing Antioxidant Power (FRAP)
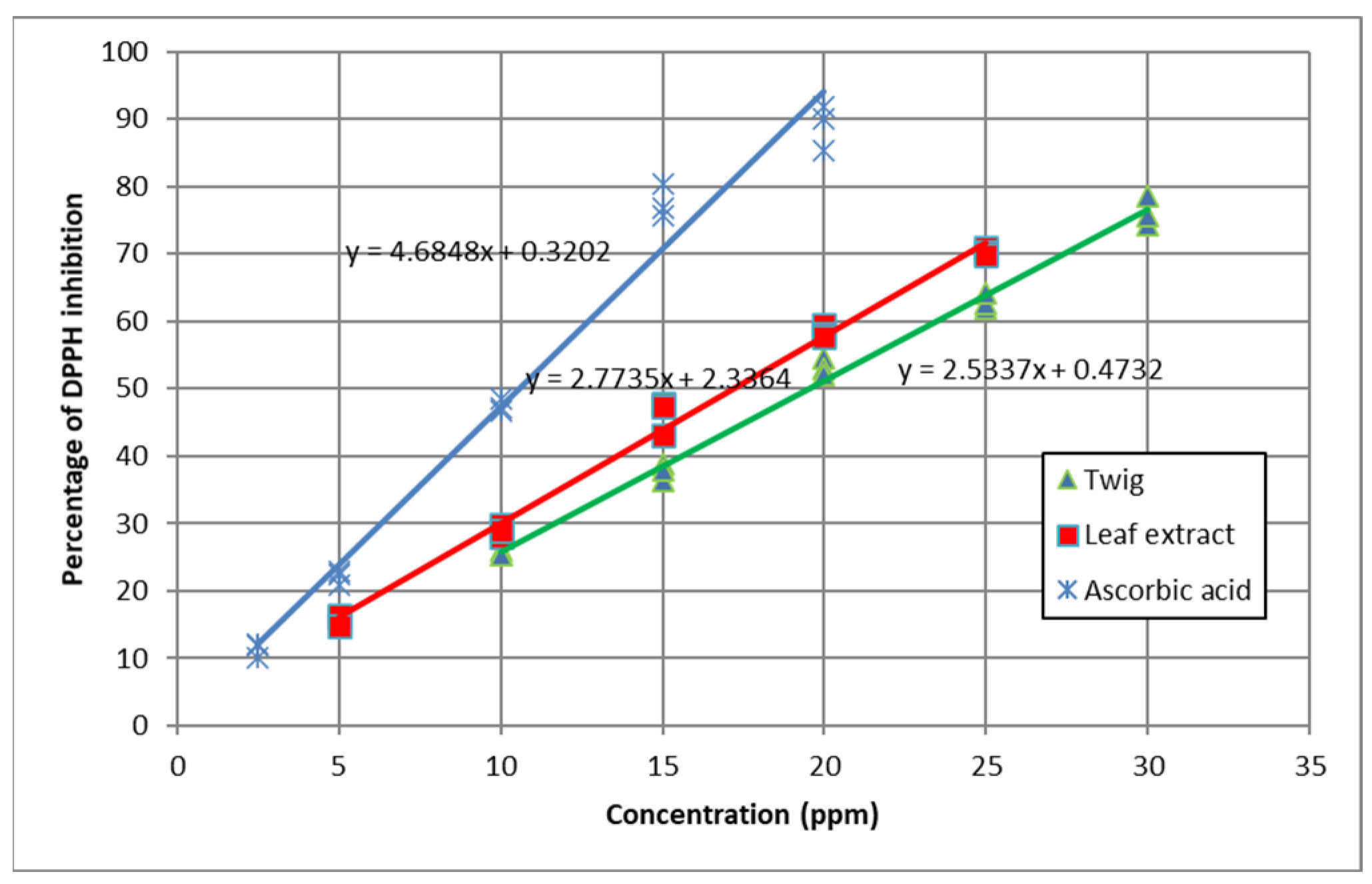
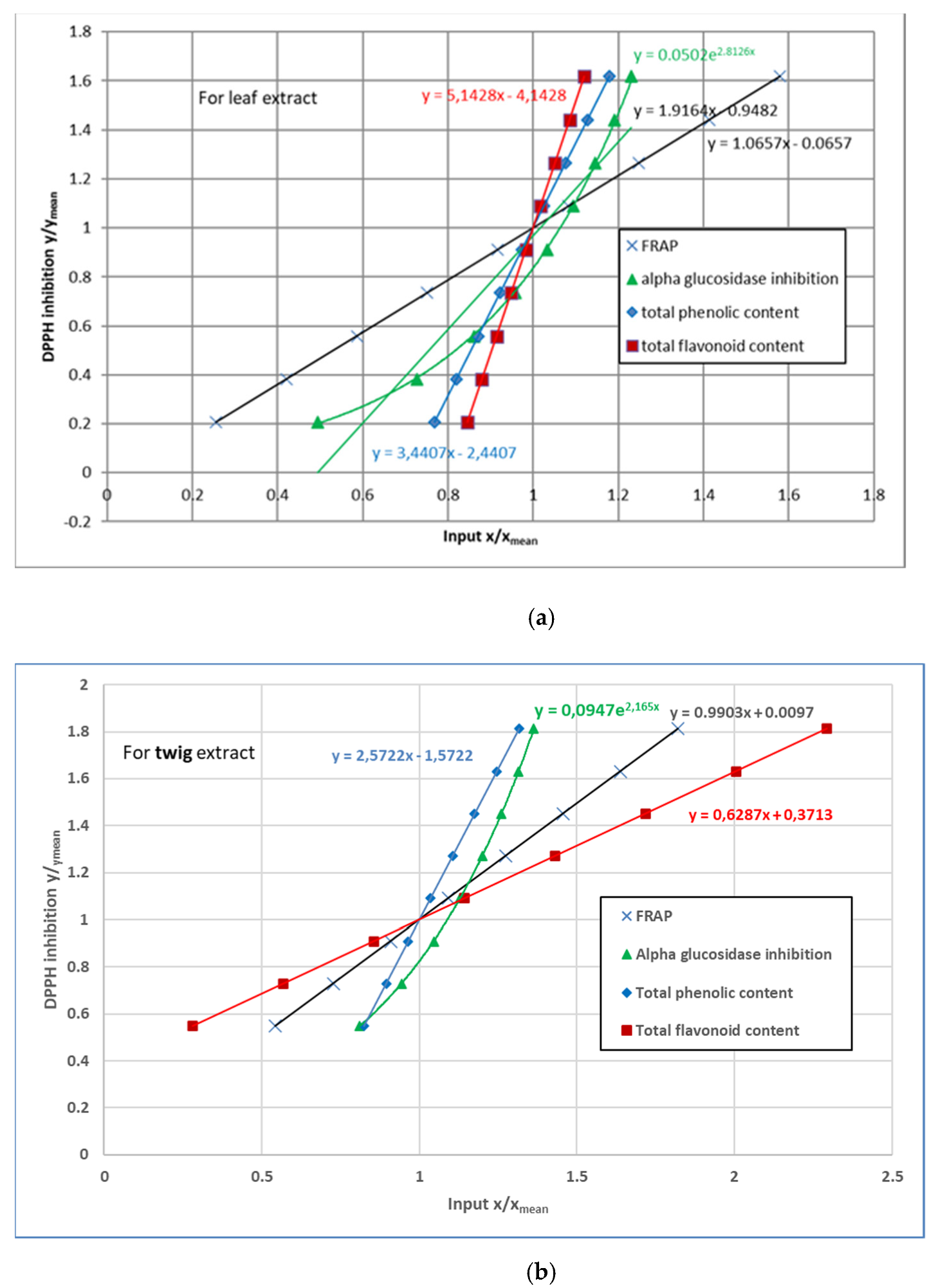
3.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
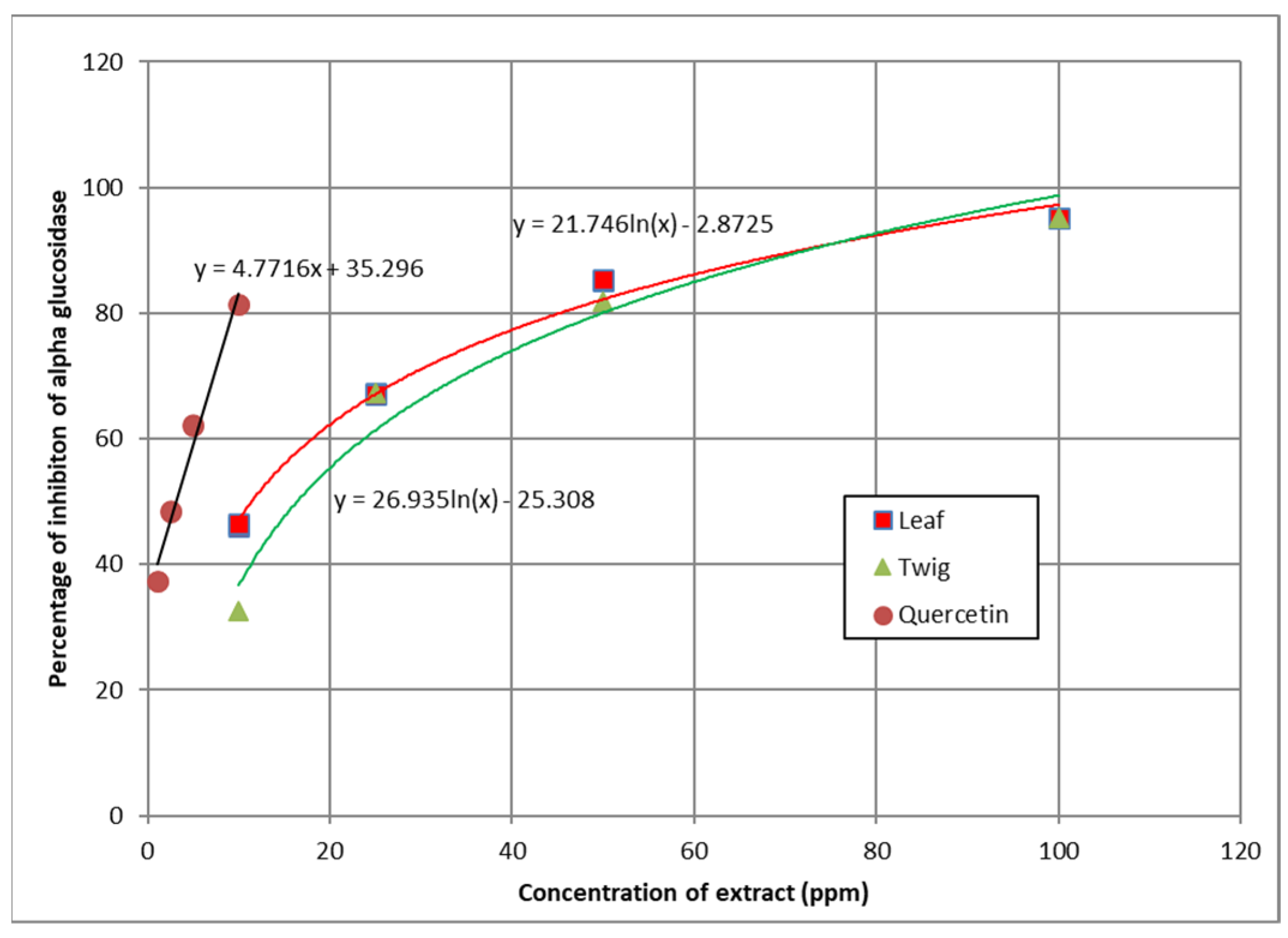
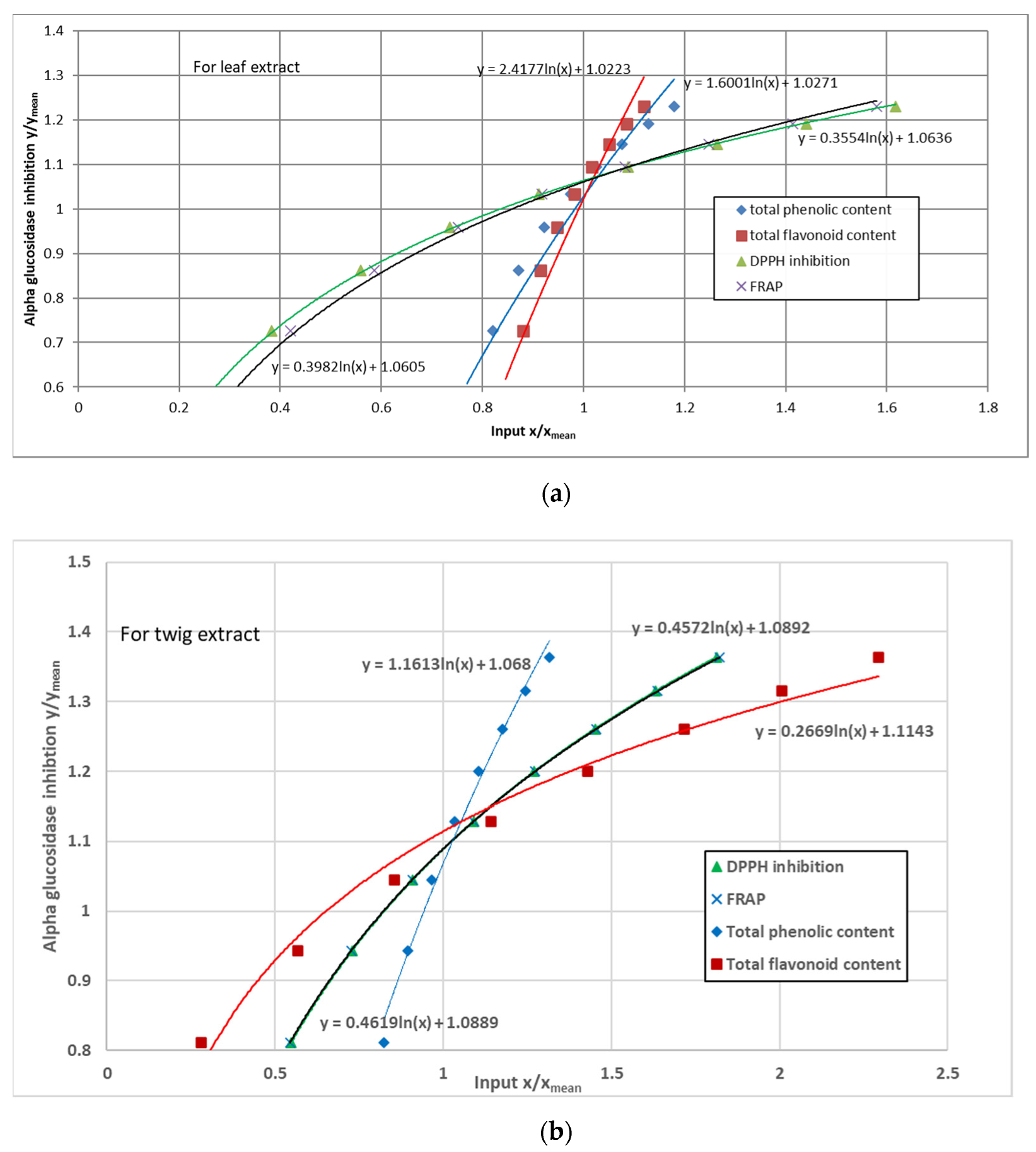
3.5. α-Glucosidase Inhibition
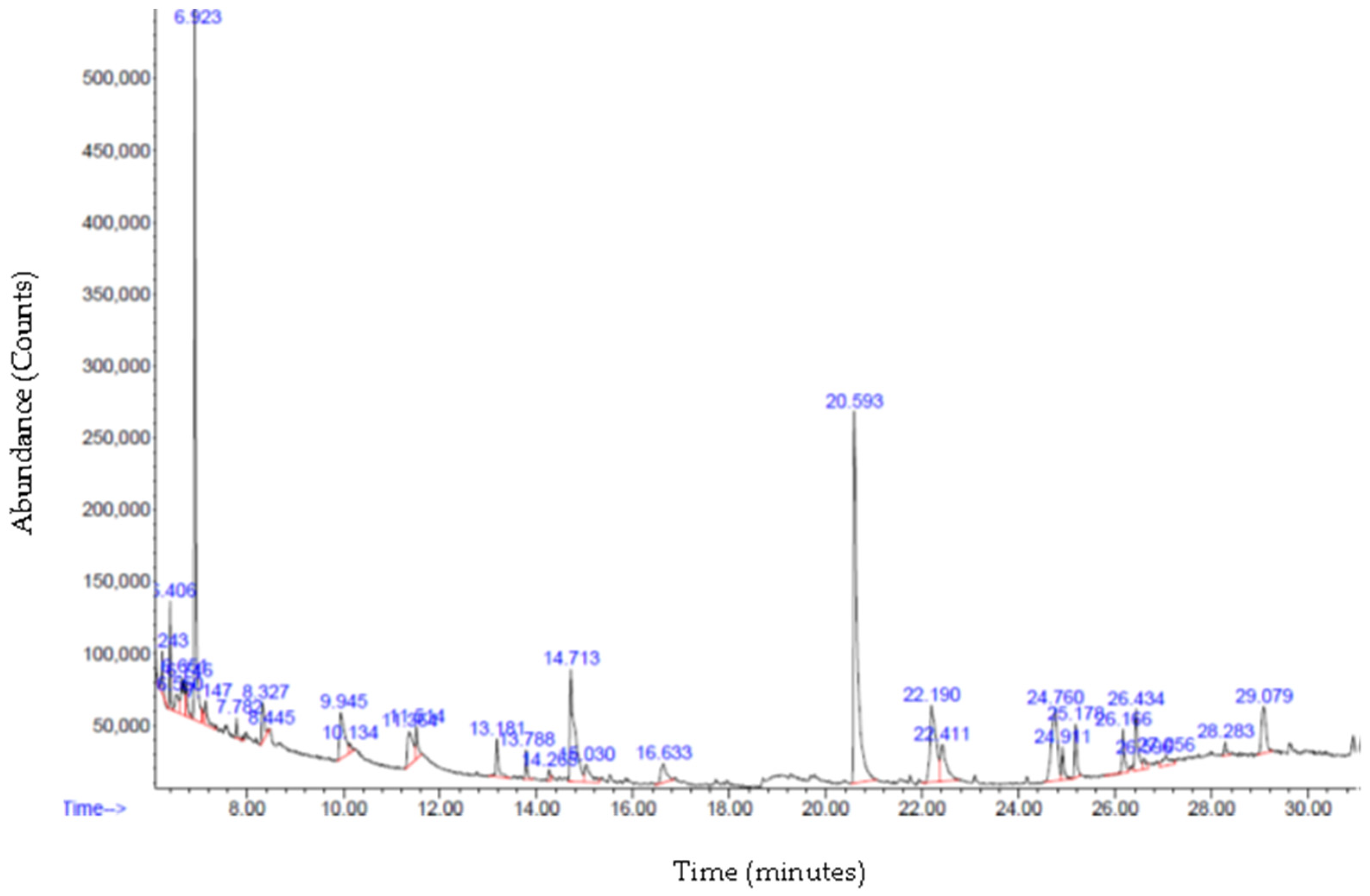
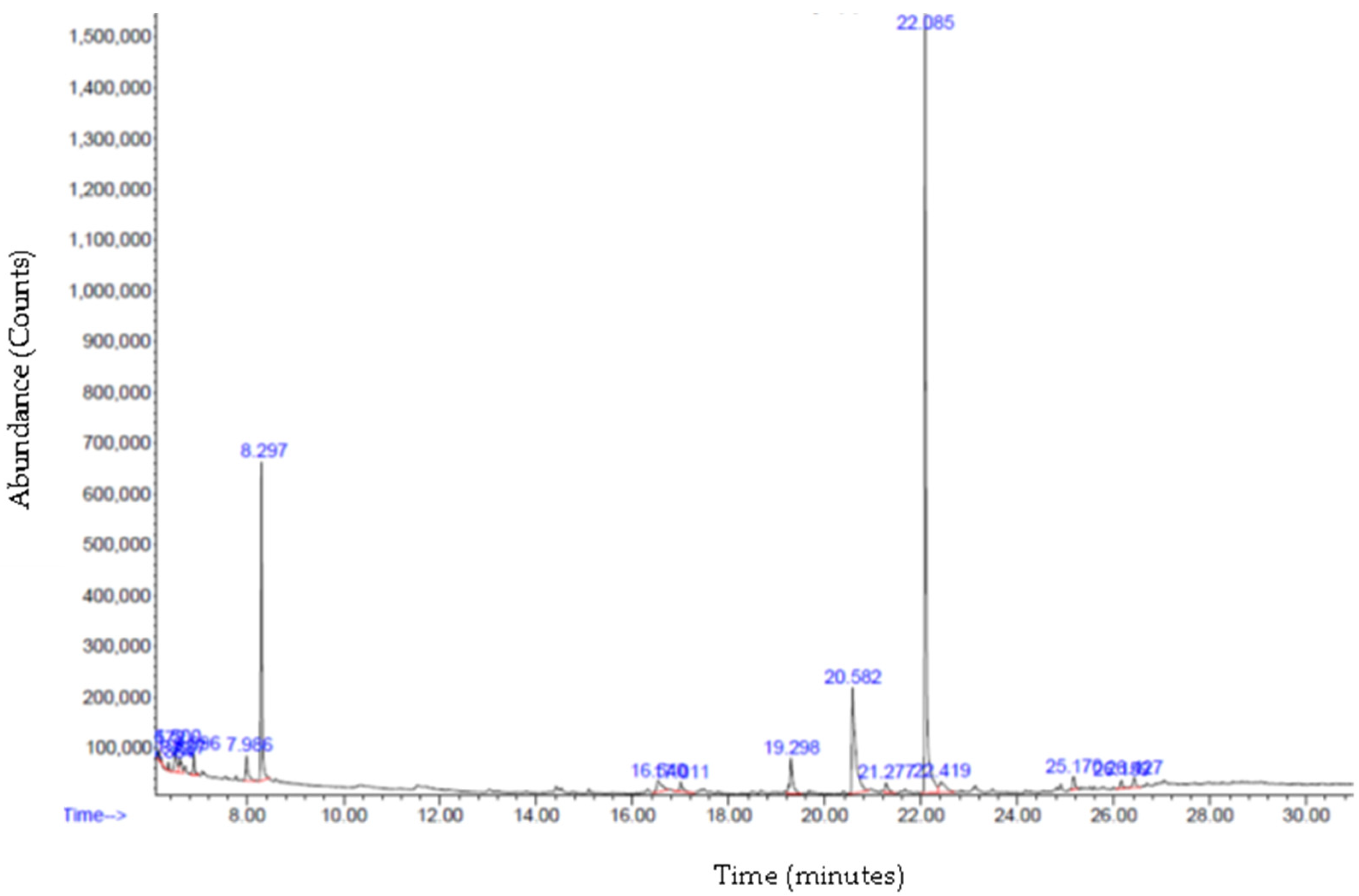
3.6. Gas Chromatography—Mass Spectrometry (GC-MS) Analysis
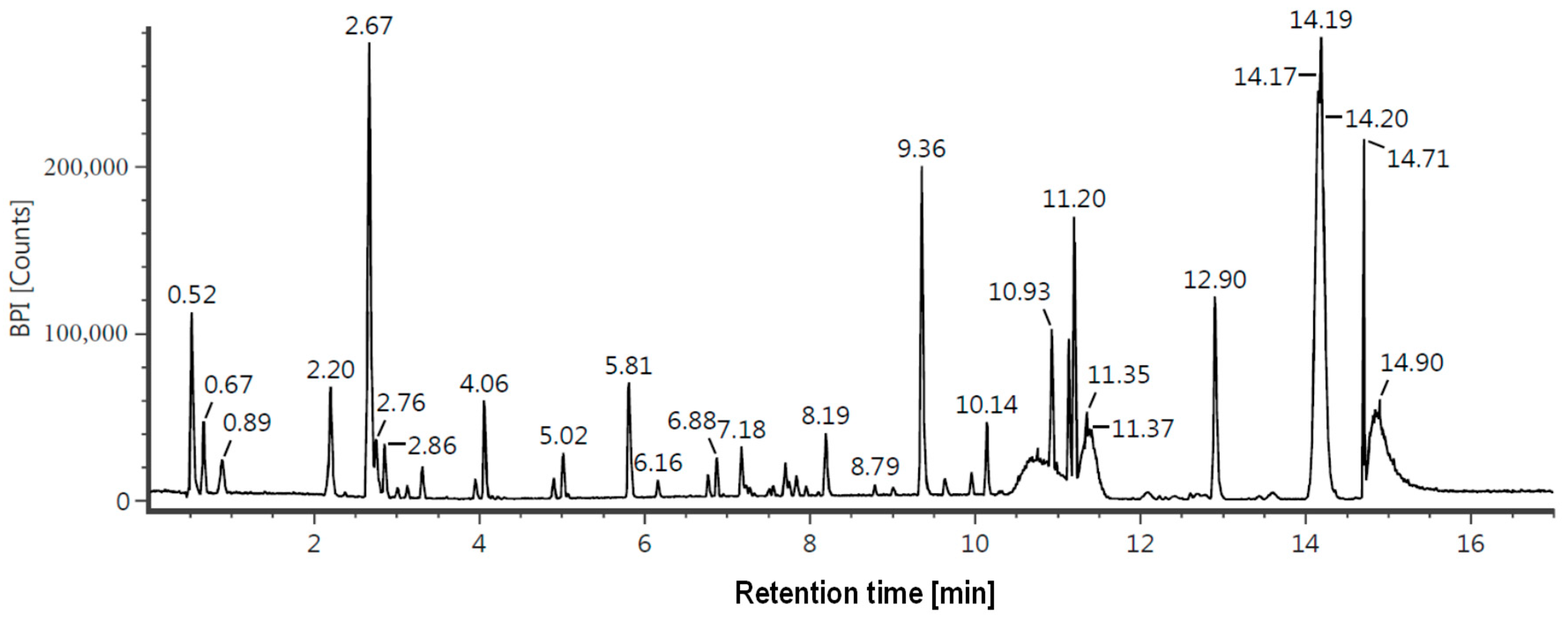
3.7. Liquid Chromatography—Mass Spectrometry (LC-MS/MS) Analysis
3.8. Molecular Docking Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, J.; Tian, J.; Tang, C.; Fang, X.; Miao, R.; Wu, H.; Wang, X.; Tong, X. The influence of different types of diabetes on vascular complications. J. Diabetes Res. 2022, 2022, 3448618. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Kumar, A.; Behera, A. Biomedicine & pharmacotherapy type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2021, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Zitzmamann, N.; Rudd, M.P.; Bock, M.T.; Dwek, A.R. Alpha glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 1998, 430, 17–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Lou, H.; Shan, L. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Oncotarget 2017, 8, 81027–81039. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, N.U.D.; Arunagirinathan, G. Efficacy and cardiovascular safety of alpha glucosidase inhibitors. Curr. Drug Saf. 2021, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J. Evolution of glucose-lowering drugs for type 2 diabetes: A new era of cardioprotection. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 429–454. ISBN 9780128120194. [Google Scholar]
- Rosa, M.M.; Dias, T. Commonly used endocrine drugs. Handb. Clin. Neurol. 2014, 120, 809–824. [Google Scholar] [CrossRef]
