Abstract
Oils, including essential oils and their constituents, are widely reported to have penetration enhancement activity and have been incorporated into a wide range of pharmaceutical formulations. This study sought to determine if there is an evidence base for the selection of appropriate oils for particular applications and compare their effectiveness across different formulation types. A systematic review of the data sources, consisting of Google Scholar, EMBASE, PubMed, Medline, and Scopus, was carried out and, following screening and quality assessment, 112 articles were included within the analysis. The research was classified according to the active pharmaceutical ingredient, dosage form, in vitro/in vivo study, carrier material(s), penetration enhancers as essential oils, and other chemical enhancers. The review identified four groups of oils used in the formulation of skin preparations; in order of popularity, these are terpene-type essential oils (63%), fatty acid-containing essential oils (29%) and, finally, 8% of essential oils comprising Vitamin E derivatives and miscellaneous essential oils. It was concluded that terpene essential oils may have benefits over the fatty acid-containing oils, and their incorporation into advanced pharmaceutical formulations such as nanoemulsions, microemulsions, vesicular systems, and transdermal patches makes them an attractive proposition to enhance drug permeation through the skin.
1. Introduction
Skin is a complicated organ that protects the human body from the surrounding environment and maintains its hydration in healthy and pathological conditions. The outermost layer of the human skin, the stratum corneum, provides the main barrier to the permeation of drugs across the skin [1,2]. Therefore, a large number of studies have focused on the identification of chemical penetration enhancers to help penetrate this layer and target the layers beneath this and even systemic circulation [3].
Over the last 30 years, more than 360 chemicals have shown promising potential for enhancing penetration through the skin [3,4]. These reviews present [1,2,3,4] an in-depth discussion of the skin barrier and pathways of permeation enhancement; however, safety concerns have limited the use of many chemicals with penetration enhancing effects [5,6]. Essential oils and their terpene constituents have been identified as a group of candidates with promising potential to be clinically useful as penetration enhancers.
Essential oils are natural oily liquids containing a mixture of volatile compounds found in different parts of plants, such as flowers, fruits, leaves, and roots. They are mainly composed of monoterpenes, sesquiterpenes, carbohydrates, alcohol, ethers, aldehydes, and ketones, and are extracted in small quantities using techniques such as steam and hydrodistillation [3,7,8,9,10,11]. Essential oils and their terpene components have been reported to enhance the penetration of various drugs successfully through the skin [12], with many demonstrating a potential for both hydrophilic and hydrophobic drugs. Among these are eucalyptus oil [13], eucalyptol [14], clove oil [15], turpentine oil [16], and peppermint oil [17]. They are generally considered to be safer and less toxic than synthetic permeation enhancers and have the ability to promote permeation to the lower layers [3].
Essential oils generally have a diverse composition that is influenced by the growing season and location, as well as the extraction method [18]. This diversity raises a challenging issue for researchers due to a lack of clear insight on how these compounds exert their activity on the transdermal permeation of various drugs [19]. Although Barry [20] proposed the lipid–protein partitioning theory as a base for the mechanism of the permeation enhancement, the theory could not specify the effectiveness of certain enhancers towards certain types of drugs. Therefore, the underlying mechanism responsible for the enhancement activity of individual essential oils is not clear and it is proposed that they use different mechanisms of action based on (1) the disintegration of the highly ordered intercellular lipid structure between corneocytes in stratum corneum, (2) interaction with intercellular domains of proteins, which induces their conformational modification, and (3) increase in the partitioning of a drug [3].
Vegetable carrier oils are natural fixed oils pressed mainly from the seeds and constitute a common constituent of pharmaceutical formulations used topically. They are a mixture of heterogeneous lipids composed mainly of triglycerides and a lower concentration of components such as free fatty acid, mono and diglycerides, sterol, phosphatides, fatty alcohol, and lipid-soluble vitamins [21]. Despite the differences between such carrier oils and essential oils, particularly in volatility, physicochemical properties, and aromatic characteristics, carrier oils play an important role in pharmaceutical formulations [22]. They are characterized by their safe profiles and common uses as emulsifying agents, stabilizing agents, and diluents for essential oils prior to topical application. In addition, studies have indicated they may impart a topical permeability enhancement effect [22,23].
Free fatty acids (saturated and unsaturated) are the key components of vegetable carrier oils. They modify the barrier of the skin reversibly by fluidizing and disintegrating stratum corneum lipids. In general, oils containing high percentages of unsaturated free fatty acids have been shown to possess a more significant skin permeation enhancement effect than those with saturated free fatty acids [24]. Carrier oils or fatty acid-containing natural oils have been reported by many studies as successful transdermal penetration enhancers for both hydrophilic and lipophilic drugs [25,26]. Among these are olive oil [25,27,28], almond oil [29], and jojoba oil [30].
This review is conducted systematically involving articles in which oils are used as penetration enhancers in pharmaceutical formulations. The aim of this review is to identify the relationship between the penetration enhancing effects of essential and carrier oils and their mechanism of action in order to inform formulation development.
2. Materials and Methods
2.1. Search Strategy
This systematic review was carried out in accordance with PRISMA [31] 2009 guidelines, and the inclusion and exclusion criteria are summarized in Figure 1. A comprehensive search plot was established using the literature databases Google Scholar, EMBASE, PubMed, Medline, and Scopus between 1 January 2011 and 30 April 2020. In order to include a wide range of studies with the potential evidence of the effect of essential oils on the permeation of drugs through the skin, a mesh list of the number of terms was used as a search strategy. The terms were: “Essential Oil” OR “Oil” OR “Essential oil for drug permeation” AND “Skin Drug Delivery” OR “Transdermal Drug Delivery” OR “Dermal Drug Delivery” OR “Drug permeation”. The titles and abstracts were screened to remove duplicates and determine eligibility. For those deemed relevant, the text was read in full.
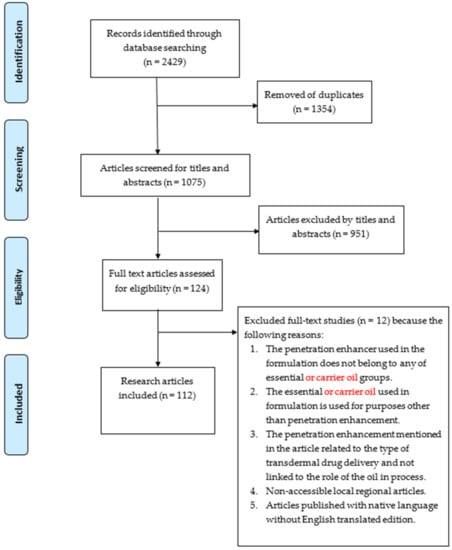
Figure 1.
Flow diagram for literature search with inclusion and exclusion criteria.
2.2. Study Selection
An assessment of the selected articles was carried out independently by two members of the review team to minimize bias. Any study reporting an essential oil as a penetration enhancer within the topical formulation was selected as an eligible study for review and with no language barrier restriction. Studies not meeting the criteria were removed, including those published only as a review, book chapter, conference presentation, letter, abstract, or commentary. The final decision over the eligibility of any disputed articles was discussed by the team based on inclusion/exclusion criteria.
2.3. Data Extraction
The following data were extracted from each of the retrieved articles: active pharmaceutical ingredient, dosage form, in vitro/in vivo studies, carrier material, essential or carrier oil as penetration enhancer, chemical penetration enhancers other than essential oils, and study characteristics. The extracted data from the eligible articles were tabulated using Microsoft Excel 2016, as shown in Table S1 (Supplementary Materials).
2.4. Quality Assessment
An assessment of the risk of bias was carried out for each included article by evaluating six domains: rationale of research, description of the methodology, characterization and testing, description of result, description of discussion, and overall conclusion, as shown in Tables S2 and S3 (Supplementary Materials). [32]. The current risk of bias framework was modified from the Cochrane Handbook for Systematic Reviews of Interventions and standard quality assessment criteria for evaluating primary research papers from a variety of fields [33,34] adopting the redundancy and mapping approach [35,36,37]. This is because of the lack of the corresponding tool of risk assessment in this special field, i.e., the pharmaceutical formulation. The process of assessment of each included study was performed independently by two members of the review team. The final decision was taken after resolving any disagreement in a process of categorization and achieving the forced agreement. The results of the assessment of the risk of bias within the included articles were tabulated as shown in Table S4 and Figure S1 (Supplementary Materials).
3. Results
The study search resulted in 1075 records from the different literature databases Google Scholar, EMBASE, PubMed, Medline, and Scopus, as well as a manual search. A total of 951 articles were excluded after screening the title and abstract of the publications, and a further 12 articles were excluded after reviewing the full text, resulting in 112 papers, as shown in Figure 1. The reasons for exclusion were mainly due to issues such as the penetration enhancer used in the formulation not being an essential or carrier oil, the oil being used in the formulation for purposes other than penetration enhancement, the mechanism of enhancement being attributed to the system rather than the oil, and, finally, non-accessible local regional articles and those without a version in English.
The study characteristics of the included articles are summarized in Table S1: active pharmaceutical ingredient, dosage form, in vitro/in vivo study, carrier material(s), and penetration enhancers as essential or carrier oils and other chemical enhancers, along with a summary of the article highlighting the main findings of the study. The review identified four groups of l oils used in the formulation of skin preparations. These are: terpene-type essential oils, which constituted 63% of the total; fatty acid-containing oils, which constituted 29% of the total; and 8% of the oils comprising of tocopherol and miscellaneous essential oils (Figure 2). The discussion is, therefore, arranged into these four groups.
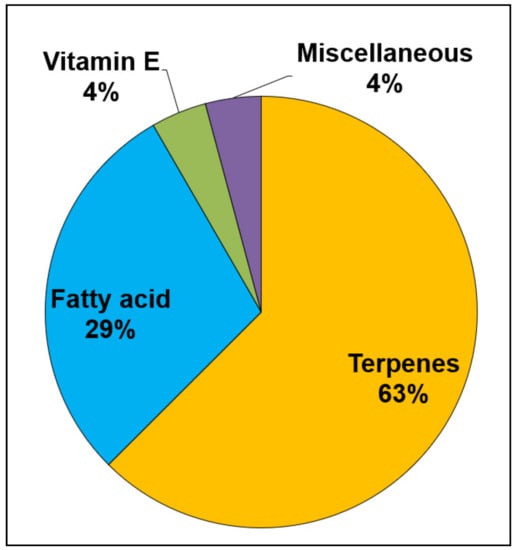
Figure 2.
The types and the percentages of essential and carrier oils used in topical and transdermal pharmaceutical dosage forms.
