Vaginal Administration of Contraceptives
Abstract
1. Introduction
2. Contraceptives
Non-Vaginal Contraceptives
3. The Vaginal Route Delivery
Vaginal Anatomy and Physiology
4. Vaginal Contraceptives
4.1. Vaginal Rings
4.2. Vaginal Spermicides as Non-Hormonal Contraceptives
4.2.1. Bactericides/Surfactant
4.2.2. Sulfhydryl Binding Agents
4.2.3. Natural Products and Their Derivatives
4.2.4. Other Synthetic Products
5. Conclusions
Funding
Conflicts of Interest
References
- Bearak, J.; Popinchalk, A.; Alkema, L.; Sedgh, G. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: Estimates from a Bayesian hierarchical model. Lancet Glob. Health 2018, 6, e380–e389. [Google Scholar] [CrossRef]
- Polis, C.B.; Curtis, K.M.; Hannaford, P.C.; Phillips, S.J.; Chipato, T.; Kiarie, J.N.; Westreich, D.J.; Steyn, P.S. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. Aids Lond. Engl. 2016, 30, 2665. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Mahamat-Saleh, Y.; Savoye, I.; Dartois, L.; Boutron-Ruault, M.; Fournier, A.; Kvaskoff, M. Oral contraceptive use and cutaneous melanoma risk: A French prospective cohort study. Int. J. Cancer 2018, 143, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R.; Nath, A.; Mishell, D.R., Jr. Contraception technology: Past, present and future. Contraception 2013, 87, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Gaffield, M.E.; Curtis, K.M.; Mohllajee, A.P.; Peterson, H.B. Medical eligibility criteria for new contraceptive methods: Combined hormonal patch, combined hormonal vaginal ring and the etonogestrel implant. Contraception 2006, 73, 134–144. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Hezaveh, H.; Muhamad, I.I.; Hashim, S.; Khairuddin, N. Montmorillonite-based polyacrylamide hydrogel rings for controlled vaginal drug delivery. Mater. Sci. Eng. C 2020, 110, 110609. [Google Scholar] [CrossRef]
- Hasanifard, M.; Ebrahimi-Hosseinzadeh, B.; Hatamian-Zarmi, A.; Rezayan, A.; Esmaeili, M. Development of thiolated chitosan nanoparticles based mucoadhesive vaginal drug delivery systems. Polym. Sci. Ser. A 2017, 59, 858–865. [Google Scholar] [CrossRef]
- Griffin, J.B.; Ridgeway, K.; Montgomery, E.; Torjesen, K.; Clark, R.; Peterson, J.; Baggaley, R.; van der Straten, A. Vaginal ring acceptability and related preferences among women in low- and middle-income countries: A systematic review and narrative synthesis. PLoS ONE 2019, 14, e0224898. [Google Scholar] [CrossRef]
- Lusti-Narasimhan, M.; Merialdi, M.; Holt, B. Multipurpose prevention technologies: Maximising positive synergies. Bjog: Int. J. Obstet. Gynaecol. 2014, 121, 251. [Google Scholar] [CrossRef]
- Hubacher, D.; Trussell, J. A definition of modern contraceptive methods. Contraception 2015, 92, 420–421. [Google Scholar] [CrossRef]