- Hossain, M.A.; Pervin, R. Current antidiabetic drugs. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 455–473. ISBN 9780128120194. [Google Scholar]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Koorbanally, N.A.; Islam, M.S. Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-glucosidase and α-amylase) by Khaya senegalensis. Acta Pharm. 2014, 64, 311–324. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Hussain, G.; Mustafa, I. Antioxidants from natural sources. In Antioxidants in Foods and Its Application; IntechOpen: London, UK, 2018; pp. 3–28. [Google Scholar] [CrossRef]
- Rasheed, A.; Fathima Abdul Azeez, R. A review on natural antioxidants. In Traditional and Complementary Medicine; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Cedeño, H.; Espinosa, S.; Andrade, J.M.; Cartuche, L.; Malagon, O. Novel flavonoid glycosides of quercetin from leaves and flowers of Gaiadendron punctatum G.Don (Violeta de Campo), used by the saraguro community in Southern Ecuador, inhibit alpha glucosidase enzyme. Molecules 2019, 24, 4267. [Google Scholar] [CrossRef] [PubMed]
- Mosihuzzman, M.; Naheed, S.; Hareem, S.; Talib, S.; Abbas, G.; Khan, S.N.; Choudhary, M.I.; Sener, B.; Tareen, R.B.; Israr, M. Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci. 2013, 92, 187–192. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; Elgamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; Alajmi, M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.N.; Gao, B.; Li, P.; Inagaki, C.; Kawabata, J. Inhibitory effect on α-glucosidase by Adhatoda vasica Nees. Food Chem. 2008, 108, 965–972. [Google Scholar] [CrossRef]
