Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome
Abstract
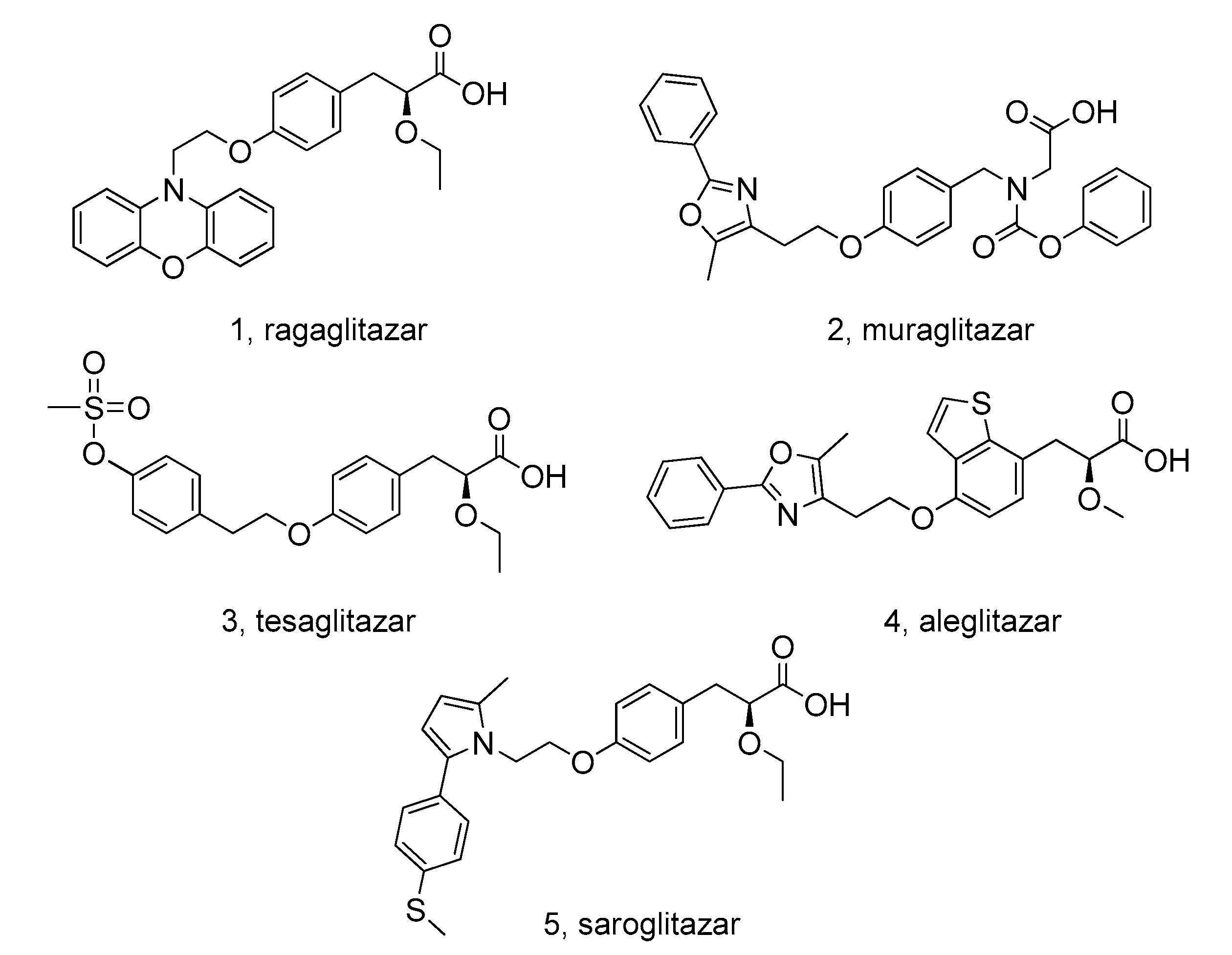
1. Introduction
2. Materials and Methods
2.1. Chemistry
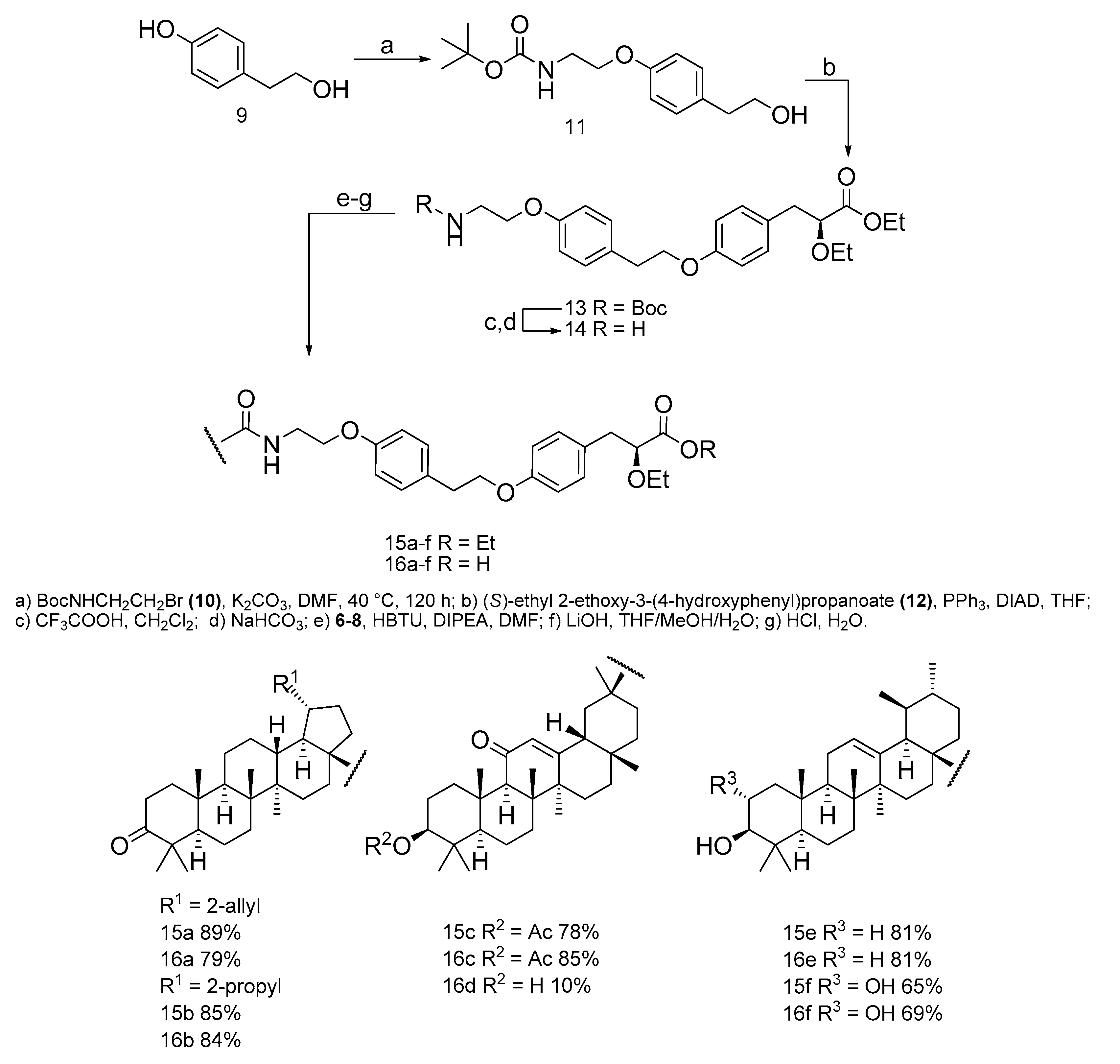
2.1.1. Synthesis of Tert-butyl 2-(4-(2-hydroxyethyl)-phenoxy)-ethylcarbamate (11)
2.1.2. Synthesis of (S)-ethyl3-(4-(4-(2-(tert-butoxycarbonylamino)-ethoxy)-phenoxy)-phenyl)-2-ethoxypropanoate (13)
2.1.3. Synthesis of 2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamine (14)
2.1.4. General Procedure for the Synthesis of [triterpenoic]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamides (15a–f)
N-[3-Oxolup-20(29)-en-28-oyl]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamide (15a)
N-[3-Oxolup-28-oyl]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamide (15b)
[3β-. acetoxy-11-oxo-18β-H-olean-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamide (15c)
[3ß-. hydroxy-urs-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamide (15e)
[2α,3ß-. dihydroxy-urs-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoate)-phenoxy)-ethyl)-phenoxy) ethanamide (15f)
2.1.5. Hydrolysis of [triterpene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamides (16a–f)