- Riley, H.E.M.; Steyn, P.S.; Achilles, S.L.; Bass, E.; Gray, A.L.; Polis, C.B.; Kiarie, J.N. Hormonal contraceptive methods and HIV: Research gaps and programmatic priorities. Contraception 2017, 96, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Claure, I.; Anderson, D.; Klapperich, C.M.; Kuohung, W.; Wong, J.Y. Biomaterials and Contraception: Promises and Pitfalls. Ann. Biomed. Eng. 2020, 48, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Narrigan, D. Women’s barrier contraceptive methods: Poised for change. J. Midwifery Women’s Health 2006, 51, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.; Creatsas, G.; Gravanis, A.; Georgoulias, V.; Psychoyos, A. Anti-STD vaginal contraceptive sponges. Ann. N. Y. Acad. Sci. 1997, 816, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Chipato, T.; Padian, N.S. Preventing disease by protecting the cervix: The unexplored promise of internal vaginal barrier devices. Aids Lond. Engl. 2001, 15, 1595–1602. [Google Scholar] [CrossRef][Green Version]
- Colquitt, C.W.; Martin, T.S. Contraceptive methods: A review of nonbarrier and barrier products. J. Pharm. Pract. 2017, 30, 130–135. [Google Scholar] [CrossRef]
- Caruso, S.; Agnello, C.; Intelisano, G.; Farina, M.; Di Mari, L.; Cianci, A. Sexual behavior of women taking low-dose oral contraceptive containing 15 microg ethinylestradiol/60 microg gestodene. Contraception 2004, 69, 237–240. [Google Scholar] [CrossRef]
- De Castro Coelho, F.; Barros, C. The Potential of Hormonal Contraception to Influence Female Sexuality. Int. J. Reprod. Med. 2019, 2019, 9701384. [Google Scholar] [CrossRef]
- Westhoff, C.L.; Pike, M.C. Hormonal contraception and breast cancer. Contraception 2018, 98, 171–173. [Google Scholar] [CrossRef]
- Smith, J.S.; Green, J.; de Gonzalez, A.B.; Appleby, P.; Peto, J.; Plummer, M.; Franceschi, S.; Beral, V. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet 2003, 361, 1159–1167. [Google Scholar] [CrossRef]
- Brynhildsen, J. Combined hormonal contraceptives: Prescribing patterns, compliance, and benefits versus risks. Ther. Adv. Drug Saf. 2014, 5, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Heffron, R.; Donnell, D.; Rees, H.; Celum, C.; Mugo, N.; Were, E.; de Bruyn, G.; Nakku-Joloba, E.; Ngure, K.; Kiarie, J.; et al. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect. Dis. 2012, 12, 19–26. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Prev. Biomark. 2013, 22, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Burkman, R.T. The transdermal contraceptive patch: A new approach to hormonal contraception. Int. J. Fertil. Womens Med. 2002, 47, 69–76. [Google Scholar] [PubMed]
- Feisullin, K.; Westhoff, C. Chapter 33-Contraception. In Principles of Gender-Specific Medicine, 2nd ed.; Legato, M.J., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 357–365. [Google Scholar]