- Sheikh, Y.; Chanu, M.B.; Mondal, G.; Manna, P.; Chattoraj, A.; Chandra Deka, D.; Chandra Talukdar, N.; Chandra Borah, J. Procyanidin A2, an anti-diabetic condensed tannin extracted from Wendlandia glabrata, reduces elevated G-6-Pase and mRNA levels in diabetic mice and increases glucose uptake in CC1 hepatocytes and C1C12 myoblast cells. RSC Adv. 2019, 9, 17211–17219. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Yashiro, K.; Matsuda, H. Kotalanol, a potent alpha glukosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic Ayurvedic medicine Salacia reticulata. Chem. Pharm. Bull. 1998, 46, 1339–1340. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nishida, N.; Shimoda, H.; Takada, M.; Kawahara, Y.; Matsuda, H. Polyphenol constituents from Salacia Species: Quantitative analysis of mangiferin with α-glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi 2001, 121, 371–378. [Google Scholar] [CrossRef]
- Hung, N.H.; Dai, D.N.; Dung, D.M.; Giang, T.T.B.; Thang, T.D.; Ogunwande, I.A. Chemical composition of essential oils of Artabotrys petelotii Merr., Artabotrys intermedius Hassk., and Artabotrys harmandii Finet & Gagnep. (Annonaceae) from Vietnam. J. Essent. Oil-Bear. Plants 2014, 17, 1105–1111. [Google Scholar] [CrossRef]
- Chen, J.; Eiadthong, W. New species and new records of Artabotrys (Annonaceae) from peninsular Thailand. PhytoKeys 2020, 151, 67–81. [Google Scholar] [CrossRef]
- Chong, J.Y.; Rajagopal, M.; Chandramanthi, S.; Ashok Kumar, B.; Sasikala, C.; Geethanjali, K. Evaluation of antibacterial activity against multidrug resistant (MDR) bacteria by the fractions of Artabotrys suaveolens (Blume). Curr. Trends Biotechnol. Pharm. 2020, 15, 262–269. [Google Scholar] [CrossRef]
- Mohan, S.K.; Veeraraghavan, V.P.; Balakrishna, J.P.; Rengasamy, G.; Rajeshkumar, S. Antidiabetic activity of methanolic extract of Artabotrys suaveolens Leaves in 3T3-L1 cell line. J. Pure Appl. Microbiol. 2020, 14, 573–580. [Google Scholar] [CrossRef]
