Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBD-Loaded Carrier Systems
2.2.1. Preparation of CBD-Loaded Carrier Systems with Propylene Glycol
2.3. Preparation of Mucoadhesive Carrier Systems
Preparation of Mucoadhesive Carrier Systems with Propylene Glycol
2.4. Drug Load Quantification
2.5. High Performance Liquid Chromatography Assay
2.6. Dissolution Experiments
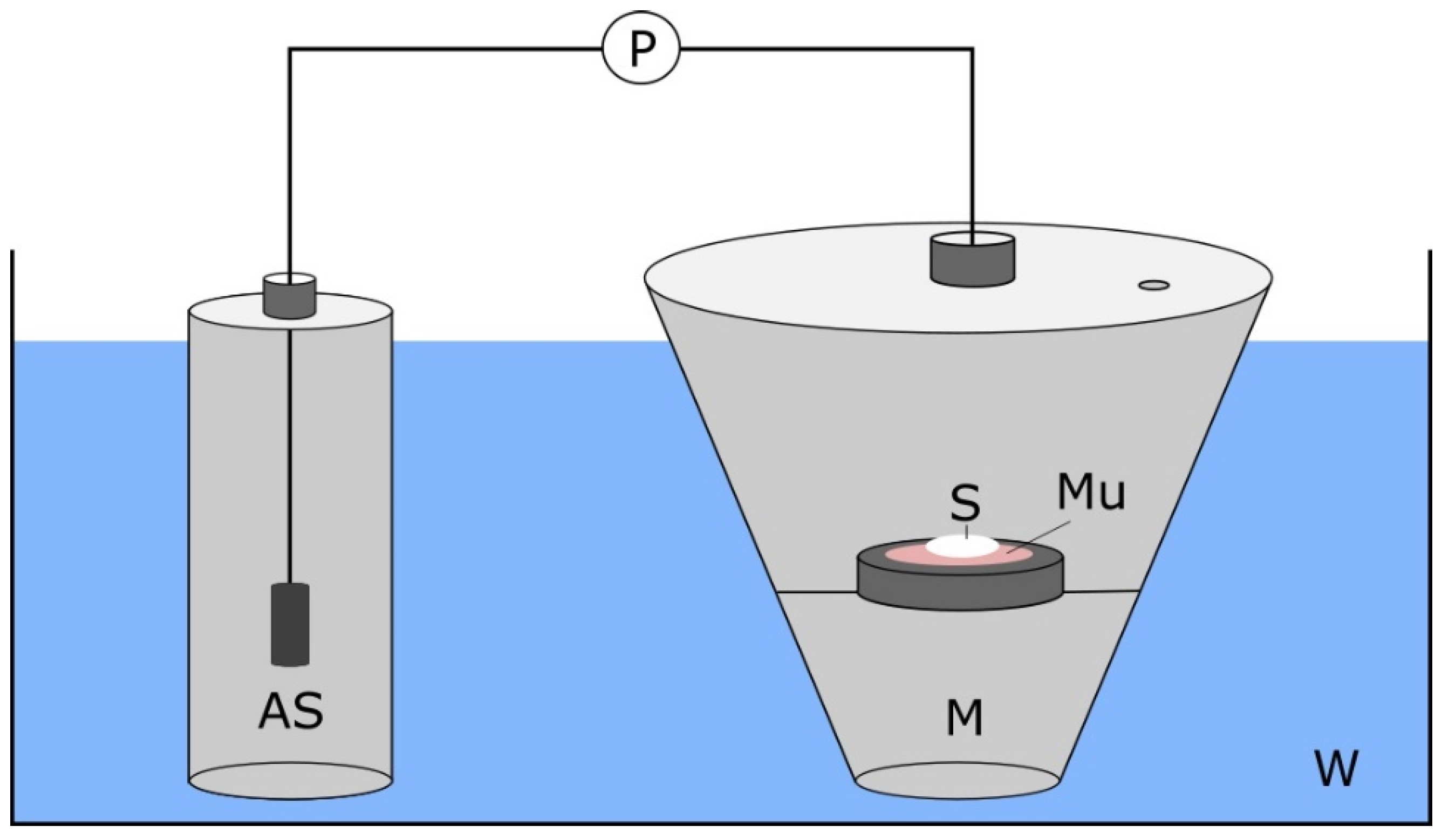
2.7. Mucoadhesion Test
2.8. Mucopenetration Studies
2.9. Microscopy
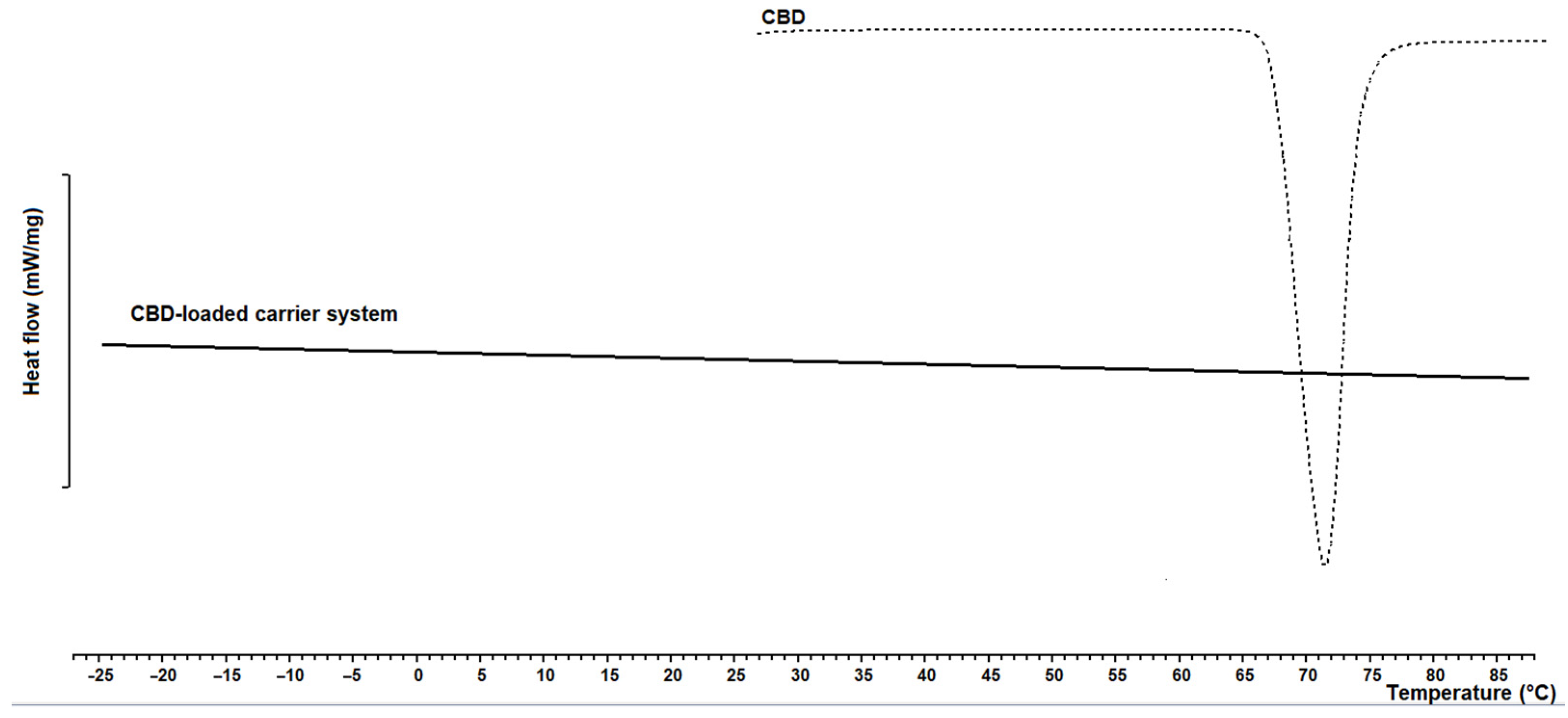
2.10. Differential Scanning Calorimetry
2.11. Statisitcal Data Analysis
3. Results and Discussion
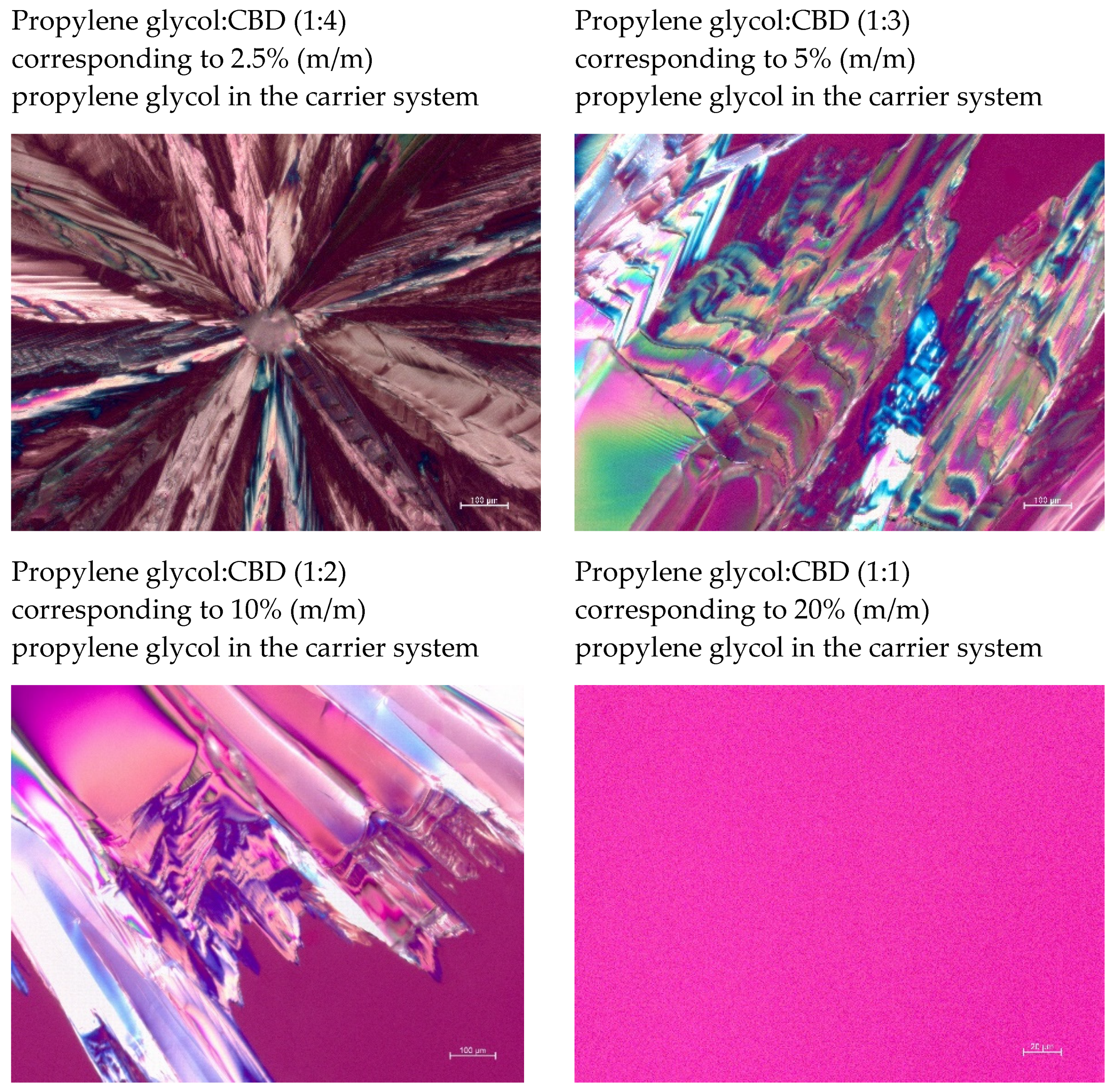
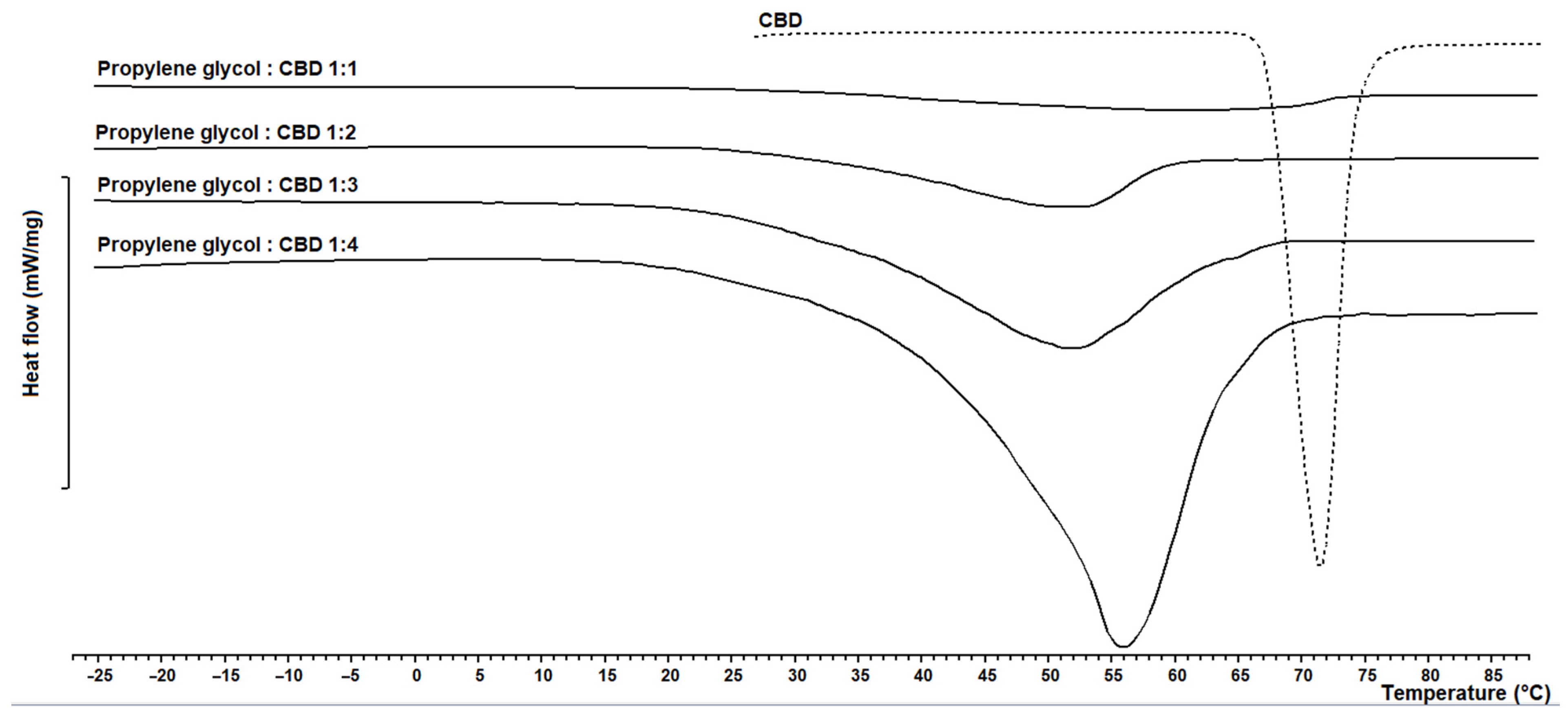
3.1. CBD-Loaded Carrier Systems
3.2. CBD-Loaded Mucoadhesive Carrier Systems
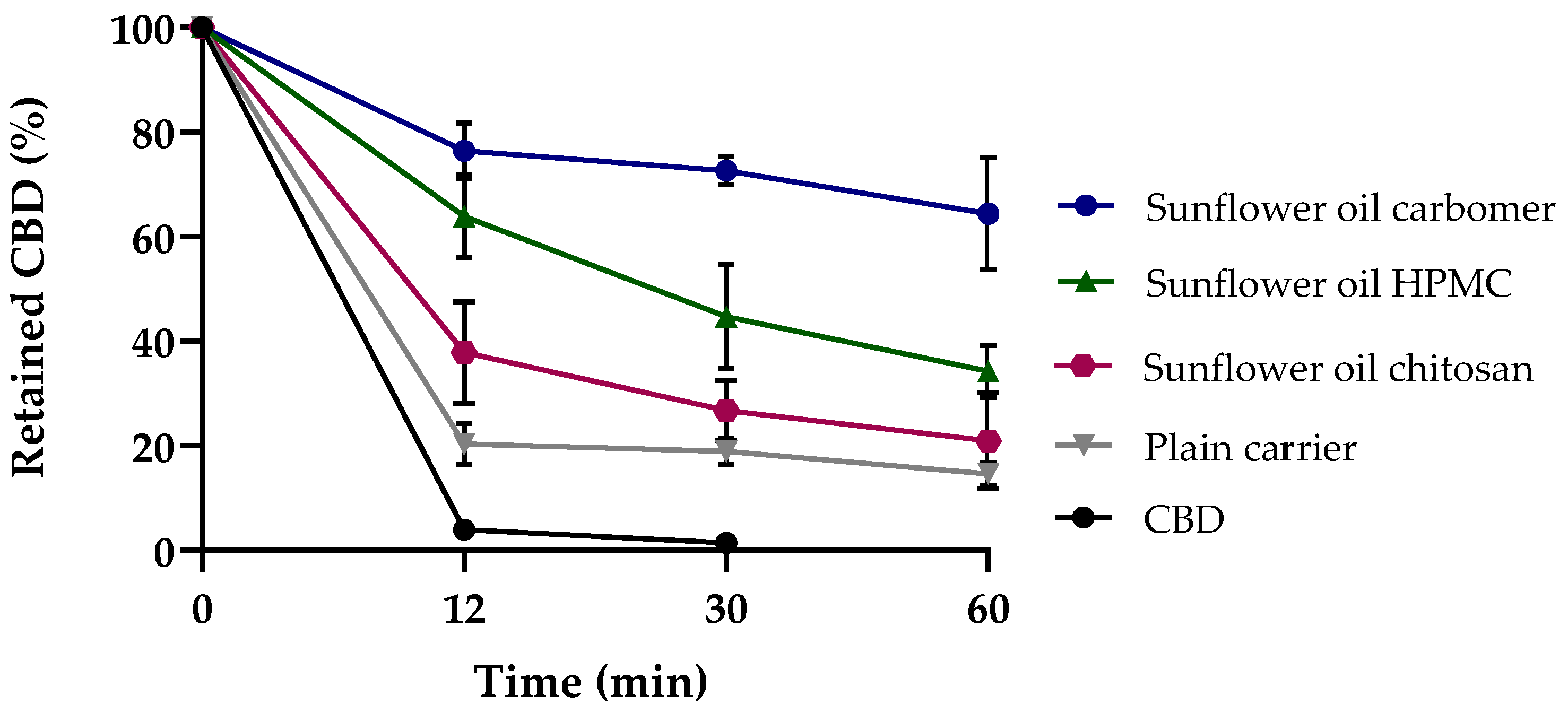
3.2.1. Mucoadhesion Test of CBD-Loaded Mucoadhesive Carrier Systems
