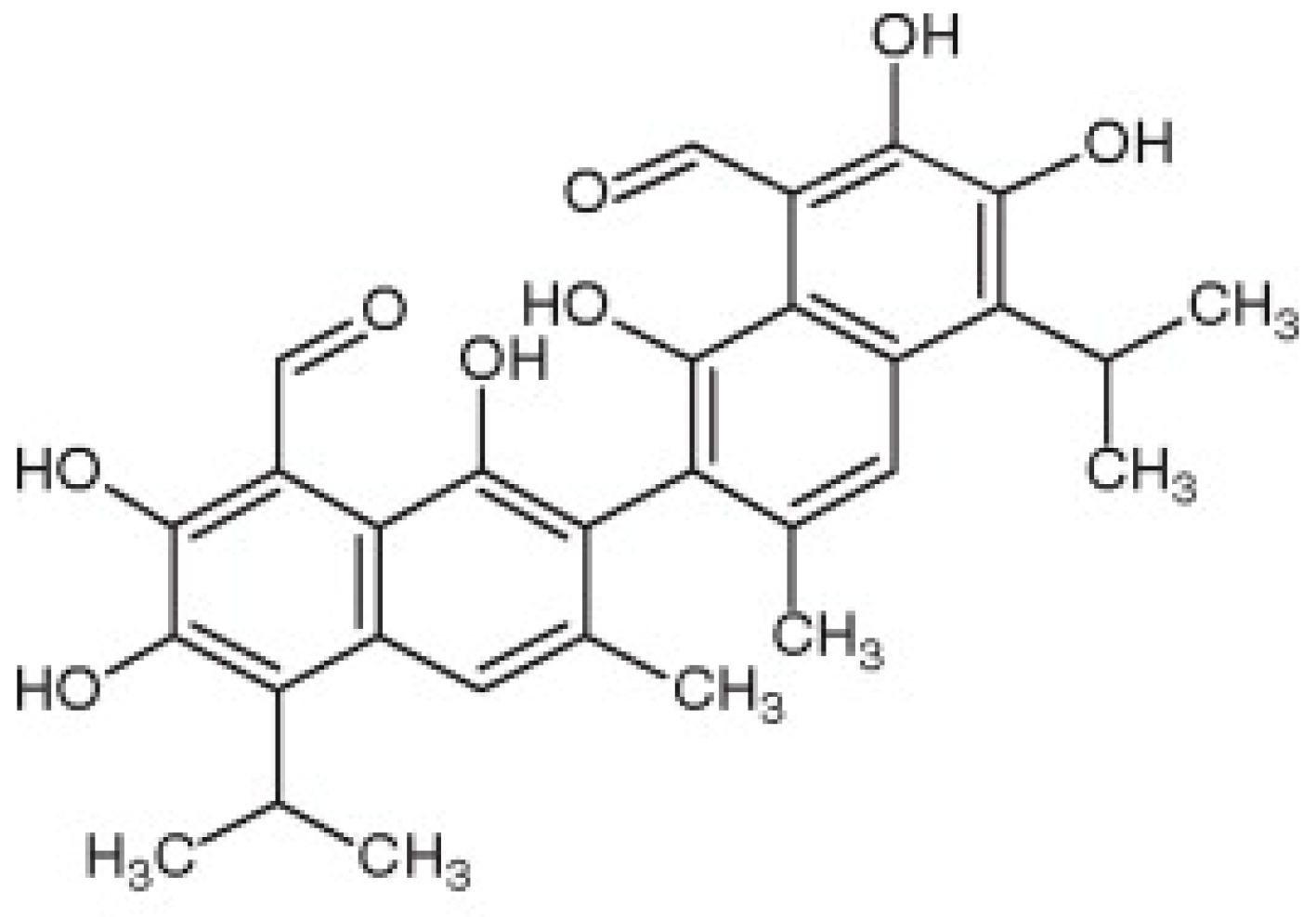
Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antimicrobial Assay
2.1.1. Bacterial Strains and Culture Requirements
2.1.2. Anti-Helicobacter pylori (HP) Activity
2.2. Enzyme Inhibition Assay
2.2.1. Instrumentation and Operational Parameter Settings
2.2.2. Anti-Helicobacter pylori Urease (HPU) Assay
2.3. Cytotoxicity Assessment
2.3.1. Cell Lines Preparation
2.3.2. Cytotoxicity Test
2.4. Molecular Docking Studies
3. Results and Discussion
3.1. Evaluation of Anti-Helicobacter pylori (HP) Properties
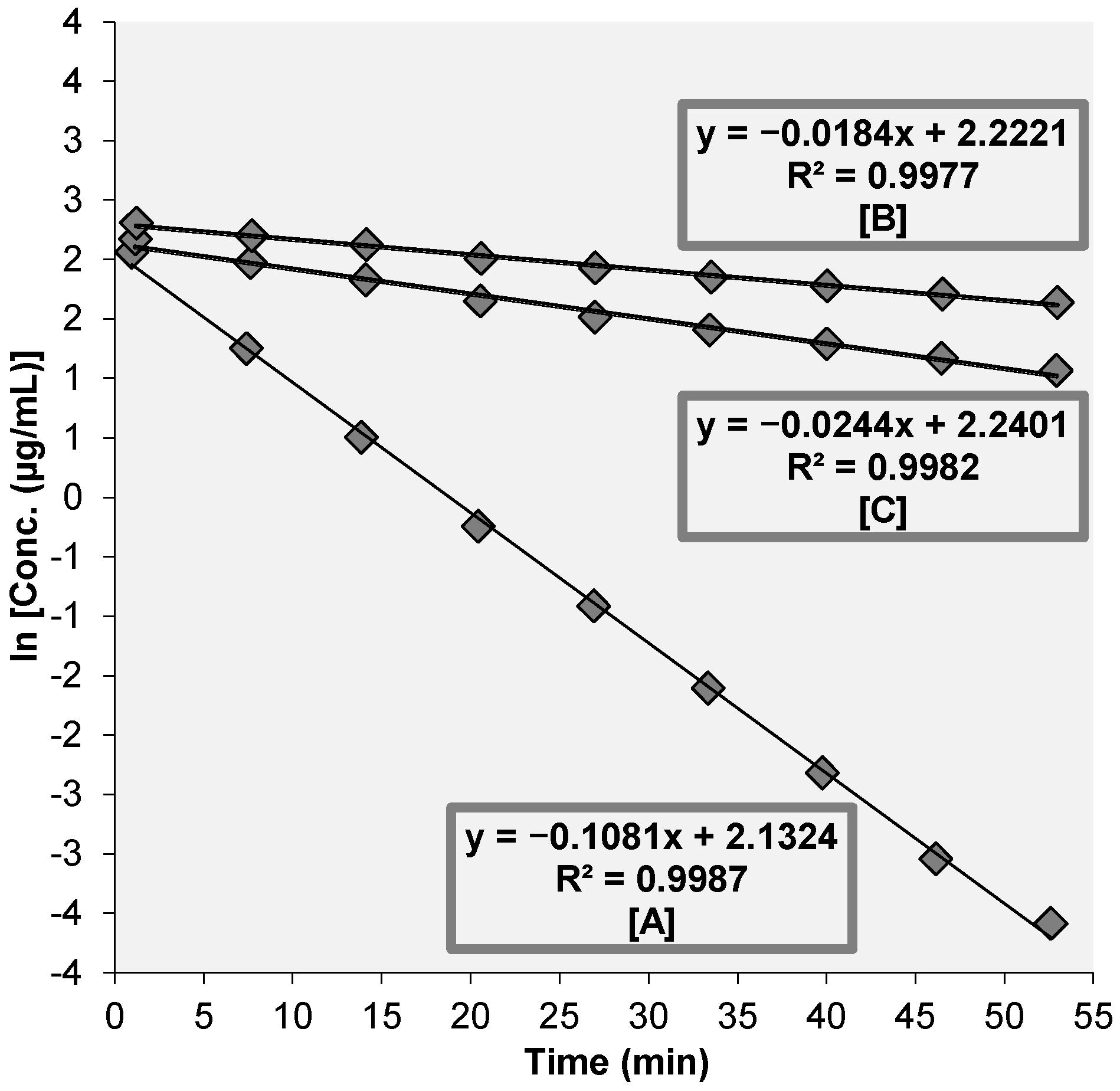
3.2. Assessment of Anti-Helicobacter pylori Urease (HPU) Activity
3.3. Evaluation of Cytotoxicity Properties
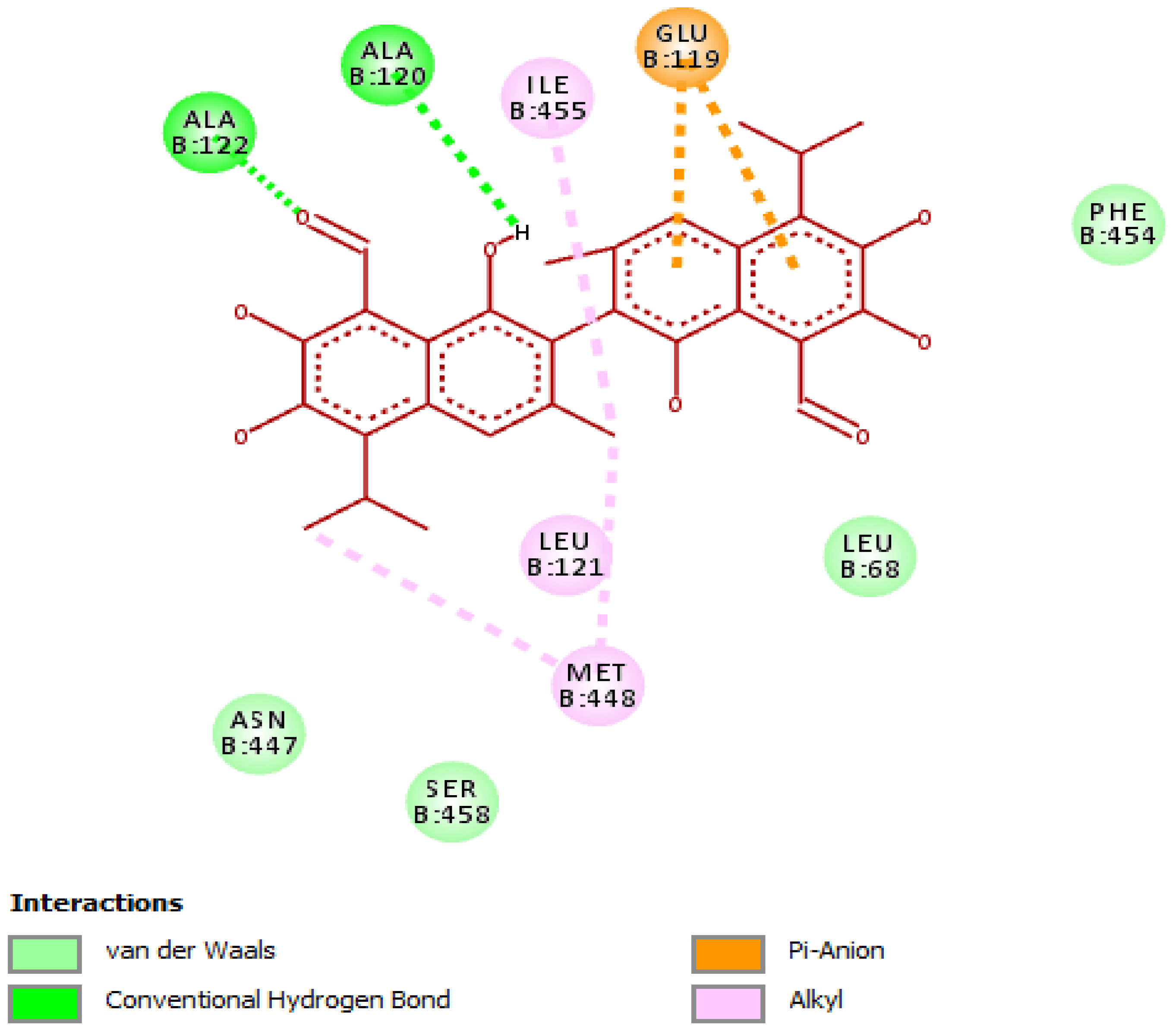
3.4. Evaluation of Molecular Docking Studies
Interaction of Gossypol with Helicobacter pylori Urease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, R.; Geissman, T.A.; Edwards, J.D. Gossypol, a Pigment of Cottonseed. Chem. Rev. 1960, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, A.; Villavicencio, H.; Montalvo, I.; Gonzalez-Garza, M.T. Gossypol Content on Leaves and Seeds from Some Wild Malvaceae Species. Afr. J. Tradit. Complementary Altern. Med. 2004, 2, 4–12. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Wierda, W.G.; Keating, M.J.; Gandhi, V. Gossypol, a BH3 Mimetic, Induces Apoptosis in Chronic Lymphocytic Leukemia Cells. Blood 2008, 112, 1971–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodou, K.; Anderson, R.J.; Small, D.A.P.; Groundwater, P.W. Investigations on Gossypol: Past and Present Developments. Expert Opin. Investig. Drugs 2005, 14, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Keshmiri-Neghab, H.; Goliaei, B. Therapeutic Potential of Gossypol: An Overview. Pharm. Biol. 2014, 52, 124–128. [Google Scholar] [CrossRef]