N-[3-Oxolup-20(29)-en-28-oyl]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16a)
N-[3-Oxolup-28-oyl]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16b)
[3β-. acetoxy-11-oxo-18β-H-olean-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16c)
[3β-. hydroxy-11-oxo-18β-H-olean-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16d)
[3ß-. hydroxy-urs-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16e)
[2α,3ß-. dihydroxy-urs-12-ene]-2-(4-(2-(4-((S)2-ethoxy-3-propanoic)-phenoxy)-ethyl)-phenoxy) ethanamide (16f)
2.2. Biology
2.2.1. Animals
2.2.2. High Fat Diet
2.2.3. Oral Glucose Tolerance Test
2.2.4. Biochemistry
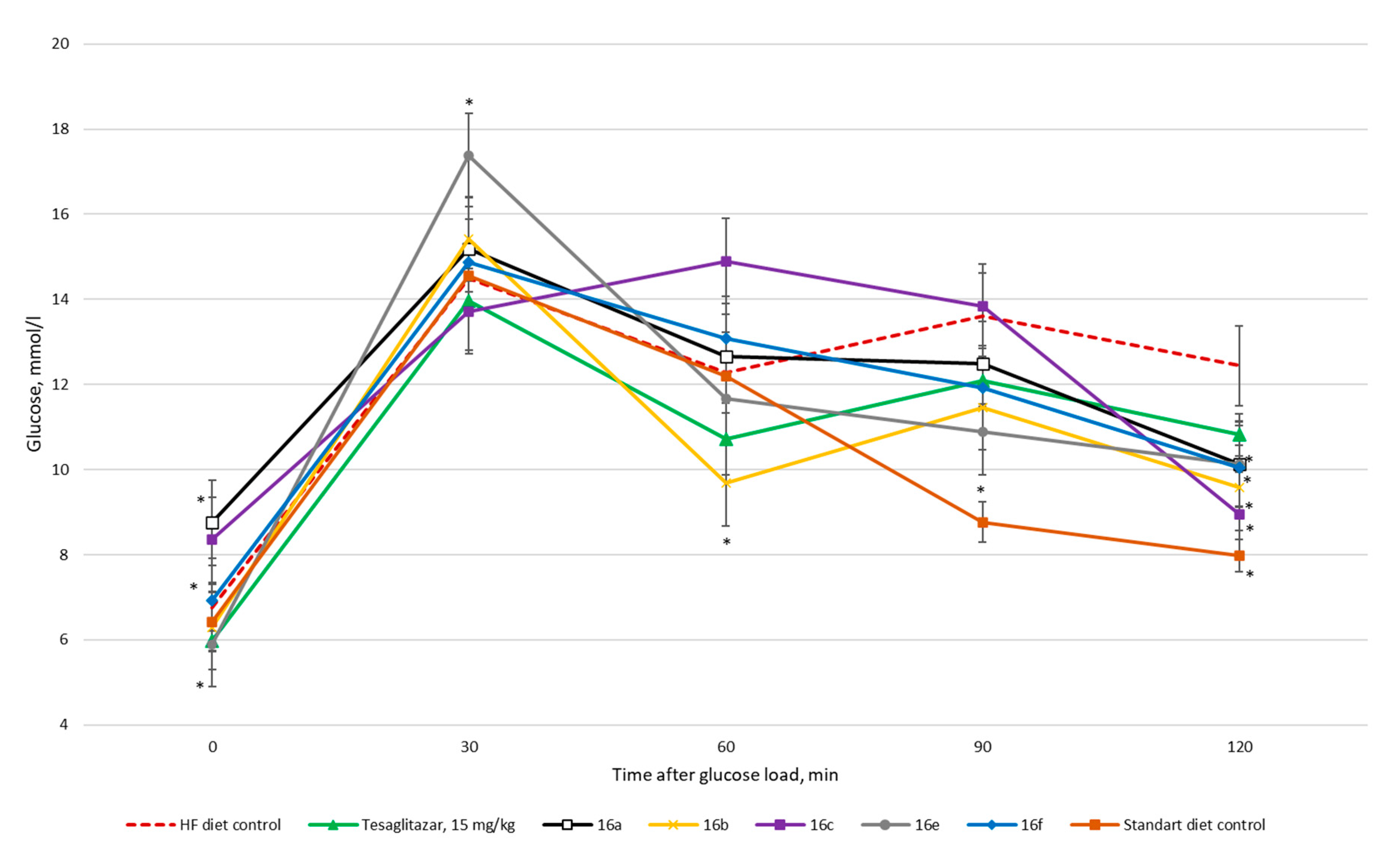
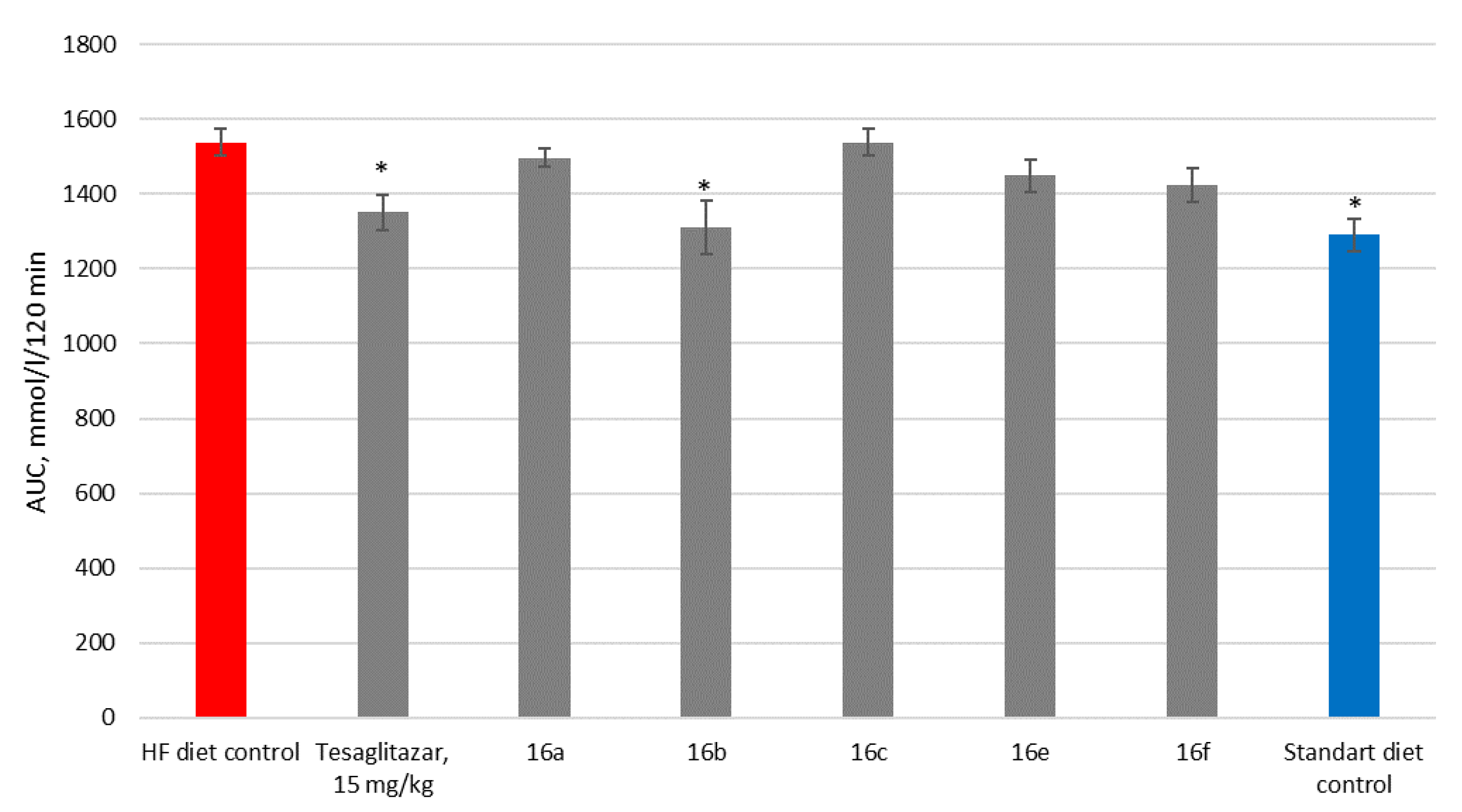
3. Results
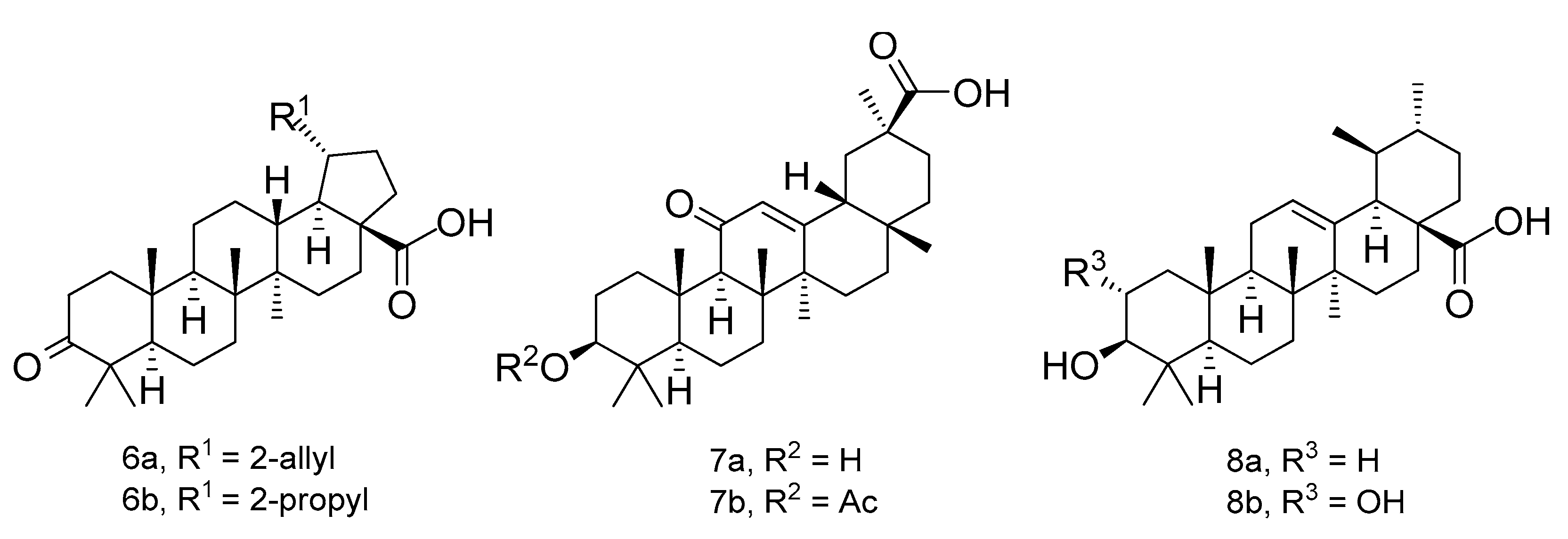
3.1. Chemistry
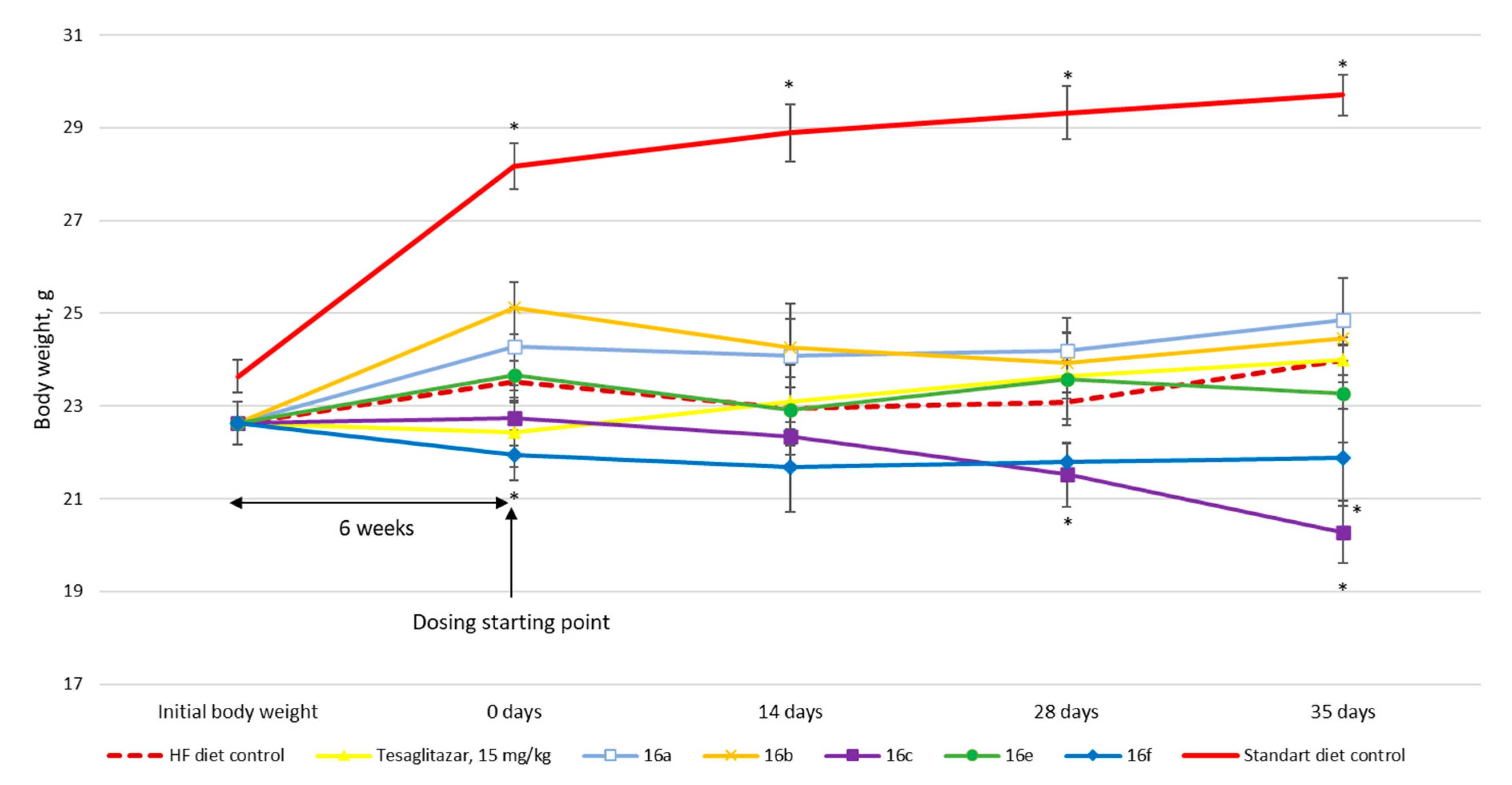
3.2. Biology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A.; Calabriso, N.; Wabitsch, M.; Storelli, C.; Wright, M.; De, C.R. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol. Res. 2016, 107, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R. The First Approved Agent in the Glitazar’s Class: Saroglitazar. Curr. Drug