3.2.2. In Vitro Release of CBD-Loaded Mucoadhesive Carrier Systems
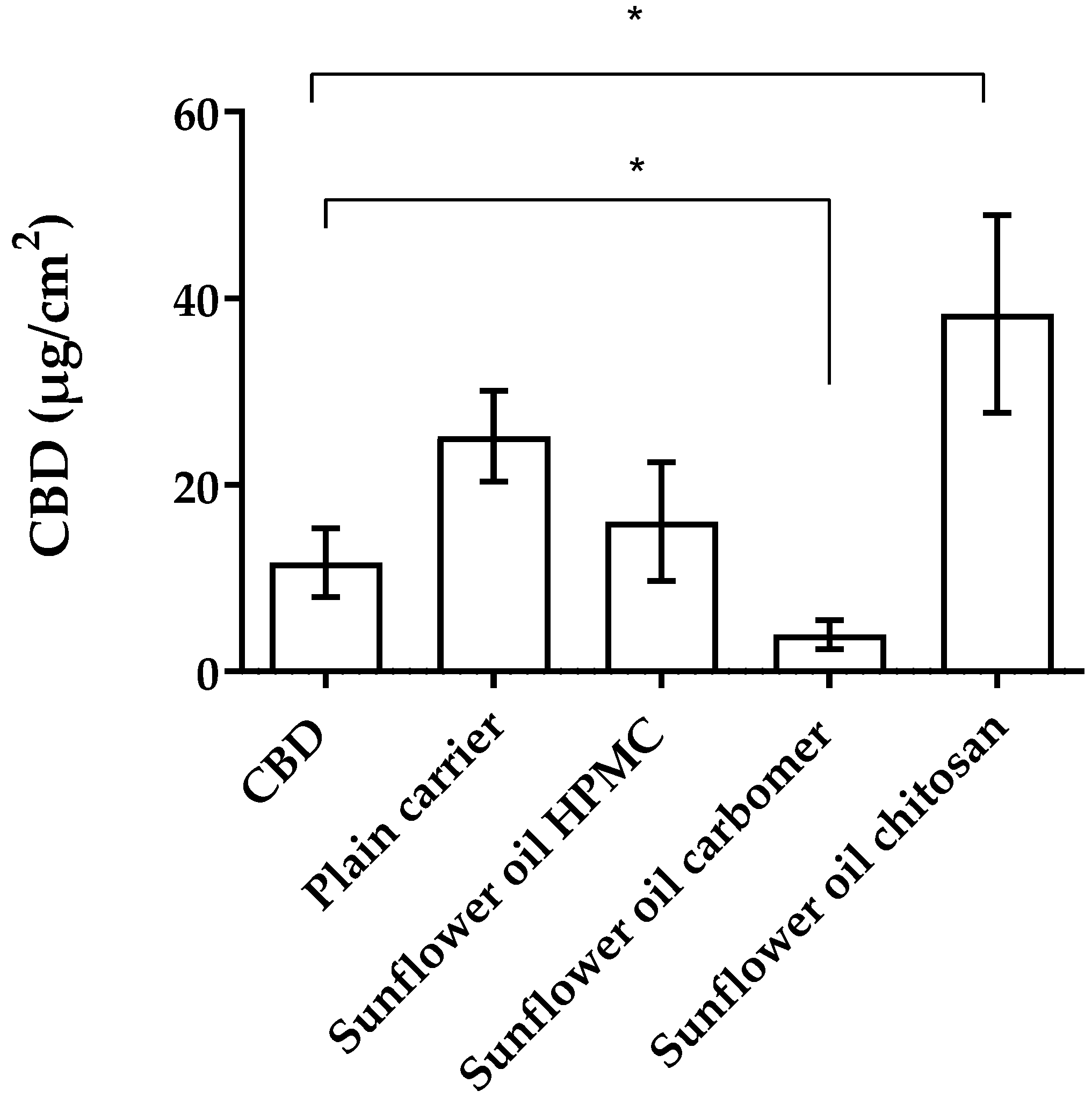
3.2.3. Mucopenetration Studies of CBD-Loaded Mucoadhesive Carrier Systems
3.3. CBD-Loaded Carrier Systems with Propylene Glykol
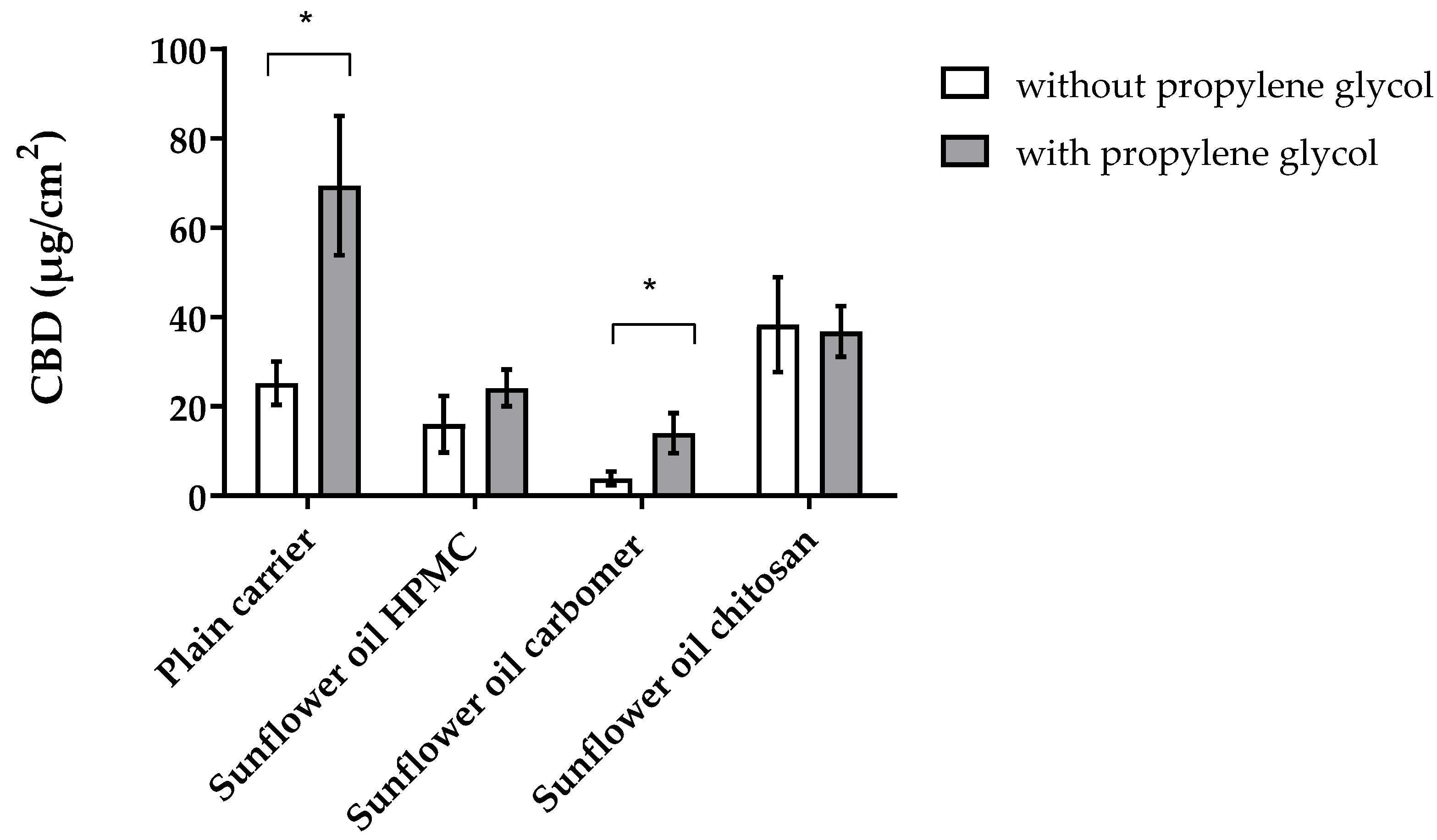
3.4. CBD-Loaded Mucoadhesive Carrier Systems with Propylene Glykol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, R.; Ali, D.W. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [CrossRef]
- Jung, B.; Lee, J.K.; Kim, J.; Kang, E.K.; Han, S.Y.; Lee, H.-Y.; Choi, I.S. Synthetic Strategies for (−)-Cannabidiol and Its Structural Analogs. Chem. Asian J. 2019, 14, 3749–3762. [Google Scholar] [CrossRef]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence from Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging Use of Epidiolex (Cannabidiol) in Epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Silmore, L.H.; Willmer, A.R.; Capparelli, E.V.; Rosania, G.R. Food