- Qian, S.Z.; Wang, Z.G. Gossypol: A Potential Antifertility Agent for Males. Annu. Rev. Pharmacol. Toxicol. 1984, 24, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of Cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Ma, J.; Xu, L.; Wu, D. Natural Product Gossypol and Its Derivatives in Precision Cancer Medicine. Curr. Med. Chem. 2019, 26, 1849–1873. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Shi, H.; Zhang, R.; Qu, K.; Hu, Y.; Qu, X.; Gan, C.; Chen, J.; Shi, X.; et al. Gastric Floating Tablet Improves the Bioavailability and Reduces the Hypokalemia Effect of Gossypol in Vivo. Saudi Pharm. J. 2021, 29, 305–314. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, Y. Resistance Mechanisms of Helicobacter pylori and Its Dual Target Precise Therapy. Crit. Rev. Microbiol. 2018, 44, 371–392. [Google Scholar] [CrossRef]
- Calvet, X.; Ramírez Lázaro, M.-J.; Lehours, P.; Mégraud, F. Diagnosis and Epidemiology of Helicobacter pylori Infection. Helicobacter 2013, 18 (Suppl. 1), 5–11. [Google Scholar] [CrossRef] [PubMed]
- Doorakkers, E.; Lagergren, J.; Engstrand, L.; Brusselaers, N. Eradication of Helicobacter pylori and Gastric Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. J. Natl. Cancer Inst. 2016, 108, djw132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Šudomová, M. Probiotics as Dietary Supplements for Eradication of Helicobacter pylori Infection in Children: A Role Beyond Infection. Children 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E. In Vitro Synergistic Effect of Hibiscus sabdariffa Aqueous Extract in Combination with Standard Antibiotics against Helicobacter pylori Clinical Isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhu, Y.; Lu, N.