The review found that olive oil was the most common essential oil used within the pharmaceutical formulations, comprising 19% of the total percentage of the essential oils, followed by eucalyptus oil at 17%, as shown in Figure 3, with 7 terpene-type essential oils and 3 fatty acid-containing essential oils as the most common essential oils used. However, the review has identified that terpene essential oils may have benefits over the fatty acid-containing oils.
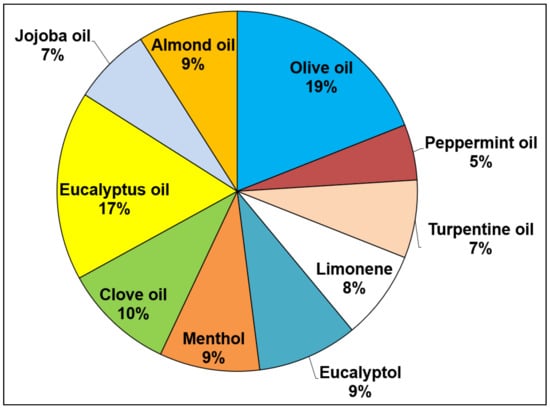
Figure 3.
The most frequently used essential and carrier oils in pharmaceutical dosage formulations from all eligible articles.
The most popular dosage form used was a topical gel utilizing either olive oil or almond oil. Moreover, ointment and vesicular system dosage forms were the least frequently represented dosage forms using palm kernel oil or Santolina insularis, respectively, as shown in Figure 4. In summary, terpene essential oils are the main essential oils used within all microemulsions, transdermal patches, nanoemulsions, and vesicular systems, while fatty acid-containing oils are mainly used in the formulation of solution and topical gel dosage forms, as shown in Figure 5.
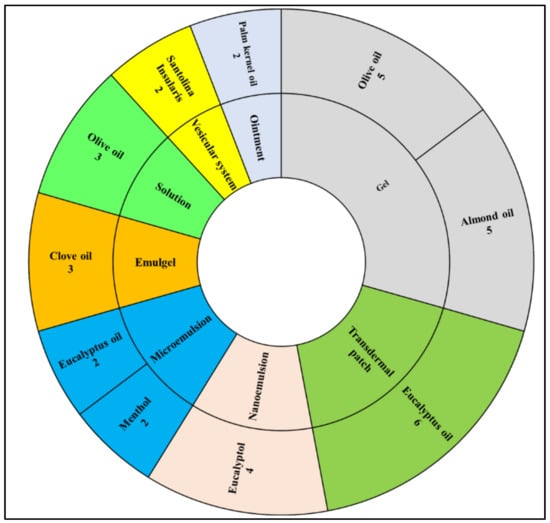
Figure 4.
The most frequently used dosage forms with the most frequently used oil from all eligible articles.
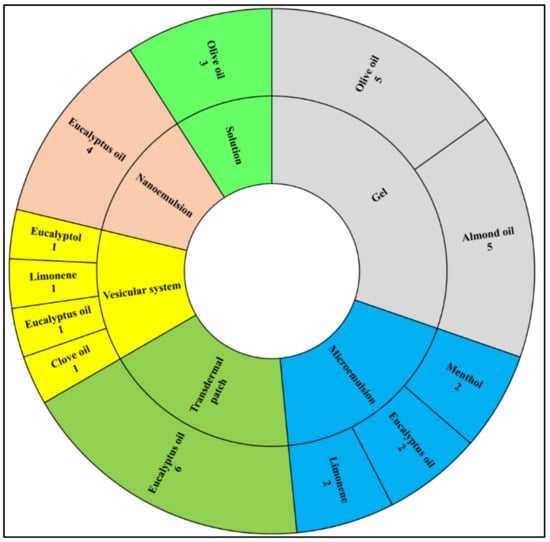
Figure 5.
The most frequent essential and carrier oils used in the most frequent dosage forms from all eligible articles.
3.1. Terpenes
Terpenes are simple hydrocarbon compounds of natural origin that are isolated from different sources such as plants and animals. They consist of basic units known as isoprene, according to which terpenes are classified, as shown in Figure 6A [38,39]. The penetration enhancement activity of terpenes has increased their utilization considerably in pharmaceutical products [8,22]. Generally, terpenes are accepted clinically and used safely as penetration enhancers for both hydrophilic and lipophilic drugs, such as 5-fluorouracil and pioglitazone [40,41].
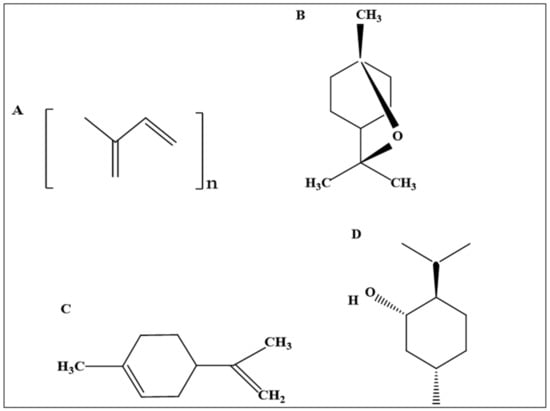
Figure 6.
The chemical structure of terpenes. (A) The structure of isoprene and classification of terpenes (n = 1, 2, 3, 4, 6, 8; hemiterpene, monoterpene, sesquiterpene, diterpene, sesterpene, triterpene, and tetraterpene, respectively), (B) eucalyptol, (C) limonene, (D) menthol.
This review identified seven terpenes as the most frequently used essential oils for their penetration enhancement capability. These are eucalyptus oil, clove oil, eucalyptol, peppermint oil, limonene, turpentine oil, and menthol.
3.1.1. Eucalyptus Oil
Eucalyptus [Eucalyptus globulus] is a plant used in traditional medicine belonging to the family of Myrtaceae [42]. It is extracted by steam distillation from the leaves of this plant [43], with the eucalyptus plant being one of the main sources of essential oils with various biological activities [42]. The main pharmaceutical constituent of eucalyptus oil is eucalyptol [1,8-cineole]. Eucalyptol, a monocyclic monoterpene ether, comprises 70–90% of the content of eucalyptus oil but it also contains minor constituents such as α-pinene, cuminylaldehyde, limonene, α-phellandrene, p-cymene, trans-pinocarveol, and terpinen-4-ol. [44]. The oil has antimicrobial activities, including antibacterial activity against Staph aureus [45], antifungal [46], and antiviral activity [47]. It has promising potential for the treatment of microbial infections, such as those encountered during wound healing, burns, and herpes infections. It also has an anti-inflammatory activity that helps relieve pain and rheumatoid arthritis [45]. Evidence of antioxidant activity has also been reported [48]. Eucalyptus oil also has an expectorant effect, which is used for cough and bronchitis treatment [49]. In addition, many studies have reported a potential role for eucalyptus oil in the formulation of topical preparations as a consequence of its permeation enhancement ability [40,50,51].
Eucalyptus oil is the terpene essential oil most frequently used in the formulation of topical skin preparations. It is used widely in the development of transdermal patch formulation; however, its penetration enhancement effect is also related to the composition of the transdermal patch. Thus, the effect of the type of polymer used [51], the amount of the polymer used [52], and the type and concentration of plasticizer used [2] can all impact its activity, and there are some contradictory reports regarding its efficacy. Although Yaqoob et al. [51], Akram et al. [50], and Zeng et al. [52] found the penetration enhancement effect of eucalyptus oil to be less than isopropyl myristate [IPM] and Azone from matrix patches of metoprolol, glimepride, and elemene, respectively, El-Nabarawi et al. [2], Shen et al. [53], and Sharma and Mehra [13] reported the opposite. They found that eucalyptus oil exerted the highest penetration enhancement effect compared with other penetration enhancers from transdermal patches of sumatriptan, tetramethylpyrazine, and celecoxib. It has been reported that eucalyptus oil is less effective in potential synergistic mixtures with other oil components such as menthol; for example, Madkaikar et al. [54] reported a synergistic effect between menthol and Tween 80 during the development of a transdermal matrix patch of ondansetron.
Many researchers have reported the superiority of eucalyptus oil over other terpenes and fatty acid-containing oils in topical gel formulations. Rajan and Vasudevan [55] and Akhlaq et al. [32] found that it was more effective than other penetration enhancers in permeation enhancement from a transferosomal gel of ketoconazole and a hydrogel of pioglitazone. Furthermore, eucalyptus oil increased the permeation and retention in dermal and epidermal layers of hydrocortisone acetate compared to clove oil and lemongrass oil within a microemulgel formulation [56]. The in vitro and ex vivo studies carried out by Sahu et al. [40] reported that eucalyptus oil could enhance the permeation of 5-fluorouracil incorporated into biodegradable polymeric nanogels.
The dominant mechanism of the penetration of eucalyptus oil is the partitioning of the essential oil into the skin, as reported by Chi-Hsien [57] during the formulation of a photosensitizer microemulsion and El-Maghraby et al. [58] during the mutual transdermal administration of indomethacin and benzocaine microemulsions. Eucalyptus oil was also used in the formulation of miconazole and ketorolac solutions and was found to exert its permeation enhancement activity by several mechanisms, i.e., lipid fluidization, disruption lipid structure, and irreversible keratin denaturation in SC, resulting in higher enhancement ratios [ER] of flux [ERF], diffusion [ERD], and permeability [ERP] as compared to hydrated skin [59,60]. The fluidization mechanism of eucalyptus oil was exploited in the enhancement of felodipine permeation from a niosome formulation [61]. An in vitro study by Kakadia [62] demonstrated the superiority of eucalyptus oil’s penetration enhancement ability over olive oil in the formulation of nanoemulsions of chlorhexidine.
The current systematic review has identified eucalyptus oil as the most popular essential oil used as a penetration enhancer in dermal and transdermal formulations. Moreover, studies reported its superior potency and efficacy compared to other terpenes and fatty acid-containing essential oils. However, the studies confirmed that eucalyptus oil shows weaker permeation activity than Azone, oleic acid, and isopropyl myristate. Figure 7A summarizes the permeation enhancement activity of other chemical enhancers compared to eucalyptus oil. This permeation property is the likely reason for its frequent inclusion in formulations targeting deeper layers of the skin, such as transdermal patches, vesicular systems, microemulsions, and nanoemulsions.
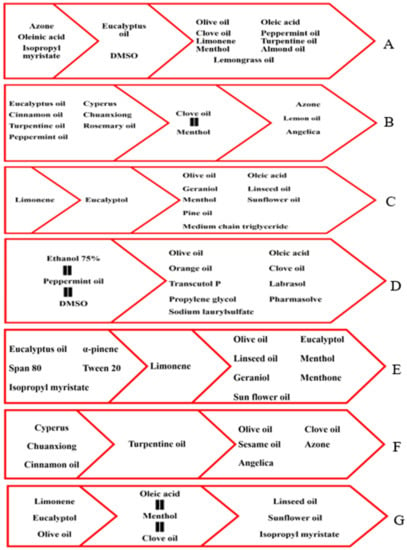
Figure 7.