- Kwan, T.K.; Shipton, F.; Nor Azman, N.S.; Hossan, S.; Jin, K.T.; Wiart, C. Cytotoxic aporphines from Artabotrys crassifolius. Nat. Prod. Commun. 2016, 11, 389–392. [Google Scholar] [CrossRef]
- Liu, Y.P.; Tang, J.Y.; Hua, Y.; Lai, L.; Luo, X.L.; Zhang, Z.J.; Yin, W.Q.; Chen, G.Y.; Fu, Y.H. Bioactive polyoxygenated seco-cyclohexenes from Artabotrys hongkongensis. Bioorg. Chem. 2018, 76, 386–391. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, Y.P.; Yan, G.; Yang, S.; Hu, S.; Hua, J.; Yin, W.Q.; Chen, G.Y.; Fu, Y.H. Bioactive eudesmane sesquiterpenes from Artabotrys hongkongensis Hance. Nat. Prod. Res. 2020, 34, 1687–1693. [Google Scholar] [CrossRef]
- Nyandoro, S.S.; Joseph, C.C.; Nkunya, M.H.H.; Hosea, K.M.M. New antimicrobial, mosquito larvicidal and other metabolites from two Artabotrys species. Nat. Prod. Res. 2013, 27, 1450–1458. [Google Scholar] [CrossRef]
- Tan, K.K.; Khoo, T.J.; Rajagopal, M.; Wiart, C. Antibacterial alkaloids from Artabotrys crassifolius Hook.f. & Thomson. Nat. Prod. Res. 2015, 29, 2346–2349. [Google Scholar] [CrossRef]
- Dewi, R.T.; Tachibana, S.; Darmawan, A. Effect on α-glucosidase inhibition and antioxidant activities of butyrolactone derivatives from Aspergillus terreus MC751. Med. Chem. Res. 2014, 23, 454–460. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Tomasina, F.; Carabio, C.; Celano, L. Analysis of two methods to evaluate antioxidants. Biochem. Mol. Biol. Educ. 2012, 40, 266–270. [Google Scholar] [CrossRef]
- Wiliantari, S.; Iswandana, R.; Elya, B. Total polyphenols, total flavonoids, antioxidant activity and inhibition of tyrosinase enzymes from extract and fraction of Passiflora ligularis Juss. Pharmacogn. J. 2022, 14, 660–671. [Google Scholar] [CrossRef]