-H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 168. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In Vitro Biological Effects of Phenolic Compounds from Morus Alba Root Bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Bren, U. Generation Times of E. coli Prolong with Increasing Tannin Concentration While the Lag Phase Extends Exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.-P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori Infections: What Are We Doing Now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.S.; Žemlička, M. Plant-Derived Urease Inhibitors as Alternative Chemotherapeutic Agents. Arch. Pharm. 2016, 349, 507–522. [Google Scholar] [CrossRef]
- Xie, J.; Lin, Z.; Xian, Y.; Kong, S.; Lai, Z.; Ip, S.; Chen, H.; Guo, H.; Su, Z.; Yang, X.; et al. (−)-Patchouli Alcohol Protects against Helicobacter pylori Urease-Induced Apoptosis, Oxidative Stress and Inflammatory Response in Human Gastric Epithelial Cells. Int. Immunopharmacol. 2016, 35, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlini, C.R.; Ligabue-Braun, R. Ureases as Multifunctional Toxic Proteins: A Review. Toxicon 2016, 110, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Šudomová, M. The Development of Urease Inhibitors: What Opportunities Exist for Better Treatment of Helicobacter pylori Infection in Children? Children 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-Enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef]
- Fiori-Duarte, A.T.; Rodrigues, R.P.; Kitagawa, R.R.; Kawano, D.F. Insights into the Design of Inhibitors of the Urease Enzyme—A Major Target for the Treatment of Helicobacter pylori Infections. Curr. Med. Chem. 2020, 27, 3967–3982. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- Šudomová, M.; Hassan, S.T.S.; Khan, H.; Rasekhian, M.; Nabavi, S.M. A Multi-Biochemical and In Silico Study on Anti-Enzymatic Actions of Pyroglutamic Acid against PDE-5, ACE, and Urease Using Various Analytical Techniques: Unexplored Pharmacological Properties and Cytotoxicity Evaluation. Biomolecules 2019, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.T.S.; Švajdlenka, E. Biological Evaluation and Molecular Docking of Protocatechuic Acid from Hibiscus sabdariffa L. as a Potent Urease Inhibitor by an ESI-MS Based Method. Molecules 2017, 22, 1696. [Google Scholar] [CrossRef] [Green Version]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 2 July 2021).
- Lu, Q.; Li, C.; Wu, G. Insight into the Inhibitory Effects of Zanthoxylum Nitidum against Helicobacter pylori Urease and Jack Bean Urease: Kinetics and Mechanism. J. Ethnopharmacol. 2020, 249, 112419. [Google Scholar] [CrossRef]
- Cunha, E.S.; Chen, X.; Sanz-Gaitero, M.; Mills, D.J.; Luecke, H. Cryo-EM Structure of Helicobacter pylori Urease with an Inhibitor in the Active Site at 2.0 Å Resolution. Nat. Commun. 2021, 12, 230. [Google Scholar] [CrossRef]
- Kafarski, P.; Talma, M. Recent Advances in Design of New Urease Inhibitors: A Review. J. Adv. Res. 2018, 13, 101–112. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Qazi, S.U.; Naz, S.; Ishtiaq, M.; Khan, K.M. A Patent Update on Therapeutic Applications of Urease Inhibitors (2012–2018). Expert Opin. Ther. Pat. 2019, 29, 181–189. [Google Scholar] [CrossRef]