Summary of permeation enhancement activity of terpene essential oils as compared to other chemical enhancers, (A) eucalyptus oil, (B) clove oil, (C) eucalyptol, (D) peppermint oil, (E) limonene, (F) turpentine oil, and (G) menthol.
3.1.2. Clove Oil
A clove is the bud of the aromatic dried flower of the Syzygium aromaticum [S. aromaticum] evergreen tree. It belongs to the Myrtaceae plant family and grows to a height ranging from 15 to 20 m. This tree is mainly grown in Asian regions such as Indonesia, Sri Lanka, India, Madagascar, and Zanzibar [63]. Clove oil is extracted from different parts of the tree, mainly by steam distillation, which results in different percentage yields. The highest percentage of clove oil obtained from the bud is (12–15%), while the lowest percentage obtains from the clove leaves (2–3%). About 5% is also obtained from the stem of the tree [64].
The major constituent of clove oil is eugenol (>80%), which gives the oil a spicy odor and flavor. It also contains caryophyllene and eugenol acetate in lesser percentages. Clove oil ranges from light yellow to brown in color and these properties are unaffected by the part of the tree from which it originates [64,65].
Clove oil is used topically as a pain killer, such as for toothache, and as an aid to wound healing. It is also used traditionally in the treatment of parasitic infections of the intestine and other digestive problems, manufacturing fragrance, and improving food stability due to its antioxidant activity [65]. Research has identified a potential role for clove oil in enhancing the permeation of different drugs intended to be administered topically [61,66,67].
Clove oil is reported to have limited permeation enhancement effects on certain types of drugs. Clove oil increases the permeation of ondansetron [54], felodipine [61], menthol and methyl salicylate [15], and mefenamic acid [68] following incorporation into a matrix-type transdermal patch, niosomes, ointment, and emulgel, respectively. Furthermore, clove oil is very effective as a permeation enhancer for ibuprofen [12] and hydrocortisone acetate [56], when formulated as a solution and microemulsion based gel, respectively, more so than angelica, Azone [12], and lemon oil [56]. However, clove oil is less effective than cinnamon oil [12,69], peppermint oil, rosemary oil [67], chuanxiong, cyperus, turpentine oil [12], and eucalyptus oil [56] when formulated in a solution, hydrogel, microemulsion, and microemulgel for the permeation enhancement of ibuprofen, fluconazole, quercetin, and hydrocortisone acetate, respectively. This might be a reason for combining clove oil with other penetration enhancers or incorporating it in only certain formulations. For instance, clove oil exhibits synergistic effects when combined with Tween 80 [70] and peppermint oil [66] in enhancing the permeation of aceclofenac and terbinafine, respectively. In addition, clove oil produces a synergistic effect on the permeation of felodipine [61] when incorporated into niosomes. However, even with this synergistic effect with Tween, it remains less effective than menthol in enhancing the permeation of aceclofenac, as reported by Magdum et al. [70].
Despite its weak penetration effect, there are no restrictions on its use in dermal dosage form, and it has been formulated as a niosome, microemulsion, and microemulgel. Figure 7B summarizes the permeation enhancement activity of other chemical enhancers compared to clove oil.
3.1.3. Eucalyptol
Eucalyptol, also known as 1,8-cineole, is a natural chiral aromatic compound composed of a cyclic ether and monoterpenoid based on cyclohexane [71], as shown in Figure 6B [72]. Eucalyptol is the main component of various natural oils, such as eucalyptus and laurel leaf oil in a concentration of 85% and 70%, respectively [64,71,73]. It is among the few fragrances produced exclusively by isolation from essential oils such as eucalyptus oil. It is also produced with high purity using fractional distillation of Eucalyptus globulus [64]. Eucalyptol is an organic colorless liquid with a smell resembling camphor. It has a spicy taste with a cooling sensation [71].
In addition to its role in pharmaceutical formulation, eucalyptol has various pharmacological activities with a range of therapeutic effects. It is included in various formulations of cough remedies and mouth gargles and in the management of asthma and chronic obstructive pulmonary disease via its inhibitory effect on cytokines. It is also used in the treatment of rhinosinusitis. The topical use of eucalyptol produces analgesia with a reduction in inflammation [73]. Recently, it has been used as a penetration enhancer in the pharmaceutical formulation of dermal and transdermal preparations [74,75,76].
It has the ability to enhance the permeation of both hydrophilic and lipophilic drugs. Shi et al. [77] confirmed the permeation enhancement activity of eucalyptol on hyperzine A and ligustrazine in a microemulsion-based transdermal patch. In addition, eucalyptol had a similar effect when incorporated into curcumin microemulsion [14].
Eucalyptol exhibits higher penetration enhancement activity than certain terpenes such as pine oil [75], menthol [74], and geraniol [78], as demonstrated during an in vitro permeation study of a curcumin nanoemulsion and a ketorolac tromethamine reservoir-type transdermal patch, respectively. Moreover, it is a more effective penetration enhancer than fatty acids and fatty acid-containing essential oils. It has been shown to enhance the rate of skin permeation more than olive oil, linseed oil and sunflower oil [74], oleic acid [76,79], and medium-chain triglycerides [75], but not as well as limonene [a terpene essential oil]. In vitro studies found that limonene increased the permeation of ketorolac tromethamine [74] and all-trans retinoic acid [80] more effectively than eucalyptol from reservoir-type transdermal patches, solid lipid nanoparticles [SLN], nanostructured lipid carriers (NLC), and nanoemulsions.
Eucalyptol has also been combined with different chemical penetration enhancers to identify any synergistic activities. Abd et al. [76] and Casey et al. [81] found a synergistic enhancement of activity when it was combined with ethanol and IPA separately in the formulation of naproxen and caffeine nanoemulsion and chlorhexidine solution, respectively. Eucalyptol also showed synergy with Tween 80 (surfactant) in increasing the permeation of sodium-fluorescein through the skin from an ultradeformable liposome [78].
It is concluded that eucalyptol presents a more effective penetration enhancement activity than other terpenes such as pine oil, menthol, and geraniol, as well as most of the fatty acid-containing oils. It can be used alone or in combination with other chemical penetration enhancers in order to target deeper skin, such as in the case of microemulsions, nanoemulsions, liposomes, and transdermal patches. Figure 7C summarizes the permeation enhancement activity of other chemical enhancers compared to eucalyptol.
3.1.4. Peppermint Oil
Mentha piperita L. belongs to the Labiatae or Lamiaceae family and is a perennial herbal plant with rhizomes growing at a height ranging from 30–90 cm. The plant is natively grown in Russia, across Europe, North America, and Australia, and has a characteristic pepper-like and strong odor. [82] Peppermint oil originates from the leaves and flowering aerial parts of the plant and is a clear liquid with colors ranging from colorless to a pale greenish-yellow. It has a cooling, minty, fresh, and sweetish odor with different compositions depending on its origin. It is mainly produced in the USA, Russia, China, and India [83].
Peppermint oil is mainly composed of menthol (29–48%) and menthone (20–31%). In addition, it includes other constituents such as limonene, isomenthone, cineole, menthyl acetate, neomenthol, menthofuran, and α- and β-pinene [84]. Currently, peppermint oil is used in the formulation of cosmetic preparations, nutritional industries, personal hygiene products, flavoring agents, and fragrances [82]. It has various purported therapeutic activities such as relieving gastric upset, toothache, headache, nausea, and muscle spasm. It has also shown promising potential in enhancing the dermal and transdermal permeation of different drugs [66,67,85].
Peppermint oil has promising pharmaceutical potential and has been used as a penetration enhancer in dermal dosage forms, including in a gel [18,66] and cream [86], as well as transdermal dosage forms such as microemulsions [67] and liposomes [85]. An in vitro permeation study found it to increase the penetration of lopinavir as compared to olive oil [85]. Moreover, Zhang et al. [18] reported peppermint oil’s ability to increase the penetration of phentolamine more effectively than Transcutol P®, Labrasol®, SDS, polyethylene glycol, Pharmasolve®, and oleic acid [18]. As well as the superiority of peppermint oil over clove oil in enhancing the permeation of quercetin through the skin from a microemulsion [67], peppermint oil combined with clove oil in an emulgel formulation enhances the permeation of terbinafine synergistically [66]. In addition, peppermint oil is more effective than orange terpeneless oil and is comparable to ethanol 75%, as well as DMSO’s effect in enhancing penetration of calcium thioglycolates and eventually decreasing tear-resistant time (TRT). TRT is the time required for the depilation of the hair using different chemical depilatories. It depends on different factors, such as the use of penetration enhancers. This might be due to the peppermint oil content of eucalyptol, limonene, and menthone that can disrupt the lipid layer of the stratum corneum, resulting in the permeation enhancement of calcium thioglycolates [86].
So, peppermint oil has a reasonable spectrum of penetration enhancement activity. This property makes it a suitable penetration enhancer used for targeting superficial and deeper skin layers when used alone or in combination with other penetration enhancing agents. Figure 7D summarizes the permeation enhancement activity of other chemical enhancers compared to peppermint oil.
Menthol
Menthol [[1S,2R,5S]-5-methyl-2-propan-2-ylcyclohexan-1-ol] is a white crystalline powder with a characteristic peppermint odor and taste and has two enantiomers, (+)-menthol and (−)-menthol, with the chemical formula C10H20O [87], as shown Figure 6D [88]. Naturally, (+)-menthol is more abundant, while (−)-menthol rarely occurs. (−)-Menthol is synthesized from various natural substances such as citronellal, cornmint, and racemic menthol. Menthol is the main component of peppermint and cornmint derived from the Mentha species such as Mentha piperita and Mentha arvensis [87].
The topical application of menthol results in a cooling feeling and tingling sensation. According to the FDA, menthol is considered the safest and an effective therapeutic for the treatment of the symptoms of the common cold. It exerts an analgesic effect through its ability to block the Ca2+ channel and has the ability to bind k-opioid receptors. Thus, menthol has been used in formulations for local anesthesia [89]. Menthol has a cooling and refreshing effect, which warrants its inclusion in toothpaste, cosmetics, and chewing gum, and is also used in liniment form [90].
Menthol has also been used for the skin permeation enhancement of water-soluble drugs [74,91]. It demonstrates limited permeation enhancement activity of active pharmaceutical ingredients through the skin and is used in combination with other chemical enhancers such as terpenes, fatty acids, and surfactants.