- Irshad, M.; Zafaryab, M.; Singh, M.; Rizvi, M.M.A. Comparative analysis of the antioxidant activity of cassia fistula extracts. Int. J. Med. Chem. 2012, 2012, 157125. [Google Scholar] [CrossRef]
- Budiarso, F.S.; Elya, B.; Hanafi, M.; Limengan, A.H.; Rahmasari, R. Antioxidant activity of methanol fractions stem bark of Kayu Sarampa (Xylocarpus moluccensis (Lam.) M. Roen)). Pharmacogn. J. 2021, 13, 1694–1701. [Google Scholar] [CrossRef]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P.R.; Dixit, G.B. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from Western Ghats of India. South African J. Bot. 2013, 88, 273–277. [Google Scholar] [CrossRef]
- Rahim, N.A.; Roslan, M.N.F.; Muhamad, M.; Seeni, A. Antioxidant activity, total phenolic and flavonoid content and LC–MS profiling of leaves extracts of Alstonia angustiloba. Separations 2022, 9, 234. [Google Scholar] [CrossRef]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. Resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, A.S.C.; Denham, A.S.; Bassett, H.H.; Curby, W.T. Differences between high- and low-affinity complexes of enzymes and non-enzymes. J. Med. Chem. 2008, 51, 6432–6441. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Z.; Zhu, Z.; Chai, Y.; Wu, Y. A Rapid high-performance liquid chromatographic method for quantitative analysis of antidiabetic-active components in Anemarrhena asphodeloides rhizomes. Chromatographia 2005, 61, 633–636. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, K.; Jiang, Y.; Yang, Z.; Hu, W.; Xiong, L.; Wang, N.; Liu, X.; Wang, W. Analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on alpha -glucosidase activity. Int. J. Biol. Macromol. 2017, 98, 829–836. [Google Scholar] [CrossRef]