Targets 2014, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Mahadevan, N.; Sambathkumar, R. A Contemporary Overview of PPARα/γ Dual Agonists for the Management of Diabetic Dyslipidemia. Curr. Mol. Pharmacol. 2019, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef]
- Newman, D.; Cragg, G. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.; Chatterjee, V.; Glass, C.; Gonzalez, F.; Grimaldi, P.; Kadowaki, T.; Lazar, M.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome Proliferator-Activated Receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Al-Assaf, H. Hepatoprotective and antioxidant effect of corosolic acid on carbon tetrachloride induced hepatotoxicity. Afr. J. Pharm. Pharmacol. 2013, 7, 673–678. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, G.; Singh, M. Ursolic acid: A natural preventive aesculapian for environmental hepatic ailments. Environ. Dis. 2017, 2, 87. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Liu, P.; Cheng, G.; Sun, M. Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Semenov, D.; Zhukova, N.; Ivanova, E.; Sorokina, I.; Baiev, D.; Nepomnyashchikh, G.; Tolstikova, T.; Biryukova, M. Hepatoprotective Properties of Betulonic Acid Amide and Heptral in Toxic Liver Injury Induced by Carbon Tetrachloride in Combination with Ethanol. Bull. Exp. Biol. Med. 2015, 158, 336–341. [Google Scholar] [CrossRef]
- Afrose, S.; Hossain, M.; Maki, T.; Tsujii, H. Karaya root saponin exerts a hypocholesterolemic response in rats fed a high-cholesterol diet. Nutr. Res. 2009, 29, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Valverde, L.; Diaz-Cedillo, F.; Lopez-Ramos, M.; Garcia-Cervera, E.; Pool-Gomez, E.; Cardena-Arredondo, C.; Ancona-Leon, G. Glibenclamide-pregnenolone derivative has greater hypoglycemic effects and biodistribution than glibenclamide-OH in alloxan-rats. Biomed. Pap. 2012, 156, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mbaze, L.; Poumale, H.; Wansi, J.; Lado, J.; Khan, S.; Iqbal, M.; Ngadjui, B.; Laatsch, H. α-Glucosidase inhibitory pentacyclic triterpenes from the stem bark of Fagaratess mannii (Rutaceae). Phytochemistry 2007, 68, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Chukwujekwu, J.; Rengasamy, K.; De Kock, C.; Smith, P.; Slavětínská, L.; Van Staden, J. Alpha-glucosidase inhibitory and antiplasmodial properties of terpenoids