Menthol was effective when used in the formulation of an aceclofenac microemulsion [92], as well as having a significant role in the enhancement of 18-β-glycyrrhetinic acid and boswellic acid permeation from matrix- and reservoir-type transdermal patches, respectively [93]. Menthol is more effective than vegetable oils such as linseed oil and sunflower oil [74] and has been shown to be more effective than olive oil, with greater enhancement activity for ketorolac from a solution and a reservoir-type transdermal patch [59,74].
When comparing menthol with limonene, eucalyptol, and eucalyptus, the in vitro permeation studies carried out by Prakash et al. [74] and Saadatzadeh et al. [59] found a lower permeation enhancement effect of menthol on ketorolac tromethamine through rat skin. In addition, an ex vivo study carried out by Khullar et al. [68] found a similar permeation effect compared to clove oil during mefenamic acid emulgel formulation. Wang et al. [94] and Liu et al. [95] reported the synergism between menthol and camphor in an in vitro permeation study of tacrolimus and glabridin from a microemulsion and microemulgel, as well as a nanoemulsion formulation, respectively. Menthol has also shown synergistic enhancement activity when combined with piperine [bioenhancer] in the formulation of an 18-β-glycyrrhetinic acid matrix-type transdermal patch and a boswellic acid reservoir-type transdermal patch, respectively [93]. In addition, menthol was combined successfully with oleic acid and Tween 80 separately in order to enhance transdermal permeation synergistically during the transdermal patch formulation of ondansetron and α-asarone, respectively [54,91].
In summary, menthol has some penetration enhancement activity when used alone; however, it shows synergistic effects with various chemical penetration enhancers. Menthol exerts its permeation activity by multiple mechanisms such as lipid disruption, lipid fluidization and extraction, and the irreversible denaturation of the protein. So, it can target deeper skin layers, as shown through its role in the formulation of microemulsions, microemulgels, nanoemulsions, and both matrix and reservoir transdermal patches. Figure 7G summarizes the permeation enhancement activity of other chemical enhancers compared to menthol.
3.1.5. Limonene
Limonene has an aliphatic hydrocarbon structure belonging to the monocyclic terpene class, as shown in Figure 6C [96]. It occurs in more than 300 plants and fruits, including grapefruit, lemons, and oranges. Limonene is a colorless aromatic liquid with a citrus odor. Limonene has three isomers, d-limonene (R or [+]), l-limonene (S or [−]), and the racemic isomer dl-limonene [83,97]. d-limonene constitutes the principal isomer and is produced mainly as a by-product during orange juice production. L-limonene occurs in relatively small quantities and is obtained by isolation from essential oils such as citrus oil, pine-needle oil, neroli oil, and cumin oil. The racemic isomer [dl-limonene] is formed as a result of the isomerization of α and β-pinene [64]. Limonene undergoes different reactions such as oxidation and dehydrogenation, resulting in the formation of carveol, carvone, and limonene oxide, as well as p-cymene, respectively. This might change the physicochemical properties of limonene [97].
Limonene has been reported to enhance the permeation of drugs delivered topically [74,80,98]. Also, it has been reported to have therapeutic effects such as hypoglycemia, antitumor, antihypertensive, lipid-lowering, and anti-inflammatory effects, as well as antioxidant activity [97]. Limonene has a wide spectrum of permeation enhancement activity with a greater ability to enhance the penetration of hydrophilic and lipophilic drugs than certain terpenes such as cineole and fatty acid-containing essential oils, including olive oil [74]. Furthermore, limonene has been incorporated successfully into transdermal patch formulations [matrix and reservoir] [50,74,80], as well as other dosage forms designed to target deeper skin tissues, such as liposomes, microemulsions, and nanoemulsions [78,98,99,100].
Limonene is more effective as a penetration enhancer than olive oil, linseed oil and sunflower oil in enhancing the permeation of ketorolac tromethamine through the skin [74]. Moreover, limonene has a greater ability than menthol [74], cineole [74,78,98], menthone [80], and geraniol [78] in the permeation enhancement of ketorolac, all-trans retinoic acid, NaFl, and ketoprofen. However, it is not as effective on the permeation of glimepride and ketoprofen as eucalyptus oil [50] and pinene [80], respectively, and an in vitro permeation study showed less enhancement with limonene than isopropyl myristate, Tween 80, and Span 80 in the transdermal permeation of glimepride [50].
Hoppel et al. [99] and Wattanasri et al. [100] incorporated limonene successfully into a microemulsion and microemulgel for the enhancement of permeation of diclofenac sodium and Kaempferia parviflora extract through the skin.
The synergistic effect of limonene was investigated and confirmed in combination with Tween 80 and a synergistic advantage was confirmed in the application of ultradeformable liposomes in enhancing the permeation of NaFl [78]. In a separate study, the researchers also reported a synergistic effect after incorporating limonene into an SLN formulation of all-trans retinoic acid [98].
In summary, limonene has effective penetration enhancement activity and is used mainly in formulations such as liposomes, microemulsions, nanoemulsions, microemulgels, and transdermal patches [matrix and reservoir] to target deeper layers within the skin. Although limonene is not as effective as some other natural and chemical enhancers such as eucalyptus oil and Tween 80, it has a better enhancement activity than certain terpenes and fatty acid-containing essential oils. Limonene is suitable to be used alone or in combination with chemical penetration enhancers such as Tween 80. Figure 7E summarizes the permeation enhancement activity of other chemical enhancers compared to limonene.
3.1.6. Turpentine Oil
Turpentine oil is an aliphatic, cyclic terpenoid composed mainly of monoterpenes such as α-pinene and β-pinene [49] and can be sourced from wood, balsam or gum, and sulfate turpentine oil [64]. It is extracted by steam distillation or the fractionation method from the trunks of cones, young twigs, chopped trees, wood, or oleoresin extracted from the Pinaceae species [49,64].
The main constituents of turpentine oil are α-pinene and β-pinene. It also contains trace amounts of careen, dipentene, terpinolene, and camphene [101].
Its pharmacological and biological activities are related to its principal constituents [α-pinene and β-pinene], thus, turpentine oil has antibacterial, antifungal, insecticidal, and antiseptic effects. In addition, it exerts anticarcinogenic and sedative effects. Turpentine oil has also been reported to exhibit pharmacological activities such as diuretic, hypoglycemic, anticholinergic, antioxidant, immunomodulatory, and xenobiotic expelling effects. Certain neurological activities, such as antistress and anticonvulsant, are also attributed to turpentine oil [102]. It has been reported to be an effective penetration enhancement effect when incorporated into topical formulations [25,103,104].
Turpentine oil enhances the permeation of NSAIDs such as diclofenac potassium and ibuprofen, as well as ketorolac tromethamine from both gel and matrix-type transdermal patches [25,104,105]. The concentration of the turpentine oil used has an impact on its permeation enhancement capacity [17,103,104,105]. Most of these studies found the optimal concentration of turpentine to be between 1–5%. Furthermore, concentrations above 5% have not been shown to be any more effective on the permeation of diclofenac potassium from a gel formulation [25].
It has been shown to be more effective in vitro than menthol [106], as well as clove oil and angelica [12] in flurbiprofen gel and ibuprofen solutions. However, it is less effective than chuanxiong, cyperus, and cinnamon oil in the formulation of ibuprofen solution [12].
With respect to other types of chemical penetration enhancers, turpentine oil is more effective in vitro than olive oil and sesame oil when incorporated into gel and matrix-type transdermal patches of diclofenac potassium and diclofenac diethanolamine, respectively [25,103]. Moreover, turpentine oil shows greater penetration enhancement ability than isopropyl palmitate, Azone, and an Azone/propylene glycol combination from a flurbiprofen gel [106] and ibuprofen solution [12].
There is a synergistic effect when combined with ethanol and propylene glycol in separate formulations. The in vitro studies found more effective permeation of diclofenac from gel and lotion dosage forms following the use of turpentine oil combined with ethanol and propylene glycol, respectively [17,25].
Turpentine oil is used alone or in combination with other chemical enhancers. It has good penetration enhancement activity compared to the number of terpenes and most fatty acid-containing oils. It is used widely in the dermal and transdermal skin preparation for the formulation of NSAIDs for pain and arthritis treatment. Figure 7F summarizes the permeation enhancement activity of other chemical enhancers compared to turpentine oil.
3.2. Fatty Acid Containing Vegetable Carrier Oils
Fatty acids play an important role in metabolic processes and represent more than 30% of the energy intake for people in developed countries. Meat and dairy products, vegetables, and fish oils are the most important sources of fatty acids [107]. They are amphiphilic molecules, with the polar end being a carboxylic group while the non-polar end is a methyl group of the general formula CH3[CH2]nCOOH. The unbranched fatty acids are classified into saturated and unsaturated according to the number of the double bonds in their structure, as shown in Figure 8 [107,108]. In addition, fatty acids can also exist in a branched structure [109].
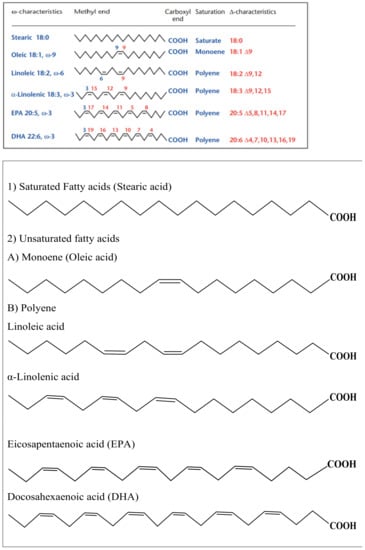
Figure 8.
The structure of unbranched fatty acids, (1) Saturated and (2) Unsaturated, (A) monoene and (B) polyene.
Fatty acids are used in the formulation of topical dosage forms as penetration enhancers, as exemplified by oleic acid (the most popular fatty acid used) [4]. In addition, they form the major component of many natural oils, such as olive oil and almond oil [29,110]. Fatty acid-containing carrier oils have been used widely in the development of transdermal dosage forms to enhance the permeation of hydrophilic and lipophilic drugs [111,112].
This systematic review has determined the fatty acid-containing carrier oils used most frequently as penetration enhancers: olive oil, almond oil, and jojoba oil.
3.2.1. Olive Oil
Olive is an evergreen traditional tree that is widely grown and cultivated in the basin of the Mediterranean region. The tree has a height of approximately 1.5–4.5 m and is also known as Olea europea L., which belongs to the family of Oleaceae [113]. It is natively cultivated in Palestine. Nowadays, it is distributed across many countries such as the European Union countries, Argentina, Australia, South Africa, and the United States. The fruit of the olive tree is the precursor of olive oil, which is extracted by mechanical or physical means at a specific temperature [114].