- Koia, J.H.; Shepherd, P. The potential of anti-diabetic rākau rongoā (Māori herbal medicine) to treat type 2 diabetes mellitus (T2DM) mate huka: A review. Front. Pharmacol. 2020, 11, 935. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.; Marston, A.; Hostettmann, K. Fitoterapia antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F.Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Manzione, M.G.; Martorell, M.; Sharopov, F.; Ganesh, N.; Venkatesh, N.; Kumar, A.; Valere, P.; Fokou, T.; Pezzani, R. Phytochemical and pharmacological properties of asperuloside, a systematic review. Eur. J. Pharmacol. 2020, 883, 173344. [Google Scholar] [CrossRef]
- Wang, Y.H. Traditional uses and pharmacologically active constituents of dendrobium plants for dermatological disorders: A review. Nat. Products Bioprospect. 2021, 11, 465–487. [Google Scholar] [CrossRef]
- Khan, K.; Firdous, S.; Ahmad, A.; Fayyaz, N.; Rasheed, M.; Faizi, S. GC-MS profile of antimicrobial and antioxidant fractions from Cordia rothii roots. Pharm. Biol. 2016, 54, 2597–2605. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, anti-inflammatory, and anti-diabetic activity of phenolic acids fractions obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef]

| R/T | Compound | Formula | MW | Peak Area% |
|---|---|---|---|---|
| 14.708 | Naphthalene decahydro-4a-methyl-1-methylene-7- (1-methylethenyl)-, [4aR-trans] | C15H24 | 204.4 | 8.8280 |
| 24.765 | 9-O-Pivaloyl-N-acetylcolchinol | C25H31NO6 | 441.5 | 6.9491 |
| 29.075 | Anthracene, 9,10-dihydro-9,9,10-trimethyl | C17H18 | 222.32 | 3.25 |
| 15.035 | Zonarene | C15H24 | 204.4 | 1.7401 |
| 13.183 | Copaene | C15H24 | 204.4 | 1.598 |
| 13.787 | Caryophyllene | C15H24 | 204.4 | 0.915 |
| 26.592 | N-Methyl-1-adamantaneacetamide | C13H21NO | 207.31 | 0.59 |