from the leaves of Buddle jasaligna Willd. J. Enzyme Inhib. Med. Chem. 2015, 31, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, P.; Deshmukh, R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.; Shamraiz, U.; Saleem, M.; Badshah, A.; Abbas, G.; Rehman, N.; Irshad, M. Therapeutic potential of glycyrrhetinic acids: A patent review (2010–2017). Expert Opin. Ther. Pat. 2018, 28, 383–398. [Google Scholar] [CrossRef]
- Teng, H.; Yuan, B.; Gothai, S.; Arulselvan, P.; Song, X.; Chen, L. Dietary triterpenes in the treatment of type 2 diabetes: To date. Trends Food Sci. Technol. 2018, 72, 34–44. [Google Scholar] [CrossRef]
- Gokaraju, G. Novel Structural Analogs of Corosolic Acid Having Anti-Diabetic And Anti-Inflammatory Properties. European Patent Office EP1773749A4, 8 July 2004. [Google Scholar]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.; et al. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure−Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Astrazeneca. Process for The Preparation of 2-Ethoxy-3-[4-(2-(Methanesulphonyloxyphenyl)-Ethoxy) Phenil] Propanoic Acid. World Patent WO2003082812A2, 8 January 2003. [Google Scholar]
- Tai, M. A Mathematical Model for the Determination of Total Area under Glucose Tolerance and Other Metabolic Curves. Diabet. Care 1994, 17, 152–154. [Google Scholar] [CrossRef]
- Meyer, M.; Foulquier, S.; Dupuis, F.; Flament, S.; Grimaud, L.; Henrion, D.; Lartaud, I.; Monard, G.; Grillier-Vuissoz, I.; Boisbrun, M. Synthesis and evaluation of new designed multiple ligands directed towards both peroxisome proliferator-activated receptor-γ and angiotensin II type 1 receptor. Eur. J. Med. Chem. 2018, 158, 334–352. [Google Scholar] [CrossRef]
- Ghosh, K.; Panja, S. Naphthalene-cholesterol conjugate as simple gelator for selective sensing of CN–ion. Supramol. Chem. 2016, 29, 350–359. [Google Scholar] [CrossRef]
- Kuranov, S.; Blokhin, M.; Borisov, S.; Khvostov, M.; Luzina, O.; Salakhutdinov, N. Synthesis and Hypoglycemic Activity of Aryl(Hetaryl)Propenoic Cyanopyrrolidine Amides. Russ. J. Bioorg. Chem. 2019, 45, 374–380. [Google Scholar] [CrossRef]
- Kuranov, S.; Luzina, O.; Onopchenko, O.; Pishel, I.; Zozulya, S.; Gureev, M.; Salakhutdinov, N.; Krasavin, M. Exploring bulky natural and natural-like periphery in the design of p-(benzyloxy)phenylpropionic acid agonists of free fatty acid receptor 1 (GPR40). Bioorg. Chem. 2020, 99, 103830. [Google Scholar] [CrossRef]