effects on the formulation, dosing, and administration of cannabidiol (CBD) in humans: A systematic review of clinical studies. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandl, M.; Bauer-Brandl, A. Oromucosal drug delivery: Trends in in-vitro biopharmaceutical assessment of new chemical entities and formulations. Eur. J. Pharm. Sci. 2019, 128, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin. Drug Deliv. 2009, 7, 97–112. [Google Scholar] [CrossRef]
- Şenel, S.; Hıncal, A. Drug permeation enhancement via buccal route: Possibilities and limitations. J. Control. Release 2001, 72, 133–144. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2019, 312, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.C.; Chaitanya, K.; Rao, Y.M. A review on bioadhesive buccal drug delivery systems: Current status of formulation and evaluation methods. DARU J. Pharm. Sci. 2011, 19, 385–403. [Google Scholar]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Hoffmann, A.; Daniels, R. A novel test system for the evaluation of oral mucoadhesion of fast disintegrating tablets. Int. J. Pharm. 2018, 551, 141–147. [Google Scholar] [CrossRef]
- Salonen, J.; Laitinen, L.; Kaukonen, A.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.T.; Lehto, V.-P. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef]
- Esim, O.; Savaser, A.; Ozkan, C.; Bayrak, Z.; Tas, C.; Ozkan, Y. Effect of polymer type on characteristics of buccal tablets using factorial design. Saudi Pharm. J. 2018, 26, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A focus on mucoadhesive polymers and their application in buccal dosage forms. J. Drug Deliv. Sci. Technol. 2016, 32, 113–125. [Google Scholar] [CrossRef]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2010, 11, 748–764. [Google Scholar] [CrossRef]