- Jain, J.; Jakimiuk, A.J.; Bode, F.R.; Ross, D.; Kaunitz, A.M. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004, 70, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Noory, A.; Khabt Aboud, H.; Essam, N.; Rezaq, A. The side effect of noristerat injection in some biochemistry parameters in al-diwaniya city. Pak. J. Biotechnol. 2018, 15, 699–702. [Google Scholar]
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Pathak, Y.V. Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Mašek, J.; Mašková, E.; Lubasová, D.; Špánek, R.; Raška, M.; Turánek, J. Nanofibers in Mucosal Drug and Vaccine Delivery, Nanomaterials-Toxicity, Human Health and Environment; Clichici, S., Filip, A., do Nascimento, G.M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef]
- Van der Bijl, P.; van Eyk, A.D. Comparative in vitro permeability of human vaginal, small intestinal and colonic mucosa. Int. J. Pharm. 2003, 261, 147–152. [Google Scholar] [CrossRef]
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Nunes, R.; Machado, A.; Sarmento, B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in Polymeric Nanoparticles for Vaginal Drug Delivery: A Review of the State of the Art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jitendra, P.K.; Bansal, S.; Banik, A. Noninvasive routes of proteins and peptides drug delivery. Indian J. Pharm. Sci. 2011, 73, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Acarturk, F. Mucoadhesive vaginal drug delivery systems. Recent Pat. Drug Deliv. Formul. 2009, 3, 193–205. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef]
- Kataria, K.; Sharma, A.; Garg, T.; Goyal, A.K.; Rath, G. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv. Lett. 2014, 4, 79–86. [Google Scholar] [CrossRef]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef]
- Zou, P.; Suo, J.; Nie, L.; Feng, S. Temperature-responsive biodegradable star-shaped block copolymers for vaginal gels. J. Mater. Chem. 2012, 22, 6316–6326. [Google Scholar] [CrossRef]
- Nie, L.; Zou, P.; Feng, S.; Suo, J. Temperature-sensitive star-shaped block copolymers hydrogels for an injection application: Phase transition behavior and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Appl. Sci. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Rathbone, M.J.; Woodrow, K.A. Vaginal Drug Delivery in Focal Controlled Drug Delivery; Domb, A.J., Khan, W., Eds.; Springer: New York, NY, USA, 2014; pp. 607–651. [Google Scholar]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Gilbert, D.; Veazey, R.S.; Wang, J.; Kendrick, S.R.; Spear, G.T. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: Implications for HIV and SIV susceptibility. Aids Res. Hum. Retrovir. 2012, 28, 76–81. [Google Scholar] [CrossRef]
- Nakano, F.Y.; Leão, R.d.B.F.; Esteves, S.C. Insights into the role of cervical mucus and vaginal pH in unexplained infertility. MedicalExpress 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O. Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front. Endocrinol. 2018, 9, 568. [Google Scholar] [CrossRef]