The composition of olive oil is variable according to its chemical, physical, and organoleptic characteristics [115]. The main constituent of olive oil is triacylglycerides (98–99%). It is composed of linoleic, α-linolenic, palmitic, and stearic acid. The minority part [2%] consists of aliphatic alcohols, triterpenic alcohol, hydrocarbons, sterol, and volatile oils with antioxidant activity such as phenols and carotenes [115,116].
Olive oil is widely used in cooking due to its unique nutritional significance. It can resist rancidity within food due to its antioxidant content [115]. Recently, the importance of olive oil has been translated into the pharmaceutical formulation of topical dosage forms, with several researchers using olive oil to enhance the permeation of different drugs through the skin [27,111,117].
Olive oil is the most successful fatty acid-containing carrier oil and is used widely in dermal and transdermal dosage forms. It is used alone or in combination with other chemical penetration enhancers in order to produce a synergistic permeation enhancement effect.
Many studies have reported the permeation enhancement capacity of olive oil as compared to formulations without penetration enhancers. Olive oil enhances the permeation of ibuprofen [118], clotrimazole [16], and flurbiprofen [111] from creams, matrix-type transdermal patches, and gels, respectively. Hussain et al. [111] reported the impact of olive oil concentration on the permeation of flurbiprofen.
In addition, olive oil has shown higher permeation enhancement activity compared to certain fatty acid-containing carrier oils, vegetable oils, mineral oils, and surfactants. In vitro permeation studies have confirmed the role of olive oil in the permeation enhancement of nemsulide from a gel as compared to almond oil [27] and flurbiprofen from a foam and gel formulation as compared to coconut, grapeseed, avocado, crocodile, and emu oils [23]. Moreover, olive oil shows greater enhancement ability than jojoba oil, groundnut oil [30], linseed, and sunflower oil [74] in the penetration of risperidone [30] and ketorolac tromethamine [74] from matrix- and reservoir-type transdermal patches, respectively. Olive oil enhances the permeation of tretenoin from a microemulsion more effectively than castor oil and isopropyl myristate [119]. Furthermore, in comparison of olive oil with certain surfactants, olive oil produces higher permeation enhancement of olanzapine [120] and curcumin [117] from a matrix-type transdermal patch and gel, respectively, as compared to benzalchonium chloride, sodium lauryl sulphate [120], and Tween 80 [117]. It has also been reported to be more effective on collagen permeation than sunflower oil, dimethyl sulfoxide [DMSO], tetrahydrofuran [THF], and coconut oil [121].
Conversely, olive oil is less effective than most terpenes, certain surfactants, and organic solvents. In vitro permeation studies have reported a lower enhancement activity of olive oil in the permeation of ketorolac as compared to limonene, cineole, menthol, and eucalyptus oil from solution and reservoir transdermal patches [59,74]. Moreover, the in vitro and ex vivo studies carried out by Hussain et al. [25] and Maniyar and Kokare [85] found it to be less effective than turpentine oil [25] and peppermint oil [85] in the permeation of diclofenac potassium and lopinavir from a gel and liposome, respectively. Kakadia [62] reported that olive oil has lower permeation enhancement activity on triclosan and chlorhexidine digluconate from SLN and nanoemulsion formulations than eucalyptus oil. In addition, in vitro permeation studies carried out by Aggarwal et al. [120] reported the superiority of Span 20 over olive oil in the permeation of olanzapine from a matrix-type transdermal patch. Moreover, analysis using confocal Raman spectroscopy found that olive oil was less effective than propylene glycol, aqueous, and absolute ethanol, as well as acetone on enhancing the penetration of cinnamaldehyde solution through the skin [110].
There are, however, several reports of its use in combination with other chemical enhancers in order to produce a synergistic effect on drug permeation. Aggarwal et al. [30] reported a successful and effective combination of olive oil with jojoba oil and groundnut oil separately, with the permeation of risperidone from a matrix-type transdermal patch due to a synergistic effect. Permeation of nimesulide from a gel was enhanced when olive oil was combined with almond oil as a penetration enhancer [27]. Combinations of acetone and olive oil also enhanced the permeation of cinnamaldehyde solution through the skin [110]. It was also reported to be more effective on collagen permeation than sunflower oil, dimethyl sulfoxide [DMSO], tetrahydrofuran [THF], and coconut oil [121]. The synergistic effect of olive oil with a ZnO nanoparticle on collagen permeation was better than olive oil alone and THF alone or in combination with ZnO nanoparticles. However, synergistic effects were lower than liquid paraffin, sunflower oil, DMSO, and coconut oil combined with ZnO nanoparticles. This might be attributed to the fatty acid composition of olive oil [121].
There are some controversial reports: the in vitro permeation study carried out by Pelikh et al. [112] found that olive oil produced a greater increase in the permeation of hesperetin and rutin from a nanocrystal as compared to urea, whereas urea is more effective at enhancing the permeation of ketorolac tromethamine from a gel than olive oil [59]. Moreover, olive oil was more effective than liquid paraffin on the permeation of flurbiprofen from foam and gel [23], whereas Shokri and Javar [121] found it to be less effective in an ex vivo permeation study in which collagen was formulated in a solution. Figure 9A summarizes the permeation enhancement activity of other chemical enhancers compared to olive oil.
Figure 9.
Summary of permeation enhancement activity of terpene essential oils as compared to other chemical enhancers, (A) olive oil, (B) almond oil, and (C) jojoba oil.
3.2.2. Almond Oil
Almond oil originates from Oleum amygdalae and its major constituent is benzaldehyde associated with some hydrogen cyanide formed by hydrolysis of glycoside amygdalin. Amygdalin is a colorless to slightly yellow liquid and has an almond-like odor with an intense cherry aroma. Amygdalin has a mild to slightly astringent taste [122]. Almonds are considered to have originated in the Middle East, however, nowadays they are grown in places such as the Mediterranean region, Australia, North America, Asia, and South Africa [123].
Almond oil has an emollient effect and sclerosant characteristics and plays a significant role in enhancing the permeation of different drugs through the skin [27,41]. Almond oil is also widely used for massage and has anti-itching effects. Additionally, almond oil has reported anti-inflammatory activity, reducing irritable bowel syndrome, anti-hepatic toxicity, and cardiovascular benefits [122].
In in vitro permeation studies, almond oil has been used to enhance the permeation of ketoprofen, clotrimazole, and pioglitazone from a gel formulation [29,41,124,125]. In vitro permeation studies have also demonstrated enhancement of timolol and ketoprofen permeation from a transdermal patch formulation containing almond oil [125,126]. Moreover, in vitro studies found it to be more effective for benzoyl peroxide and nimesulide formulations compared to wheat germ oil [127] and olive oil [27], respectively. This effect is concentration-dependent [41,124,126].
On the other hand, almond oil was less effective on the permeation of benzoyl peroxide and pioglitazone than sesame and jojoba oil [127], as well as eucalypus oil [41], respectively. Despite this, Mahmood et al. [27] and Nawaz et al. [124] reported the synergistic combinations of almond oil with other chemical penetration enhancers such as olive oil and Tween 80. The in vitro permeation studies carried out by Mahmood et al. [27] and Nawaz et al. [124] showed the enhancement activity of almond oil in permeation of nimesulide and clotrimazole from gel formulations.
Choe et al. [128] determined a similar penetration effect of almond oil and jojoba oil in disturbing the lateral and lamellar packing of intercellular lipids [ICL] at 30% and 70–90% of the stratum corneum thickness. Furthermore, almond oil is semi-occlusive when applied to the skin surface, represented by its slight reduction in transepidermal water loss [TEWL] similar to paraffin oil [129]. This means that almond oil is less able to penetrate the deeper layers of skin.
Almond oil is a fatty acid-containing oil with weak permeation enhancement activity. It is used mainly in dosage forms targeting the upper layers of the skin, such as solutions and gels. However, it is also used in the formulation of matrix transdermal patches and SLN. Almond oil has a weaker topical permeation activity than most terpenes, certain mineral oils, and vegetable oils. Figure 9B summarizes the permeation enhancement activity of other chemical enhancers compared to almond oil.
3.2.3. Jojoba Oil
Jojoba is the desert plant known as Simmondsia chinensis. It is an evergreen perennial plant with a woody shrub growing to 2–3 ft in height. It is natively grown in northwestern Mexico, southern Arizona, and California [130]. Jojoba oil is a liquid, unsaturated wax composed mainly of an ester of long carbon chain fatty acid and alcohol (C20–C22). Jojoba oil exerts a dermal anti-inflammatory effect in addition to its inclusion in cosmetic formulations such as moisturizers and sunscreens [131]. Many studies have reported that jojoba oil contributes to the skin barrier in addition to acting as a permeation enhancer [30,127,132].
Jojoba oil is a fatty acid-containing oil with weak penetration enhancement activity. However, it is used alone or in combination with other essential oils in the formulation of different topical dosage forms such as solutions, solid nanoemulsions, matrix type transdermal patches, and emulgels.
Jojoba oil is more effective as a permeation enhancer of benzoyl peroxide from an emulgel formulation than wheat germ oil and almond oil [127]. In addition, it was more effective in an in vitro study on the permeation of curcumin than almond oil, paraffin oil, and petrolatum [129]. Confocal Raman microscopy showed that it changed the ICL lamellar and lateral packing at depths of 30% and 70–90% in the stratum corneum as compared to paraffin oil and petrolatum. This might be attributed to its fatty acid composition [128]. Ex vivo tape stripping and Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) studies reported deeper penetration of jojoba oil (33%) than sunflower oil (16%) into the stratum corneum [132]. This is in agreement with Choe et al. [128], who reported the penetration depth of jojoba oil into 30% of stratum corneum based on confocal raman microscopy. Moreover, laser scanning microscopy (LSM) and TEWL measurements revealed that jojoba oil caused no change in TEWL because of its ability to pass through the upper layers of skin to deeper parts without occlusion of the skin [129].
Jojoba oil has been reported to be a less effective enhancer for imoquimod from solid nanoemulsions as compared to avocado oil, squalene, tocopherol, and laurocapram [133]. Also, an in vitro permeation study by Thakur et al. [127] reported the superiority of sesame oil for benzoyl peroxide from gellified emulsions as compared to jojoba oil. It has also been less effective in enhancing the permeation of olanzapine and risperidone from matrix-type transdermal patches compared to corn oil and groundnut oil [26], as well as olive oil [120], respectively, in rat skin. Furthermore, Vater et al. [132] and Gogoll et al. [133] reported the deeper permeation of medium-chain triglyceride (52%) of stratum corneum and greater permeation of imiquimod from solid nanoemulsion as compared to jojoba oil.
It has been reported to have synergistic activity with olive oil and groundnut oil [115]. Figure 9C summarizes the permeation enhancement activity of other chemical enhancers compared to jojoba oil.