- Hintze, K.; Benninghoff, A.; Cho, C.; Ward, R. Modeling the Western Diet for Preclinical Investigations. Adv. Nutr. 2018, 9, 263–271. [Google Scholar] [CrossRef]
| TC, mmol/L | TG, mmol/L | HDL, mg/dL | LDL, mg/dL | GlU, mmol/L | ALP, U/L | |
|---|---|---|---|---|---|---|
| HF diet | 4.29 ± 0.16 | 0.57 ± 0.07 | 213.51 ± 16.61 | 19.84 ± 2.57 | 6.76 ± 0.32 | 196.54 ± 8.86 |
| 3 | 4.88 ± 0.18 * | 0.39 ± 0.02 * | 201.60 ± 5.73 | 18.54 ± 0.93 | 7.55 ± 0.54 | 492.76 ± 34.74 * |
| 16a | 4.03 ± 0.22 | 0.37 ± 0.04 * | 173.79 ± 10.85 | 24.93 ± 1.49 | 7.62 ± 0.77 | 190.33 ± 13.71 |
| 16b | 3.71 ± 0.23 * | 0.64 ± 0.09 | 156.43 ± 13.05 * | 17.76 ± 1.89 | 5.51 ± 0.44 * | 184.25 ± 9.49 |
| 16c | 3.88 ± 0.27 | 0.57 ± 0.04 | 153.81 ± 11.02 * | 18.03 ± 2.69 | 6.72 ± 0.48 | 208.80 ± 26.77 |
| 16e | 4,28 ± 0.47 | 0.40 ± 0.04 | 184.95 ± 20.59 | 20.72 ± 6.87 | 5.71 ± 0.64 | 342.70 ± 80.70 |
| 16f | 3.83 ± 0.29 | 0.65 ± 0.05 | 158.378 ± 10.15 * | 17.60 ± 1.12 | 6.71 ± 0.49 | 223.06 ± 22.87 |