- Manallack, D.T. The p K a distribution of drugs: Application to drug discovery. Perspect. Med. Chem. 2007, 1, 1177391X0700100003. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 500–514. [Google Scholar] [CrossRef]
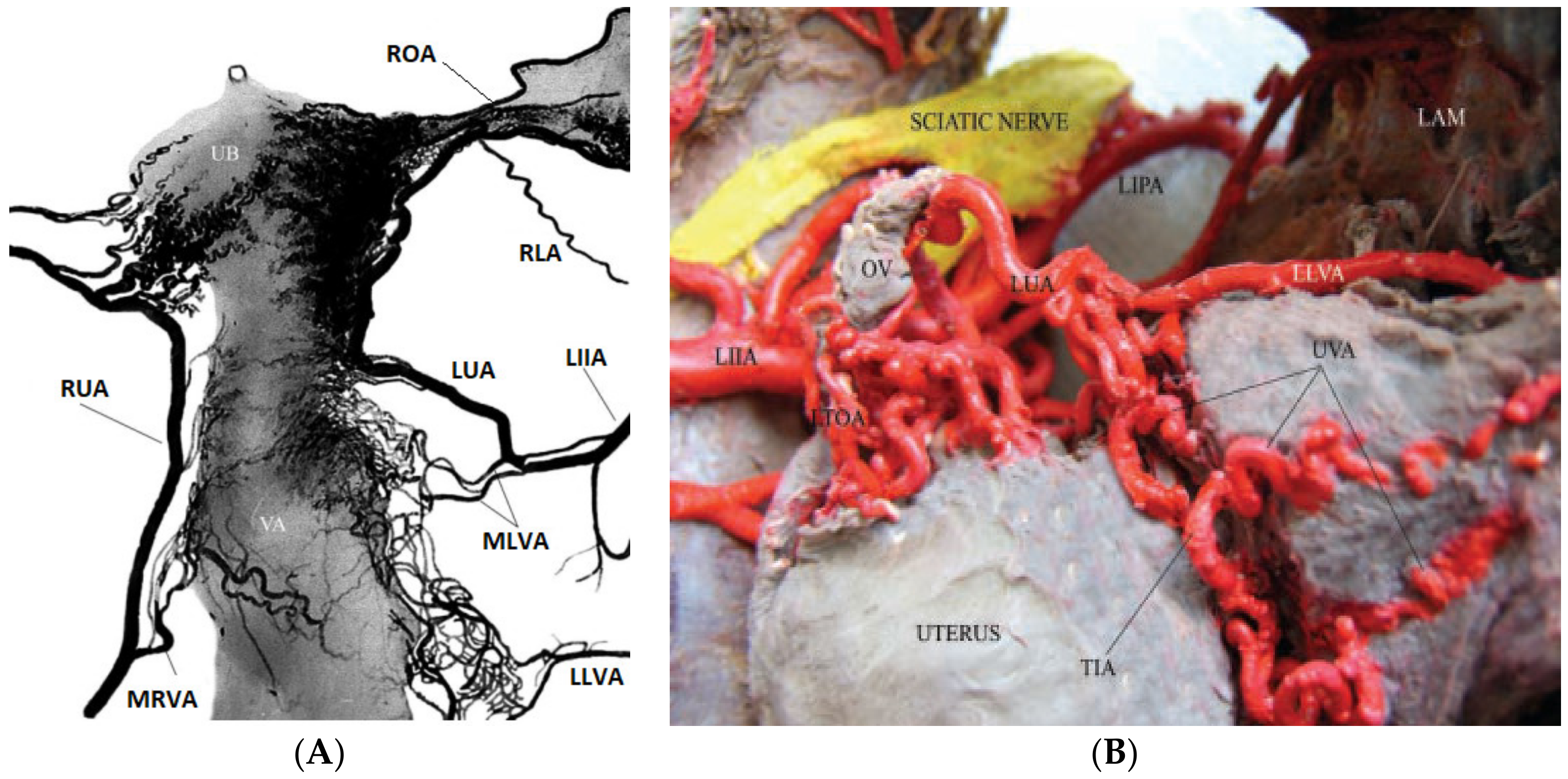
- Jaraquemada, J.M.P.; Mónaco, R.G.; Barbosa, N.E.; Ferle, L.; Iriarte, H.; Conesa, H.A. Lower uterine blood supply: Extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet. Gynecol. Scand. 2007, 86, 228–234. [Google Scholar] [CrossRef]
- Faundes, A.; Brache, V.; Alvarez, F. Pros and cons of vaginal rings for contraceptive hormone delivery. Am. J. Drug Deliv. 2004, 2, 241–250. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Gallo, M.F.; Stockton, L.L.; Schulz, K.F. Skin patch and vaginal ring versus combined oral contraceptives for contraception. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Graham, C.A.; Bass, J.L.; Bancroft, J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception 2001, 64, 51–58. [Google Scholar] [CrossRef]
- Brache, V.; Faundes, A. Contraceptive vaginal rings: A review. Contraception 2010, 82, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Novák, A.; de la Loge, C.; Abetz, L.; van der Meulen, E.A. The combined contraceptive vaginal ring, NuvaRing®: An international study of user acceptability. Contraception 2003, 67, 187–194. [Google Scholar] [CrossRef]
- Kestelyn, E.; Van Nuil, J.I.; Umulisa, M.M.; Umutoni, G.; Uwingabire, A.; Mwambarangwe, L.; Uwineza, M.; Agaba, S.; Crucitti, T.; van de Wijgert, J. High acceptability of a contraceptive vaginal ring among women in Kigali, Rwanda. PLoS ONE 2018, 13, e0199096. [Google Scholar] [CrossRef]
- Santibenchakul, S.; Jaisamrarn, U. Acceptability, tolerability, and satisfaction of a contraceptive vaginal ring (the NuvaRing) among Thai women. Asian Biomed. 2017, 10, 235–241. [Google Scholar]
- Brache, V.; Payan, L.J.; Faundes, A. Current status of contraceptive vaginal rings. Contraception 2013, 87, 264–272. [Google Scholar] [CrossRef]
- Temmerman, M. A new woman-controlled contraceptive vaginal ring: A global step forward. Lancet Glob. Health 2019, 7, e986–e987. [Google Scholar] [CrossRef]
- Roumen, F.J. Review of the combined contraceptive vaginal ring, NuvaRing®. Ther. Clin. Risk Manag. 2008, 4, 441. [Google Scholar] [CrossRef]
- Helbling, I.M.; Ibarra, J.C.; Luna, J.A. Evaluation and optimization of progesterone release from intravaginal rings using response surface methodology. J. Drug Deliv. Sci. Technol. 2015, 29, 218–225. [Google Scholar] [CrossRef]
- FDA. FDA Approves New Vaginal Ring for One Year of Birth Control. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-vaginal-ring-one-year-birth-control (accessed on 25 December 2020).