| 14.266 | Lavandulyl isobutyrate | C15H24 | 204.4 | 0.3354 |
| R/T | Compound | Formula | MW | Peak Area% |
|---|---|---|---|---|
| 19.295 | 7,9-Di-tert-butyl-1-oxaspiro(4,5) deca-6,9-diene-2,8-dione | C17H24O3 | 276.4 | 4.07 |
| 7.990 | D- Limonene | C10H16 | 136.2 | 2.29 |
| 21.274 | Octacosane | C28H58 | 394.8 | 1.09 |
| 17.014 | Octadecane | C18H38 | 254.5 | 1.03 |
| R/T | Compound | Formula | Observed MW | Adduct | Response |
|---|---|---|---|---|---|
| 2.68 | Mangiferin | C19H18O11 | 423.09 | +H, +Na | 443421 |
| 3.23 | Kaempferol 7-O-α-L-rhamnoside | C21H20O10 | 433.11 | +H | 157434 |
| 12.91 | Stigmastan-3,6-dione | C29H48O2 | 429.4 | +H | 108634 |
| 2.24 | Neomangiferin | C25H28O16 | 585.15 | +H, +Na | 100578 |
| 2.87 | Asperuloside | C18H22O11 | 437.1 | +Na | 88206 |
| R/T | Compound | Formula | Observed MW | Adduct | Response |
|---|---|---|---|---|---|
| 2.67 | Mangiferin | C19H18O11 | 423.09 | +H, +Na | 374611 |
| 9.36 | Trichosanic acid | C29H48O2 | 279.23 | +H | 212382 |
| 2.21 | Neomangiferin | C25H28O16 | 585.15 | +H, +Na | 193055 |
| 12.91 | Stigmastan-3,6-dione | C29H48O2 | 429.4 | +H | 162276 |
| 4.07 | Moupinamide | C21H20O10 | 433.11 | +H | 70497 |
| No. | Compound | Free Binding Energy (kcal/mol) | |
|---|---|---|---|
| 1 | Neomangiferin | −16.27 | 1.18 pM |
| 2 | Kaempferol-7-O-α-L-rhamnoside | −12.86 | 372.97 pM |
| 3 | Asperuloside | −12.39 | 829.30 pM |
| 4 | Mangiferin | −12.23 | 1.08 nM |
| 5 | Moupinamide | −10.61 | 16.65 nM |
| 6 | 9-O-Pivaloyl-N-acetylcolchinol | −10.02 | 45.40 nM |
| 7 | Stigmastan 3,6-dione | −9.65 | 84.60 nM |
| 8 | 7,9-Di-tert-butyl-1-oxaspiro(4,5) deca-6,9-diene-2,8-dione | −8.98 | 262.18 nM |
| 9 | Trichosanic acid | −7.81 | 1.89 μM |
| 10 | N-Methyl-1-adamantaneacetamide | −7.67 | 2.40 μM |