| SD control | 2.41 ± 0.18 * | 0.79 ± 0.05 * | 108.64 ± 6.11 * | 11.01 ± 1.18 * | 6.38 ± 0.28 | 180.53 ± 7.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, V.; Blokhin, M.; Kuranov, S.; Khvostov, M.; Baev, D.; Borisova, M.S.; Luzina, O.; Tolstikova, T.G.; Salakhutdinov, N.F. Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome. Sci. Pharm. 2021, 89, 4. https://doi.org/10.3390/scipharm89010004
Fomenko V, Blokhin M, Kuranov S, Khvostov M, Baev D, Borisova MS, Luzina O, Tolstikova TG, Salakhutdinov NF. Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome. Scientia Pharmaceutica. 2021; 89(1):4. https://doi.org/10.3390/scipharm89010004
Chicago/Turabian StyleFomenko, Vladislav, Mikhail Blokhin, Sergey Kuranov, Mikhail Khvostov, Dmitriy Baev, Marina S. Borisova, Olga Luzina, Tatyana G. Tolstikova, and Nariman F. Salakhutdinov. 2021. "Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome" Scientia Pharmaceutica 89, no. 1: 4. https://doi.org/10.3390/scipharm89010004
APA StyleFomenko, V., Blokhin, M., Kuranov, S., Khvostov, M., Baev, D., Borisova, M. S., Luzina, O., Tolstikova, T. G., & Salakhutdinov, N. F. (2021). Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome. Scientia Pharmaceutica, 89(1), 4. https://doi.org/10.3390/scipharm89010004