- Algorta, J.; Diaz, M.; de Benito, R.; Lefebvre, M.; Sicard, E.; Furtado, M.; Regidor, P.A.; Ronchi, C. Pharmacokinetic bioequivalence, safety and acceptability of Ornibel®, a new polymer composition contraceptive vaginal ring (etonogestrel/ethinylestradiol 11.00/3.474 mg) compared with Nuvaring®(etonogestrel/ethinylestradiol 11.7/2.7 mg). Eur. J. Contracept. Reprod Health Care 2017, 22, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Santen, R. Vaginal administration of estradiol: Effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015, 18, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; Tiitinen, A.; Affandi, B.; Brache, V.; Croxatto, H.B.; Diaz, S.; Ginsburg, J.; Gu, S.; Holma, P.; Johansson, E.; et al. Norplant® Consensus Statement and Background Review 22The Consensus Statement follows the end of Appendix B. Contraception 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Croxatt, H.B. Progestin implants for female contraception. Contraception 2002, 65, 15–19. [Google Scholar] [CrossRef]
- Coutinho, E.M. One year contraception with a single subdermal implant containing nomegestrol acetate (Uniplant). Contraception 1993, 47, 97–105. [Google Scholar] [CrossRef]
- Ernst, D. FDA Approves New Contraceptive Patch; Haymarket Media, Inc.: London, UK, 2020. [Google Scholar]
- Merkatz, R.B.; Plagianos, M.; Hoskin, E.; Cooney, M.; Hewett, P.C.; Mensch, B.S. Acceptability of the nestorone®/ethinyl estradiol contraceptive vaginal ring: Development of a model; implications for introduction. Contraception 2014, 90, 514–521. [Google Scholar] [CrossRef]
- Micks, E.A.; Jensen, J.T. A technology evaluation of Annovera: A segesterone acetate and ethinyl estradiol vaginal ring used to prevent pregnancy for up to one year. Expert Opin. Drug Deliv. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Stifani, B.M.; Plagianos, M.; Vieira, C.S.; Merkatz, R.B. Factors associated with nonadherence to instructions for using the Nestorone®/ethinyl estradiol contraceptive vaginal ring. Contraception 2018, 97, 415–421. [Google Scholar] [CrossRef]
- Larner, J.; Reel, J.; Blye, R. Circulating concentrations of the antiprogestins CDB-2914 and mifepristone in the female rhesus monkey following various routes of administration. Hum. Reprod. 2000, 15, 1100–1106. [Google Scholar] [CrossRef]
- Brache, V.; Sitruk-Ware, R.; Williams, A.; Blithe, D.; Croxatto, H.; Kumar, N.; Kumar, S.; Tsong, Y.-Y.; Sivin, I.; Nath, A. Effects of a novel estrogen-free, progesterone receptor modulator contraceptive vaginal ring on inhibition of ovulation, bleeding patterns and endometrium in normal women. Contraception 2012, 85, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Saxena, B.B.; Han, Y.A.; Fu, D.; Rathnam, P.; Singh, M.; Laurence, J.; Lerner, S. Sustained release of microbicides by newly engineered vaginal rings. Aids 2009, 23, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Wieder, D.R.; Pattimakiel, L. Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing®). Int. J. Womens Health 2010, 2, 401. [Google Scholar] [CrossRef] [PubMed]
- Roumen, F.J. The contraceptive vaginal ring compared with the combined oral contraceptive pill: A comprehensive review of randomized controlled trials. Contraception 2007, 75, 420–429. [Google Scholar] [CrossRef]
- Dieben, T.O.; Roumen, F.J.; Apter, D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet. Gynecol. 2002, 100, 585–593. [Google Scholar] [PubMed]