3.3. Vitamin E Derivatives
Vitamin E is a lipid-soluble antioxidant that has been used in dermatological preparations for decades. It is extracted from plant-based dietary products such as wheatgerm oil and palm oil. [134] Vitamin E is composed of a chromanal ring linked at position C2 with a side chain. It is classified into eight classes: α, β, γ, and δ tocopherol with their analogues, as shown in Figure 10 [135].
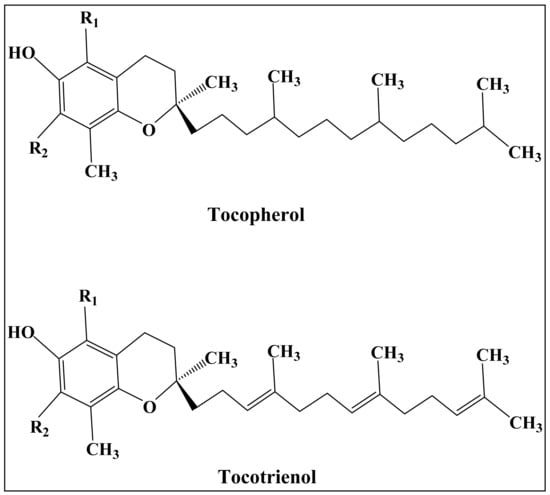
Figure 10.
The chemical structure of Vitamin E analogues, type (R1, R2): α (CH3, CH3), β (CH3, H), γ (H, CH3), and δ (H, H).
Wheat germ oil is considered a rich source of vitamin E with a wide range of topical applications. The permeation enhancement activity of wheat germ oil was investigated by Thakur et al. [127] Although it was used in the formulation of benzoyl peroxide as a gellified emulsion, it had less of an impact on the release and permeation of drugs than sesame oil, jojoba oil, and almond oil [127]. Natural oils rich with vitamin E, such as crude palm oil (CRO) and the tocotrienol rich fraction (TRF) of palm oil, were reported to significantly enhance the permeation of ibuprofen across human skin in an in vitro study [136].
3.4. Miscellaneous Essential and Carrier Oils
3.4.1. Aloe Vera
Aloe vera is an evergreen perpetual plant belonging to the plant family Asphodelaceae. It is a traditional medicine used commonly in the treatment of arthritis, constipation, diabetes, dermal inflammation, eczema, burns, and skin cancer [22,137]. In addition, Aloe vera is used as a penetration enhancer in the preparation of the dermal and transdermal formulations [137]. An in vitro skin study found it to be a better permeation enhancer for losartan than rosemary oil, clove oil, and tea tree oil and resulted in the achievement of a therapeutic dose of losartan transdermally [138].
3.4.2. Chuanxiong
Chuanxiong is an essential oil extracted from Ligusticum chuanxiong Hort. Ligusticum chuanxiong Hort is a traditional Chinese medicine prescribed for the treatment of cardiovascular disease [139]. Chuanxiong oil is used in balneotherapy and as a penetration enhancer in topical formulations for various drugs such as flurbiprofen [140]. Jiang et al. [12] reported the superiority of Chuanxiong oil in the permeation of ibuprofen compared to Azone, turpentine, cyperus, angelica, cinnamon, and clove oil.
3.4.3. White Mustard Seed (Sinapis alba L.) Volatile Oil
Sinapis alba L. (white or yellow mustard) is an annual plant that belongs to the family Brassicaceae and is also known as Brassica hirta. It is grown originally in the Mediterranean Sea region and distributed worldwide [141]. White mustard is used in the treatment of deep abscess disease and numbness of the joints [142]. Sinapis alba L. oil (SVO) is a volatile essential oil extracted from this plant mechanically by cold pressing [141]. Ruan et al. [142] reported that SVO was a more effective enhancer of both hyrophillic and lipophilic drugs such as 5-fluorouracil, osthole, and paeonol, compared to Azone.
4. Conclusions
The purpose of this review was to systematically explore the potential of essential and carrier oils in enhancing the permeation of active pharmaceutical ingredients from dermal and transdermal pharmaceutical formulations. The review found that four major types of oils are currently used alone or in combination with other penetration enhancers. By including all the accessible, translated relevant studies published over the last 10 years and following revised PRISMA guidelines, we reduced the risk biases.
The findings were that terpenes are the most widely and frequently used essential oil, followed by fatty acid-containing l oils. In addition, the review included other minor oil types such as vitamin E derivatives and miscellaneous oils. The successful incorporation of terpenes into advanced pharmaceutical formulations such as nanoemulsions, microemulsions, vesicular systems, and transdermal patches makes them more attractive compared to fatty acid-containing oils. Therefore, they are recommended to be used as a first-choice safe penetration enhancer for topical formulations. Terpenes offer promising potential for the permeation of hydrophilic and lipophilic drugs through/into skin using different mechanisms and are likely to play a significant role in the development of effective dermal and transdermal formulation in the future.
This review concludes that terpene-type essential oils are effective chemical penetration enhancers. They have been used in the successful development of dermal and transdermal formulations. However, the selection of the best terpene is an important factor for consideration. Despite the similarity in the chemical structure of the terpene-type essential oils, there is a difference in the mechanisms of the permeation enhancement of drugs through/into the skin, influencing the selection of specific terpenes commonly reported in the formulation of certain topical formulations [dermal or transdermal dosage forms]. For example, eucalyptus oil has demonstrated higher activity in the formulation of transdermal dosage forms such as transdermal patches, microemulsions, nanoemulsions, and vesicular systems, as shown in Figure 4 and Figure 5. So, understanding the relationship between the chemical structure of terpenes and their mechanisms of action is a key factor in the successful development of dermal or transdermal formulations. Also, the interaction of the terpenes with other terpenes, or other excipients such as surfactants, is affected by the type of formulation being developed.
This review has highlighted the significant roles of the most common fatty acid-containing oils in topical formulations. The permeation enhancement activity of these oils for the active ingredients through/into the skin is attributed to the chemical structure of the fatty acid content. Although this kind of oil is not as effective as terpenes, it is used widely in different types of pharmaceutical formulations such as transdermal patches, gels, and solutions. Oleic acid represents the cornerstone in the chemical structure of these oils; in other words, the permeation enhancement activity of these oils is attributed to the content of this fatty acid. In addition, the saturation degree and the branching structure within the fatty acid structure have brought some complexity in the behavior of the fatty acid-containing oils and the extent of their effect on the stratum corneum.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/scipharm90010014/s1. Table S1, The summary of study characteristics; Table S2, Criteria used for the risk assessment of biasness within included studies in this systematic review (23); Table S3, The interpretation of risk of bias of studies included in this Systematic Review (within and across study) (23); Table S4, The risk of biasness assessment of the included literature; and Figure S1, The risk of biasness of eligible articles.
Author Contributions
Conceptualization, B.R.C. and M.U.G.; methodology, B.A. and M.U.G.; validation, B.A. and M.U.G.; formal analysis, B.A. and M.U.G.; investigation, B.A., B.R.C. and M.U.G.; writing—original draft preparation, B.A.; writing—review and editing, B.A., B.R.C. and M.U.G.; supervision, B.R.C.; project administration, B.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aggarwal, S.; Agarwal, S.; Jalhan, S. Essential oils as novel human skin penetration enhancer for transdermal drug delivery: A review. Int. J. Pharm. Bio Sci. 2013, 4, 857–868. [Google Scholar]
- El-Nabarawi, M.; El Meshad, A.N.; Moutasim, M.Y. Assessment of bioavailability of sumatriptan transdermal delivery systems in rabbits. Int. J. Pharm. Pharm. Sci. 2013, 5, 225–240. [Google Scholar]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration en-hancers for transdermal drug delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- de Matos, S.P.; Teixeira, H.F.; de Lima, A.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Ait, A.E.H.; Casabianca, H.; Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, L.; Moghaddam, M. Essential Oils: Biological Activity and Therapeutic Potential. In Therapeutic, Probiotic, and Unconventional Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 167–179. [Google Scholar]
- Osuntokun, O.T. Prospects of essential oils in drug discovery. Adv. Cytol. Pathol. 2017, 2, 00010. [Google Scholar] [CrossRef][Green Version]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mehra, G.R. Preparation, characteriszation, in vitro and in vivo evaluation of transdermal matrix of Celecoxib. Acta Pharm. Sci. 2011, 53, 67–76. [Google Scholar]
- Liu, C.-H.; Chang, F.Y. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem. Pharm. Bull. 2011, 59, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Patadiya, N.; Patel, J.; Shastri, D.; Shelat, P. Development and evaluation of antiarthritic herbal ointment. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 221–228. [Google Scholar]
- Shah, S.N.H.; Hussain, T.; Khan, I.U.; Asghar, S.; Shahzad, Y. Formulation study of topically applied lotion: In vitro and in vivo evaluation. BioImpacts 2013, 3, 11–19. [Google Scholar]
- Zhang, Y.; Yang, N.; Lv, J.; Song, H.; Duan, X.; Leng, J.; Bo, J.; Liu, N.; Huang, Y. A Preclinical Study of Novel Phentolamine Formulation and in Vitro/in Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2014, 24, 22–26. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Barry, B. Lipid-protein-partitioning theory of skin penetration enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Gunstone, F. (Ed.) Vegetable Oils in Food Technology: Composition, Properties and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fox, L.T.; Gerber, M.; Du Plessis, J.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Viljoen, J.; Cowley, A.; du Preez, J.; Gerber, M.; Du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, G.M.; Khan, N.R.; Khan, A.; Rehman, S.U.; Asif, H.M.; Akram, M.; Ali, Y. Trans-dermal diclofenac potassium gels natural penetration enhancers can be effective. Lat. Am. J. Pharm. 2015, 34, 1022–1029. [Google Scholar]
- Aggarwal, G.; Dhawan, S.L.; Hari, K.S. Natural oils as skin permeation enhancers for transdermal delivery of olanzapine: In vitro and in vivo evaluation. Curr. Drug Deliv. 2012, 9, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.S.; Alaayedi, M.; Ashoor, J.A.; Alghurabi, H. The enhancement effect of olive and almond oils on permeability of nimesulide as transdermal gel. Int. J. Pharm. Res. 2019, 11, 1200–1206. [Google Scholar]