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol Toxicity from Cottonseed Products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J. Plumbagin, a Plant-Derived Compound, Exhibits Antifungal Combinatory Effect with Amphotericin B against Candida Albicans Clinical Isolates and Anti-Hepatitis C Virus Activity. Phytother. Res. 2016, 30, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B Interacts Synergistically with Antibiotics against Staphylococcus aureus Clinical Isolates and Exhibits Antiviral Activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Ha, N.C.; Oh, S.T.; Sung, J.Y.; Cha, K.A.; Lee, M.H.; Oh, B.H. Supramolecular Assembly and Acid Resistance of Helicobacter pylori Urease. Nat. Struct. Biol. 2001, 8, 505–509. [Google Scholar] [CrossRef]
- Mazzei, L.; Musiani, F.; Ciurli, S. The Structure-Based Reaction Mechanism of Urease, a Nickel Dependent Enzyme: Tale of a Long Debate. J. Biol. Inorg. Chem. 2020, 25, 829–845. [Google Scholar] [CrossRef]
- Furlan, V.; Konc, J.; Bren, U. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 2018, 23, 3351. [Google Scholar] [CrossRef] [Green Version]
- Lešnik, S.; Bren, U. Mechanistic Insights into Biological Activities of Polyphenolic Compounds from Rosemary Obtained by Inverse Molecular Docking. Foods 2021, 11, 67. [Google Scholar] [CrossRef]
| HP Strains | MIC (µg/mL) | |||
|---|---|---|---|---|
| Gossypol | Clarithromycin | Metronidazole | Levofloxacin | |
| HP43504 a | 4.14 ± 0.13 (9.5 ± 3.31) | 0.26 ± 0.02 | 7.91 ± 0.13 | 1.12 ± 0.13 |