- Oddsson, K.; Leifels-Fischer, B.; de Melo, N.R.; Wiel-Masson, D.; Benedetto, C.; Verhoeven, C.H.; Dieben, T.O. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: A 1-year randomized trial. Contraception 2005, 71, 176–182. [Google Scholar] [CrossRef]
- Patra, A.; Roy, A.K.; Gupta, G.; Maikhuri, J.P.; Kumar, M.; Shukla, P.K.; Jain, R.K.; Batra, S.; Singh, V. Discovery of substituted isoxazolecarbaldehydes as potent spermicides, acrosin inhibitors and mild anti-fungal agents. Hum. Reprod. 2005, 20, 2301–2308. [Google Scholar] [CrossRef][Green Version]
- Maikhuri, J.P.; Dwivedi, A.K.; Dhar, J.D.; Setty, B.S.; Gupta, G. Mechanism of action of some acrylophenones, quinolines and dithiocarbamate as potent, non-detergent spermicidal agents. Contraception 2003, 67, 403–408. [Google Scholar] [CrossRef]
- Bernstein, G.S. Physiological aspects of vaginal contraception. Contraception 1974, 9, 333–345. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Dixit, S. Novel spermicidal agent—A review. Der Pharma Chem. 2010, 4, 278–297. [Google Scholar]
- Reddy, K.V.R.; Aranha, C.; Gupta, S.M.; Yedery, R.D. Evaluation of antimicrobial peptide nisin as a safe vaginal contraceptive agent in rabbits: In vitro and in vivo studies. Reproduction 2004, 128, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Batar, I. State-of-the-art of non-hormonal methods of contraception: II. Chemical barrier contraceptives. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2010, 15, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tepper, N.K.; Krashin, J.W.; Curtis, K.M.; Cox, S.; Whiteman, M.K. Update to CDC’s US medical eligibility criteria for contraceptive use, 2016: Revised recommendations for the use of hormonal contraception among women at high risk for HIV infection. Mmwr. Morb. Mortal. Wkly. Rep. 2017, 66, 990. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.A.; Ratcliffe, S.J.; Sammel, M.D.; Whittaker, P.G. A self-assessment efficacy tool for spermicide contraceptive users. Am. J. Obstet. Gynecol. 2016, 214, 264.e261–264.e267. [Google Scholar] [CrossRef]
- Vignini, A.; Buldreghini, E.; Nanetti, L.; Amoroso, S.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L.; Balercia, G. Free thiols in human spermatozoa: Are Na+/K+-ATPase, Ca2+-ATPase activities involved in sperm motility through peroxynitrite formation? Reprod. Biomed. Online 2009, 18, 132–140. [Google Scholar] [CrossRef]
- Mendez, F.; Castro, A.; Ortega, A. Use effectiveness of a spermicidal suppository containing benzalkonium chloride. Contraception 1986, 34, 353–362. [Google Scholar]
- Xiao-Hui, D.; Qi, C.; Waller, D.P.; Kaminski, J.; Zaneveld, L.J.D. Comparison of the spermicidal activity and acute toxicity of nonoxynol-9 and agent 741[alkylphenoxy polyethoxy ethanol(10)]. Contraception 1986, 33, 1–5. [Google Scholar] [CrossRef]
- Livingston, G.M.; Thornburgh, D.B.; Longmore, J. Antiseptic Spermicidal Composition and Means for Its Application. U.S. Patent 8,518,434, 27 August 2013. [Google Scholar]
- D’Cruz, O.J.; Uckun, F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef]
- Digenis, G.A.; Nosek, D.; Mohammadi, F.; Darwazeh, N.B.; Anwar, H.S.; Zavos, P.M. Novel vaginal controlled-delivery systems incorporating coprecipitates of nonoxynol-9. Pharm. Dev. Technol. 1999, 4, 421–430. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chien, Y.W. Development and evaluation of a mucoadhesive drug delivery system for dual-controlled delivery of nonoxynol-9. J. Control. Release 1996, 39, 93–103. [Google Scholar] [CrossRef]