- Nawaz, A.; Khan, G.M.; Shah, S.U.; Shah, K.U. Preparation and Evaluation of Clotrimazole Matrix Type Patch: Effect of Olive Oil on Drug Penetration Across Rabbit Skin. Proc. Pak. Acad. Sci. 2011, 48, 95–100. [Google Scholar]
- Hasan, Z.A.; Al-Mousawy, J.M.M.; Alghurabi, H.S.K. The effect of almond oil on the permeability of ketoprofen hydrogel. Int. J. Appl. Pharm. 2019, 12, 65–69. [Google Scholar] [CrossRef]
- Aggarwal, G.; Dhawan, S.; Hari, K.S. Formulation, in vitro and in vivo evaluation of transdermal patches containing risperidone. Drug Dev. Ind. Pharm. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Khizer, Z.; Sadia, A.; Sharma, R.; Farhaj, S.; Nirwan, J.S.; Kakadia, P.G.; Hussain, T.; Yousaf, A.; Shahzad, Y.; Conway, B.; et al. Drug delivery approaches for managing overactive bladder [OAB]: A systematic review. Pharmaceuticals 2021, 14, 409. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. Cochrane Handbook For Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Educ. Res. Arch. 2004, 1–4. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Conway, B.R.; Ghori, M.U. Global prevalence and risk factors of gastro-oesophageal reflux disease [GORD]: Systematic review with meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Downe, S. Appraising the quality of qualitative research. Midwifery 2006, 22, 108–119. [Google Scholar] [CrossRef]
- Korakakis, V.; Whiteley, R.; Tzavara, A.; Malliaropoulos, N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: A systematic review including quantification of patient-rated pain reduction. Br. J. Sports Med. 2018, 52, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Sapra, B.; Jain, S.; Tiwary, A. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120–132. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A. Introductory Chapter: Terpenes and Terpenoids. In Terpenes Terpenoids; BoD-Books on Demand: London, UK, 2018; pp. 1–12. [Google Scholar]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef]
- Akhlaq, M.; Siddiqua, A.; Ullah, H.; Akram, M.; Abdur, R.S.; Khan, M.; Nazir, R.; Imran, M.; Sherazi, M.; Baloch, M.; et al. Development of semi-solid formulation for skin administration of pioglitazone. Lat. Am. J. Pharm. 2019, 38, 771–779. [Google Scholar]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Lu, H.; Shao, X.; Cao, J.; Ou, C.; Pan, D. Antimicrobial activity of eucalyptus essential oil against Pseudomonas in vitro and potential application in refrigerated storage of pork meat. Int. J. Food Sci. Technol. 2016, 51, 994–1001. [Google Scholar] [CrossRef]
- Higgins, C.; Palmer, A.; Nixon, R. Eucalyptus oil: Contact allergy and safety. Contact Derm. 2015, 72, 344–346. [Google Scholar] [CrossRef]
- Sugumar, S.; Clarke, S.; Nirmala, M.; Tyagi, B.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of euca-lyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Med. 2015, 25, e128–e133. [Google Scholar] [CrossRef] [PubMed]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Said, Z.B.-O.S.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Preedy, V.R. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Akram, M.R.; Ahmad, M.; Abrar, A.; Sarfraz, R.M.; Mahmood, A. Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride. Drug Des. Dev. Ther. 2018, 12, 349–364. [Google Scholar] [CrossRef]
- Yaqoob, A.; Ahmad, M.; Mahmood, A.; Sarfraz, R.M. Preparation, in vitro and in vivo characterization of hydrophobic patches of a highly water soluble drug for prolonged plasma half life: Effect of permeation enhancers. Acta Pol. Pharm. 2016, 73, 1639–1648. [Google Scholar]
- Zeng, Z.-W.; Lin, J.; Li, H.; Xi, T.; Zhou, W.; Fan, H. Effect of the matrices and penetration enhancers in elemene transdermal drug delivery system. Afr. J. Pharm. Pharmacol. 2011, 5, 879–886. [Google Scholar]
- Shen, T.; Xu, H.; Weng, W.; Zhang, J. Development of a reservoir-type transdermal delivery system containing eucalyptus oil for tetramethylpyrazine. Drug Deliv. 2013, 20, 19–24. [Google Scholar] [CrossRef]
- Madkaikar, N.; Shirodker, A.; Bhangle, S.; Gude, R. Formulation, optimization and evaluation of matrix type transdermal drug delivery system of antiemetic drug using essential oils and non-ionic surfactant as permeation enhancers. Indian Drugs 2018, 55, 77–78. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112–116. [Google Scholar]
- Parney, S.; Dhurke, R.K. Effect of natural penetration enhancers on dermal delivery of hydrocortisone acetate. J. Pharm. Investig. 2014, 44, 365–380. [Google Scholar] [CrossRef]
- Rout, B.; Liu, C.H.; Wu, W.C. Enhancement of photodynamic inactivation against Pseudomonas aeruginosa by a nano-carrier approach. Colloids Surf. B Biointerfaces 2016, 140, 472–480. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M.; Arafa, M.F.; Osman, M.A. Microemulsion for simultaneous transdermal delivery of benzocaine and indomethacin: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2014, 40, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Saadatzadeh, A.; Salimi, A.; Zarooni, M. Influence of permeation enhancers on the in vitro skin permeation of ketorolac tromethamine through excised rat skin: A mechanistic study. Asian J. Pharm. Clin. Res. 2018, 11, 242–247. [Google Scholar] [CrossRef]
- Christensen, L.; Turner, R.; Weaver, S.; Caserta, F.; Long, L.; Ghannoum, M.; Brown, M. Evaluation of the ability of a novel miconazole formulation to penetrate nail by using three in vitro nail models. Antimicrob. Agents Chemother. 2017, 61, e02554-16. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.K.; Essa, E.A.; El Maghraby, G.M. Essential oils in niosomes for enhanced transdermal delivery of felodipine. Pharm. Dev. Technol. 2019, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, P.G. Formulation and Evaluation of Nanoencapsulated Antimicrobial Agents for Dermal Delivery; University of Huddersfield: Huddersfield, UK, 2016. [Google Scholar]
- Nurdjannah, N.; Bermawie, N.C. Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 197–215. [Google Scholar]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Barboza, J.N.; Filho, C.D.S.M.B.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Arora, R.; Khan, R.; Ojha, A.; Chopra, H.; Upadhyaya, K. Formulation and characterization of terbinafine emulgel for superficial fungal infections. Res. J. Pharm. Technol. 2018, 11, 5029–5036. [Google Scholar] [CrossRef]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of essential oil-based microemulsions for im-proving the solubility, pH stability, photostability, and skin permeation of quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Muţ, A.; Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Stănciulescu, C.; Mitu, M.A.; Szabadi, Z.; Lupuliasa, D. Chitosan/HPMC-based hydrogels containing essential oils for topical delivery of fluconazole: Preliminary studies. Farmacia 2018, 66, 248–256. [Google Scholar]
- Magdum, S.S.; Dounde, P.; Kamble, D.D.; Patil, S.V. Design and characterization of novel emulgel for pain management. Indian Drugs 2016, 53, 16–20. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mishra, S. Plant Monoterpenoids [Prospective Pesticides]. In Ecofriendly Pest Management for Food Security; Elsevier: Amsterdam, The Netherlands, 2016; pp. 507–524. [Google Scholar]
- PubChem. Eucalyptol Cited 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2758 (accessed on 1 December 2021).
- Galan, D.M.; Ezeudu, N.E.; Garcia, J.; Geronimo, C.A.; Berry, N.M.; Malcolm, B.J. Eucalyptol [1,8-cineole]: An underutilized ally in respiratory disorders? J. Essent. Oil Res. 2020, 32, 103–110. [Google Scholar] [CrossRef]
- Prakash, G.; Venkala, R.I.; Doddayya, H. Comparative evaluation of selected vegetable oils and terpenes on transdermal permeation of ketorolac tromethamine. Int. J. Drug. Dev. Res. 2011, 3, 178–188. [Google Scholar]
- Nikolić, I.; Mitsou, E.; Pantelic, I.; Randjelovic, D.; Markovic, B.; Papadimitriou, V.; Xenakis, A.; Lunter, D.J.; Zugic, A.; Savic, S. Microstructure and biopharmaceutical performances of curcumin-loaded low-energy nanoemulsions containing eucalyptol and pinene: Terpenes’ role overcome penetration enhancement effect? Eur. J. Pharm. Sci. 2020, 142, 105135. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef]
- Shi, J.; Cong, W.; Wang, Y.; Liu, Q.; Luo, G. Microemulsion-based patch for transdermal delivery of huperzine A and ligustrazine phosphate in treatment of Alzheimer’s disease. Drug Dev. Ind. Pharm. 2012, 38, 752–761. [Google Scholar] [CrossRef]
- Ubongkot, T.; Duangjit, S.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Ultradeformable liposomes with terpenes for delivery of hydrophilic compound. J. Liposome Res. 2012, 22, 254–262. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.A.E.; Roberts, M.; Grice, J.E. Minoxidil Skin Delivery from Nanoemulsion Formulations Containing Eucalyptol or Oleic Acid: Enhanced Diffusivity and Follicular Targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Thipwichai, S.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S. Development and Evaluation of Ketoprofen Acrylic Transdermal Patches. Trop. J. Pharm. Res. 2012, 11, 553–560. [Google Scholar] [CrossRef][Green Version]
- Casey, A.L.; Karpanen, T.J.; Conway, B.R.; Worthington, T.; Nightingale, P.; Waters, R. Enhanced chlor-hexidine skin penetration with 1,8-cineole. BMC Infect. Dis. 2017, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Herro, E.; Jacob, S.E. Mentha piperita [peppermint]. Dermatitis 2010, 21, 327–329. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; Routledge: London, UK, 2021. [Google Scholar]
- PubChem. Peppermint Oil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6850741 (accessed on 1 December 2021).
- Maniyar, M.G.; Kokare, C.R. Formulation and evaluation of spray dried liposomes of lopinavir for topical application. J. Pharm. Investig. 2018, 49, 259–270. [Google Scholar] [CrossRef]
- Moghimi, H.R.; Jamali, B.; Farahmand, S.; Shafaghi, B. Effect of essential oils, hydrating agents, and ethanol on hair removal efficiency of thioglycolates. J. Cosmet. Dermatol. 2013, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. Menthol and related cooling compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Menthol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1254 (accessed on 1 December 2021).