| 11 | Anthracene, 9,10-dihydro-9,9,10-trimethyl | −7.62 | 2.59 μM |
| 12 | Naphthalene decahydro-4a-methyl-1-methylene-7- (1-methylethenyl)-, [4aR-trans] | −7.51 | 3.10 μM |
| 13 | Copaene | −7.34 | 4.18 μM |
| 14 | Zonarene | −7.15 | 5.78 μM |
| 15 | (β-)Caryophyllene | −7.00 | 6.12 μM |
| 16 | Lavandulyl isobutyrate | −6.67 | 12.93 μM |
| 17 | D limonene | −5.72 | 63.83 μM |
| 18 | Octadecane | −5.53 | 88.13 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, D.; Elya, B.; Hanafi, M.; Khatib, A.; Surya, M.I. In Vitro and In Silico Screening Analysis of Artabotrys sumatranus Leaf and Twig Extracts for α-Glucosidase Inhibition Activity and Its Relationship with Antioxidant Activity. Sci. Pharm. 2023, 91, 2. https://doi.org/10.3390/scipharm91010002
Rosa D, Elya B, Hanafi M, Khatib A, Surya MI. In Vitro and In Silico Screening Analysis of Artabotrys sumatranus Leaf and Twig Extracts for α-Glucosidase Inhibition Activity and Its Relationship with Antioxidant Activity. Scientia Pharmaceutica. 2023; 91(1):2. https://doi.org/10.3390/scipharm91010002
Chicago/Turabian StyleRosa, Dela, Berna Elya, Muhammad Hanafi, Alfi Khatib, and Muhammad Imam Surya. 2023. "In Vitro and In Silico Screening Analysis of Artabotrys sumatranus Leaf and Twig Extracts for α-Glucosidase Inhibition Activity and Its Relationship with Antioxidant Activity" Scientia Pharmaceutica 91, no. 1: 2. https://doi.org/10.3390/scipharm91010002
APA StyleRosa, D., Elya, B., Hanafi, M., Khatib, A., & Surya, M. I. (2023). In Vitro and In Silico Screening Analysis of Artabotrys sumatranus Leaf and Twig Extracts for α-Glucosidase Inhibition Activity and Its Relationship with Antioxidant Activity. Scientia Pharmaceutica, 91(1), 2. https://doi.org/10.3390/scipharm91010002