- Malcolm, K.; Woolfson, D.; Russell, J.; Andrews, C. In vitro release of nonoxynol-9 from silicone matrix intravaginal rings. J. Control. Release 2003, 91, 355–364. [Google Scholar] [CrossRef]
- Miari, V.F.; Ison, C.A. Is There a Role for Topical Antiseptics in the Treatment of Gonorrhoea? BMJ Publishing Group Ltd.: London, UK, 2017. [Google Scholar]
- Roddy, R.E.; Zekeng, L.; Ryan, K.A.; Tamoufé, U.; Tweedy, K.G. Effect of nonoxynol-9 gel on urogenital gonorrhea and chlamydial infection: A randomized controlled trial. JAMA 2002, 287, 1117–1122. [Google Scholar] [CrossRef]
- Wilkinson, D.; Ramjee, G.; Tholandi, M.; Rutherford, G.W. Nonoxynol-9 for preventing vaginal acquisition of sexually transmitted infections by women from men. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Florez, M.; Díaz, E.S.; Brito, I.; González, J.; Morales, P. N,N′–Dithiobisphthalimide, a disulfide aromatic compound, is a potent spermicide agent in humans. Syst. Biol. Reprod. Med. 2011, 57, 309–317. [Google Scholar] [CrossRef]
- Schill, W.B.; Wolff, H.H. Ultrastructure of human spermatozoa in the presence of the spermicide nonoxinol-9 and a vaginal contraceptive containing nonoxinol-9. Andrologia 1981, 13, 42–49. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Tucker, L.D.; Anderson, D.J. The Molecular Basis of Nonoxynol-9-Induced Vaginal Inflammation and Its Possible Relevance to Human Immunodeficiency Virus Type 1 Transmission. J. Infect. Dis. 2001, 184, 418–428. [Google Scholar] [CrossRef]
- Pandey, R.R.; Srivastava, A.; Pachauri, S.D.; Khandelwal, K.; Naqvi, A.; Malasoni, R.; Kushwaha, B.; Kumar, L.; Maikhuri, J.P.; Pandey, G.; et al. Design and synthesis of gamma-butyrolactone derivatives as potential spermicidal agents. Bioorganic Med. Chem. Lett. 2014, 24, 3903–3906. [Google Scholar] [CrossRef]
- Nelson, A.L. An overview of properties of Amphora (Acidform) contraceptive vaginal gel. Expert Opin. Drug Saf. 2018, 17, 935–943. [Google Scholar] [CrossRef]
- Yang, M.; Zhi, R.; Lu, L.; Dong, M.; Wang, Y.; Tian, F.; Xia, M.; Hu, J.; Dai, Q.; Jiang, S. A CCR5 antagonist-based HIV entry inhibitor exhibited potent spermicidal activity: Potential application for contraception and prevention of HIV sexual transmission. Eur. J. Pharm. Sci. 2018, 117, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ceña-Diez, R.; Martin-Moreno, A.; de la Mata, F.J.; Gómez-Ramirez, R.; Muñoz, E.; Ardoy, M.; Muñoz-Fernández, M.Á. G1-S4 or G2-S16 carbosilane dendrimer in combination with Platycodin D as a promising vaginal microbicide candidate with contraceptive activity. Int. J. Nanomed. 2019, 14, 2371. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Yiv, S.H.; Waurzyniak, B.; Uckun, F.M. Contraceptive efficacy and safety studies of a novel microemulsion-based lipophilic vaginal spermicide. Fertil. Steril. 2001, 75, 115–124. [Google Scholar] [CrossRef]