- Stanos, S.P.; Tyburski, M.D.; Parikh, S.S. Minor and Short-Acting Analgesics, Including Opioid Combination Products. In Practical Management of Pain; Elsevier: Amsterdam, The Netherlands, 2014; pp. 508–529. [Google Scholar]
- Choulis, N. Miscellaneous Drugs, Materials, Medical Devices and Techniques. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2014; Volume 36, pp. 725–746. [Google Scholar]
- Yu, Z.; Liang, Y.; Liang, W. Development and evaluation of alpha-asarone transdermal patches based on hot-melt pressure-sensitive adhesives. AAPS PharmSciTech 2013, 14, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Ahmed, A.B. Effect of menthol on the transdermal permeation of aceclofenac from microemulsion formulation. Int. J. Appl. Pharm. 2019, 11, 117–122. [Google Scholar]
- Patel, P.M. Formulation Development and Evaluation of Bioadhesive Drug Delivery System Containing Selected Phytopharmaceuticals. Ph.D. Thesis, Gujarat Technology University, Ahmedabad, Gujarat, India, 2017. [Google Scholar]
- Wang, Y.; Cao, S.; Yu, K.; Yang, F.; Yu, X.; Zhai, Y.; Wu, C.; Xu, Y. Integrating tacrolimus into eutectic oil-based microemulsion for atopic dermatitis: Simultaneously enhancing percutaneous delivery and treatment efficacy with relieving side effects. Int. J. Nanomed. 2019, 14, 5849–5863. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Sui, H.; Zhao, Q.; Zhang, X.; Wang, W. Enhanced skin permeation of glabridin using eutectic mixture-based nanoemulsion. Drug Deliv. Transl. Res. 2017, 7, 325–332. [Google Scholar] [CrossRef]
- PubChem. Limonene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/22311 (accessed on 1 December 2021).
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Charoenputtakun, P.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Terpene composited lipid nanoparticles for enhanced dermal delivery of all-trans-retinoic acids. Biol. Pharm. Bull. 2014, 37, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Caneri, M.; Glatter, O.; Valenta, C. Self-assembled nanostructured aqueous dispersions as dermal delivery systems. Int. J. Pharm. 2015, 495, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Wattanasri, P. Development of microemulsions, microemulgels and organogels for transdermal delivery of Kaempferia parviflora extract. AAPS PharmSciTech 2016, 19, 2058–2067. [Google Scholar]
- Trease, G.; Evans, M. Pharmacopoeial and Related Drugs of Biological Origin. A Textbook of Pharmacognosy, 15th ed.; WB Saunders: London, UK, 2001; pp. 262–270. [Google Scholar]
- Mercier, B.; Prost, J.; Prost, M. The essential oil of turpentine and its major volatile fraction [α-and β-pinenes]: A review. Int. J. Occup. Med. Environ. Health 2009, 22, 331–342. [Google Scholar] [CrossRef]
- Xu, P.-G.; Lei, X.-F.; Ren, B.-D.; Lv, S.-Y.; Zhang, J.-L. Diclofenac transdermal patch versus the sustained release tablet: A randomized clinical trial in rheumatoid arthritic patients. Trop. J. Pharm. Res. 2017, 16, 477. [Google Scholar] [CrossRef][Green Version]
- Khan, N.; Khan, G.M.; Wahab, A.; Khan, A.R.; Hussain, A.; Nawaz, A.; Akhlaq, M. Formulation, and physical, in vitro and ex vivo evaluation of transdermal ibuprofen hydrogels containing turpentine oil as penetration enhancer. Pharmazie 2011, 66, 849–852. [Google Scholar]
- Wang, F.L.; Ji, H.M.; Zhu, J.Y.; Xu, G.J.; Guan, Y.Z.; Chen, Y.J. Penetration enhancement effect of turpentine oil on transdermal film of ketorolac. Trop. J. Pharm. Res. 2015, 14, 1341. [Google Scholar] [CrossRef][Green Version]
- Wang, H.B.; Yang, F.F.; Gai, X.M.; Cheng, B.C.; Li., J.Y.; Pan, H.; Yang, X.-G.; Pan, W.-S. A pH-independent instantaneous release of flurbiprofen: A study of the preparation of complexes, their characterization and in vitro/in vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1460–1471. [Google Scholar] [CrossRef]
- Rustan, A.; Drevon, C. Fatty Acids: Structures and Properties. In Encyclopedia of Life Sciences; Nature Publishing: London, UK, 2005. [Google Scholar]
- Kumar, L.; Verma, S.; Kumar, S.; Prasad, D.N.; Jain, A.K. Fatty acid vesicles acting as expanding horizon for transdermal delivery. Artif. Cells Nanomed. Biotechnol. 2016, 45, 251–260. [Google Scholar] [CrossRef]
- Mittal, A.; Sara, U.; Ali, A.; Aqil, M. Status of fatty acids as skin penetration enhancers-a review. Curr. Drug Deliv. 2009, 6, 274–279. [Google Scholar] [CrossRef]
- Bonnist, E.; Gorce, J.-P.; Mackay, C.; Pendlington, R.; Pudney, P. Measuring the Penetration of a Skin Sensitizer and Its Delivery Vehicles Simultaneously with Confocal Raman Spectroscopy. Skin Pharmacol. Physiol. 2011, 24, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Khan, G.M.; Jan, S.U.; Shah, S.; Shah, K.U.; Akhlaq, M.; Khan, N.; Nawaz, A.; Wahab, A. Effect of olive oil on transdermal penetration of flurbiprofen from topical gel as enhancer. Pak. J. Pharm. Sci. 2012, 25, 365–369. [Google Scholar] [PubMed]
- Pelikh, O.; Stahr, P.-L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for improved dermal drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Kiritsakis, A.; Markakis, P. Olive oil: A review. Adv. Food Res. 1988, 31, 453–482. [Google Scholar]
- Tsimidou, M.; Blekas, G.; Boskou, D. Olive oil. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: London, UK, 2003; pp. 4252–4260. [Google Scholar]
- Calabriso, N.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A. Olive Oil. In The Mediterranean Diet; Elsevier: Amsterdam, The Netherlands, 2015; pp. 135–142. [Google Scholar]
- PubChem. Olive Oil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24753271 (accessed on 1 December 2021).
- Nawaz, A.; Khan, G.M.; Akhlaq, M.; Zeb, A.; Khan, A.; Hussain, A.; Dayo, A. Formulation and in-vitro evaluation of topically applied curcumin hydrogel. Lat. Am. J. Pharm. 2012, 31, 671–677. [Google Scholar]
- Khan, M.R.U. Formulation design, optimization and enhancement of skin permeation of ibuprofen cream by using olive oil as permeation enhancer. Indo Am. J. Pharm. Sci. 2018, 5, 17–23. [Google Scholar]
- Mortazavi, S.A.; Pishrochi, S. Formulation and in-vitro evaluation of tretinoin microemulsion as a potential carrier for dermal drug delivery. Iran. J. Pharm. Res. IJPR 2013, 12, 599. [Google Scholar]
- Aggarwal, G.; Dhawan, S.; Harikumar, S.L. Formulation, in vitro, and in vivo evaluation of matrix-type transdermal patches containing olanzapine. Pharm. Dev. Technol. 2013, 18, 916–925. [Google Scholar] [CrossRef]
- Shokri, N.; Javari, H.A. Using a synergistic combination of two enhancers for dermal delivery of collagen in pharmaceutical and cosmetic products. J. Pharm. Res. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Ahmad, Z. The uses and properties of almond oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Nawaz, A.; Jan, S.U.; Khan, N.; Hussain, A.; Khan, G.M. Formulation and in vitro evaluation of clotrimazole gel containing almond oil and tween 80 as penetration enhancer for topical application. Pak. J. Pharm. Sci. 2013, 26, 617. [Google Scholar] [PubMed]
- Hussain, A.; Khan, G.M.; Shah, S.U.; Shah, K.U.; Khan, N.; Wahab, A.; Rehman, A.-U. Development of a novel ketoprofen transdermal patch: Effect of almond oil as penetration enhancers on in-vitro and ex-vivo penetration of ketoprofen through rabbit skin. Pak. J. Pharm. Sci. 2012, 25, 227–232. [Google Scholar]
- Parveen, K.; Shrivastava, B.; Gupta, M.M.; Sharma, A.K. Optimization and preparation of solid lipid nano-particle incorporated transdermal patch of timolol maleate using factorial design. Int. J. Appl. Pharm. 2019, 11, 100–107. [Google Scholar] [CrossRef]
- Thakur, N.K.; Bharti, P.; Mahant, S.; Rao, R. Formulation and characterization of benzoyl peroxide gellified emulsions. Sci. Pharm. 2012, 80, 1045–1060. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth profiles of the stratum corneum lipid organization influenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Wisniak, T. Jojoba oil and derivates. Prog. Chem. Fats Other Lipids 1977, 15, 167–218. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Ghassemi, M.; Kazerouni, A.; Rafeie, E.; Jamshydian, N. Jojoba in dermatology: A succinct review. G. Ital. Dermatol. 2013, 148, 687–691. [Google Scholar]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichon, M.A.; Klang, V.; Elbe-Burger, A.; Wirth, M.; Valenta, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef]
- Gogoll, K.; Stein, P.; Lee, K.; Arnold, P.; Peters, T.; Schild, H.; Radsak, M.; Langguth, P. Solid nanoemulsion as antigen and immunopotentiator carrier for transcutaneous immunization. Cell. Immunol. 2016, 308, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; Royal Society of Chemistry: London, UK, 2019; pp. 1–11. [Google Scholar] [CrossRef]
- Ingh, I.; Nair, R.S.; Gan, S.; Cheong, V.; Morris, A. An evaluation of crude palm oil (CPO) and tocotrienol rich fraction (TRF) of palm oil as percutaneous permeation enhancers using full-thickness human skin. Pharm. Dev. Technol. 2019, 24, 448–454. [Google Scholar]
- Sharma, K.; Mittal, A.; Chauhan, N. Aloe vera as penetration enhancer. Int. J. Drug Dev. Res. 2015, 7, 31–43. [Google Scholar]
- Vashisth, I.; Ahad, A.; Aqil, M.; Agarwal, S.P. Investigating the potential of essential oils as penetration enhancer for transdermal losartan delivery: Effectiveness and mechanism of action. Asian J. Pharm. Sci. 2014, 9, 260–267. [Google Scholar] [CrossRef]
- Ran, X.; Ma, L.; Peng, C.; Zhang, H.; Qin, L.-P. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm. Biol. 2011, 49, 1180–1189. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Gao, L.-H.; Hu, J.-H.; Yan, C.-X.; Zhu, Q.-G. Enhancing effect of essential oils from Ligusticum chuanxiong Hort. On the permeation of flurbiprofen through isolated rat skin. Pharm. Care Res. 2006, 6, 413. [Google Scholar]
- Mitrović, P.M.; Stamenković, O.S.; Banković-Ilić, I.; Djalović, I.G.; Nježić, Z.B.; Farooq, M.; Kadambot, H.M.S.; Velijovic, V.B. White mustard [Sinapis alba L.] oil in biodiesel production: A review. Front. Plant Sci. 2020, 11, 299. [Google Scholar] [CrossRef]
- Ruan, S.; Wang, Z.; Xiang, S.; Chen, H.; Shen, Q.; Liu, L.; Wu, W.; Cao, S.; Wang, Z.; Yang, Z.; et al. Mechanisms of white mustard seed (Sinapis alba L.) volatile oils as transdermal penetration enhancers. Fitoterapia 2019, 138, 104195. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).