- Chaudhari, S.; Gupte, A. Mesoporous Silica as a Carrier for Amorphous Solid Dispersion. Br. J. Pharm. Res. 2017, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J.T. Drug Delivery Formulations of Ordered and Nonordered Mesoporous Silica: Comparison of Three Drug Loading Methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Chun, M.-K.; Choi, H.-K. Preparation of an extended-release matrix tablet using chitosan/Carbopol interpolymer complex. Int. J. Pharm. 2008, 347, 39–44. [Google Scholar] [CrossRef]
- Esim, O.; Savaser, A.; Ozkan, C.K.; Tas, C.; Ozkan, Y. Investigation of the mucoadhesivity, swelling, and drug release mechanisms of indomethacin buccal tablets: Effect of formulation variables. Drug Dev. Ind. Pharm. 2020, 46, 1979–1987. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Froehlich, C.; Kalia, Y.N.; Guy, R.H. Supersaturation: Enhancement of skin penetration and permeation of a lipophilic drug. Pharm. Res. 2001, 18, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; Kremer, M.J.; Kaş, S.; Wertz, P.W.; Hıncal, A.A.; Squier, C.A. Effect of Chitosan in Enhancing Drug Delivery across Buccal Mucosa. Advances in Chitin Science; University of Potsdam: Postdam, Germany, 2000; pp. 254–258. [Google Scholar]
- Sohi, H.; Ahuja, A.; Ahmad, F.; Khar, R.K. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal penetration enhancers—How do they really work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A. Transdermal Drug Delivery: Penetration Enhancement Techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
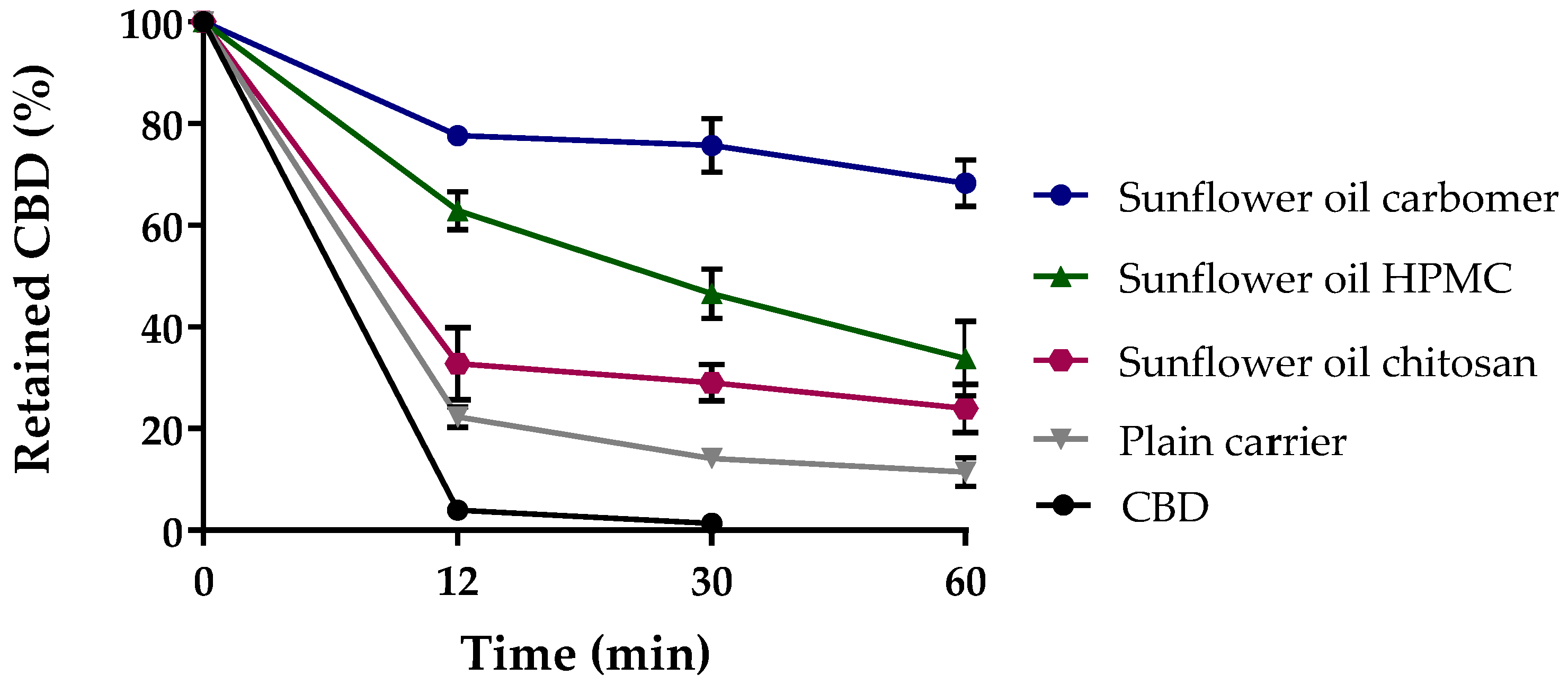
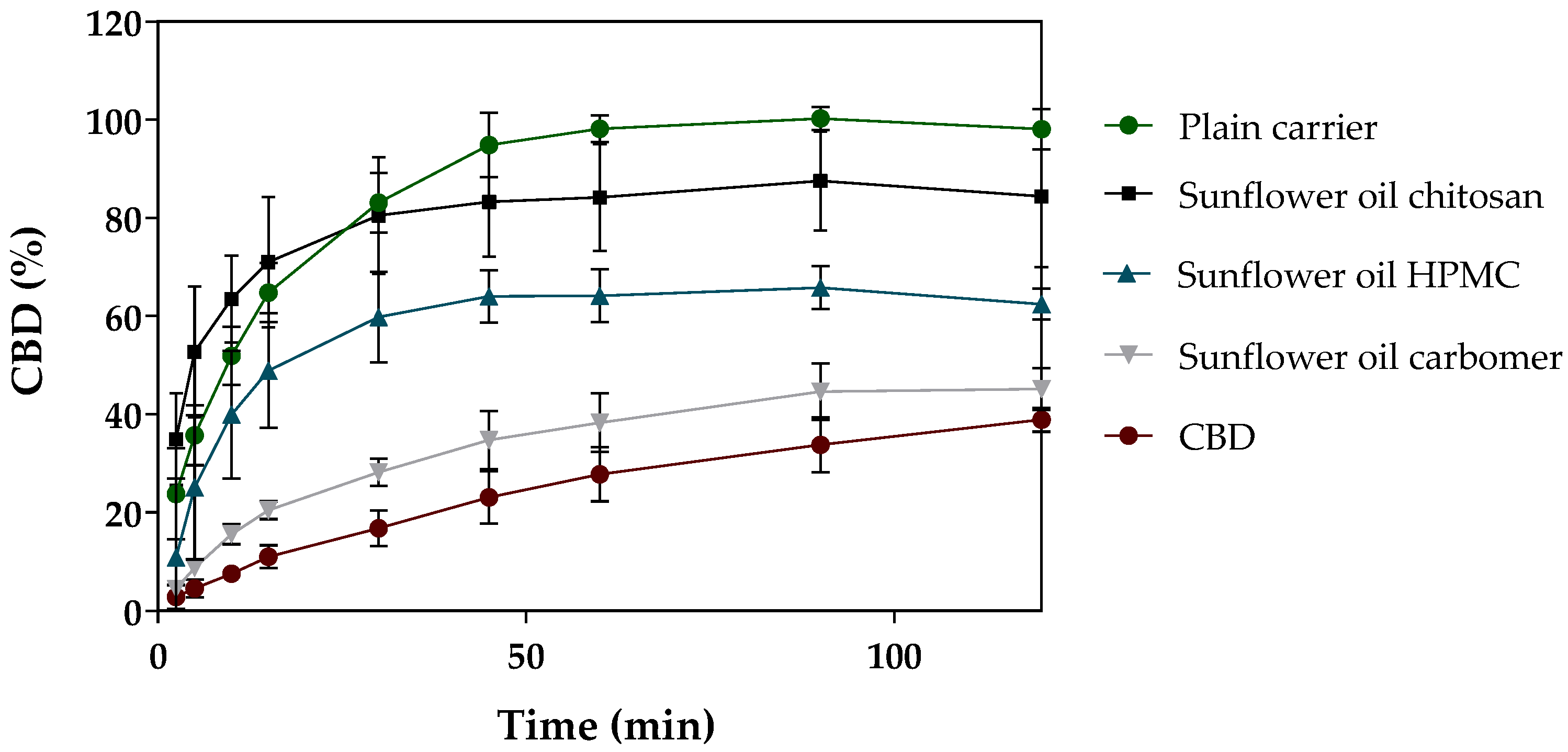
| Formulation | Mucoadhesion [%] | Mucoadhesion Coefficient |
|---|---|---|
| CBD | 3.94 ± 0.85 | 1.00 |
| Plain carrier system | 22.31 ± 1.98 | 5.66 |