| HP1 b | 3.91 ± 0.12 (10.1 ± 3.58) | 0.27 ± 0.03 | 8.11 ± 0.13 | 1.11 ± 0.12 |
| HP2 b | 3.92 ± 0.14 (10.0 ± 3.07) | 0.24 ± 0.03 | 7.94 ± 0.15 | 0.92 ± 0.03 |
| HP3 b | 4.11 ± 0.15 (9.6 ± 2.87) | 0.23 ± 0.03 | 8.10 ± 0.14 | 1.12 ± 0.13 |
| HP4 b | 3.82 ± 0.12 (10.3 ± 3.58) | 0.24 ± 0.03 | 8.13 ± 0.10 | 1.13 ± 0.14 |
| HP5 b | 3.81 ± 0.14 (10.3 ± 3.07) | 0.28 ± 0.03 | 8.12 ± 0.12 | 0.91 ± 0.04 |
| HP6 b | 4.12 ± 0.12 (9.54 ± 3.58) | 0.28 ± 0.03 | 8.20 ± 0.12 | 0.94 ± 0.04 |
| HP7 b | 3.91 ± 0.12 (10.1 ± 3.58) | 0.25 ± 0.04 | 7.96 ± 0.14 | 1.10 ± 0.11 |
| HP8 b | 3.73 ± 0.13 (10.5 ± 3.31) | 0.26 ± 0.02 | 8.14 ± 0.13 | 1.10 ± 0.11 |
| HP9 b | 3.72 ± 0.12 (10.6 ± 3.58) | 0.24 ± 0.03 | 8.00 ± 0.15 | 1.13 ± 0.14 |
| HP10 b | 3.51 ± 0.15 (11.2 ± 2.87) | 0.25 ± 0.04 | 8.13 ± 0.13 | 1.11 ± 0.12 |
| Compound | IC50 (µg/mL) |
|---|---|
| Gossypol | 39.32 ± 0.43 |
| Cisplatin | 6.21 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šudomová, M.; Hassan, S.T.S. Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments. Sci. Pharm. 2022, 90, 29. https://doi.org/10.3390/scipharm90020029
Šudomová M, Hassan STS. Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments. Scientia Pharmaceutica. 2022; 90(2):29. https://doi.org/10.3390/scipharm90020029
Chicago/Turabian StyleŠudomová, Miroslava, and Sherif T. S. Hassan. 2022. "Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments" Scientia Pharmaceutica 90, no. 2: 29. https://doi.org/10.3390/scipharm90020029
APA StyleŠudomová, M., & Hassan, S. T. S. (2022). Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments. Scientia Pharmaceutica, 90(2), 29. https://doi.org/10.3390/scipharm90020029