| Sunflower oil chitosan | 32.82 ± 7.09 | 8.33 |
| Sunflower oil HPMC | 62.87 ± 3.76 | 15.96 |
| Sunflower oil carbomer | 77.65 ± 1.10 | 19.71 |
| Formulation | Penetrated CBD [µg/ cm2] | PE Ratio |
|---|---|---|
| CBD | 11.70 ± 3.70 | 1.00 |
| Aeroperl 300 CBD 0% Propylene glycol | 25.23 ± 4.85 | 2.16 |
| Aeroperl 300 CBD 2.5% Propylene glycol | 34.01 ± 8.90 | 2.91 |
| Aeroperl 300 CBD 5% Propylene glycol | 70.47 ± 15.63 | 6.02 |
| Aeroperl 300 CBD 10% Propylene glycol | 72.99 ± 11.78 | 6.24 |
| Aeroperl 300 CBD 20% Propylene glycol | 77.95 ± 6.44 | 6.66 |
| Formulation | without Propylene Glycol | with Propylene Glycol | ||
|---|---|---|---|---|
| Penetrated CBD [µg/cm2] | PE | Penetrated CBD [µg/cm2] | PE | |
| CBD | 11.70 ± 3.70 | 1.00 | - | - |
| Plain carrier | 25.23 ± 4.85 | 2.16 | 69.39 ± 15.62 | 5.93 |
| Sunflower oil HPMC | 16.06 ± 6.35 | 1.37 | 24.15 ± 4.14 | 2.06 |
| Sunflower oil carbomer | 3.91 ± 1.54 | 0.33 | 14.06 ± 4.52 | 1.20 |
| Sunflower oil chitosan | 38.31 ± 10.81 | 3.27 | 36.79 ± 5.67 | 3.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söpper, U.; Hoffmann, A.; Daniels, R. Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Sci. Pharm. 2021, 89, 35. https://doi.org/10.3390/scipharm89030035
Söpper U, Hoffmann A, Daniels R. Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Scientia Pharmaceutica. 2021; 89(3):35. https://doi.org/10.3390/scipharm89030035
Chicago/Turabian StyleSöpper, Ulrike, Anja Hoffmann, and Rolf Daniels. 2021. "Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery" Scientia Pharmaceutica 89, no. 3: 35. https://doi.org/10.3390/scipharm89030035
APA StyleSöpper, U., Hoffmann, A., & Daniels, R. (2021). Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Scientia Pharmaceutica, 89(3), 35. https://doi.org/10.3390/scipharm89030035