- Jangir, S.; Bala, V.; Lal, N.; Kumar, L.; Sarswat, A.; Kumar, L.; Kushwaha, B.; Singh, P.; Shukla, P.K.; Maikhuri, J.P.; et al. A unique dithiocarbamate chemistry during design & synthesis of novel sperm-immobilizing agents. Org. Biomol. Chem. 2014, 12, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lal, N.; Kumar, L.; Verma, V.; Kumar, R.; Kumar, L.; Singh, V.; Mishra, R.K.; Sarswat, A.; Jain, S.K.; et al. Novel trichomonacidal spermicides. Antimicrob. Agents Chemother. 2011, 55, 4343–4351. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Chaki, S.P.; Misro, M.M. Assessment of human sperm function after hydrogen peroxide exposure. development of a vaginal contraceptive. Contraception 2002, 66, 187–192. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Venkatachalam, T.K.; Uckun, F.M. Novel Thiourea Compounds as Dual-Function Anti-HIV and Spermicidal Agents. Fertil. Steril. 2000, 74, S72–S73. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Sharma, V.L.; Kumaria, N.; Kiran Kumar, S.T.V.S.; Srivastava, P.K.; Ansari, A.H.; Maikhuri, J.P.; Gupta, G.; Dhar, J.D.; Roy, R.; et al. Synthesis of disulfide esters of dialkylaminocarbothioic acid as potent, non-detergent spermicidal agents. Bioorganic Med. Chem. 2007, 15, 6642–6648. [Google Scholar] [CrossRef]
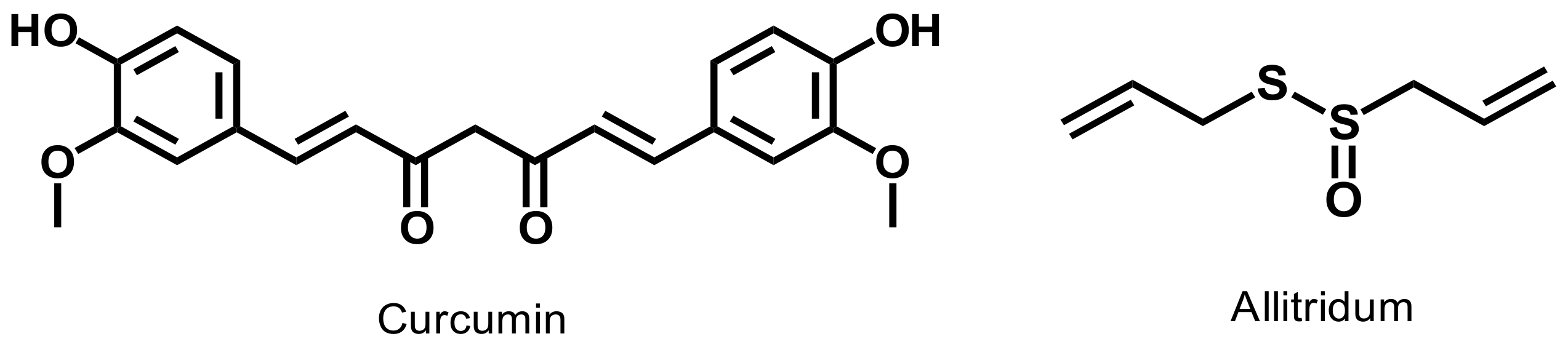
- Naz, R.K.; Lough, M.L.; Barthelmess, E.K. Curcumin: A novel non-steroidal contraceptive with antimicrobial properties. Front. Biosci. Elite Ed. 2016, 8, 113–128. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Unamba-Oparah, I.C.; Odoemenam, V.U.; Etuk, I.F.; Okoli, I.C. The potentiality of medicinal plants as the source of new contraceptive principles in males. N. Am. J. Med. Sci. 2011, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Datta, M.; Biswas, S.; Pal, A.K.; Malakar, D.; Bhattacharyya, A.K.; Bhattacharya, S.; Kobayashi, H. Immotilin, a novel sperm immobilizing protein. Fertil. Steril. 2003, 79, 1673–1675. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Sutyak, K.E.; Anderson, R.A.; Dover, S.E.; Feathergill, K.A.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Spermicidal Activity of the Safe Natural Antimicrobial Peptide Subtilosin. Infect. Dis. Obstet. Gynecol. 2008, 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Aranha, C.; Gupta, S.; Reddy, K.V. Contraceptive efficacy of antimicrobial peptide Nisin: In vitro and in vivo studies. Contraception 2004, 69, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaur, I.P.; Prabha, V. Evaluation of antifertility effect of gel formulation containing sperm immobilizing factor: In vitro and in vivo studies. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 81, 67–74. [Google Scholar] [CrossRef]
- Kaur, S.; Prabha, V.; Sarwal, A. Receptor Mediated Agglutination of Human Spermatozoa by Spermagglutinating Factor Isolated From Staphylococcus aureus. J. Urol. 2010, 184, 2586–2590. [Google Scholar] [CrossRef]
- Suthutvoravut, S.; Kamyarat, O. Spermicidal effects of lemon juice and juices from other natural products. Agric. Nat. Resour. 2016, 50, 133–138. [Google Scholar] [CrossRef][Green Version]
- Lakshmi, V.; Saxena, A.; Mishra, S.K.; Raghubir, R.; Srivastava, M.N.; Jain, R.K.; Maikhuri, J.P.; Gupta, G. Spermicidal Activity of Bivittoside D from Bohadschia vitiensis. Arch. Med. Res. 2008, 39, 631–638. [Google Scholar] [CrossRef]
- Saha, P.; Majumdar, S.; Pal, D.; Pal, B.C.; Kabir, S.N. Evaluation of spermicidal activity of MI-saponin A. Reprod. Sci. 2010, 17, 454–464. [Google Scholar] [CrossRef]
- Zhou, B.; Qiu, Z.; Liu, G.; Liu, C.; Zhang, J. Spermicidal and antigonococcal effects of tannins from pomegranate rind. J. Med. Plants Res. 2012, 6, 1334–1339. [Google Scholar] [CrossRef]
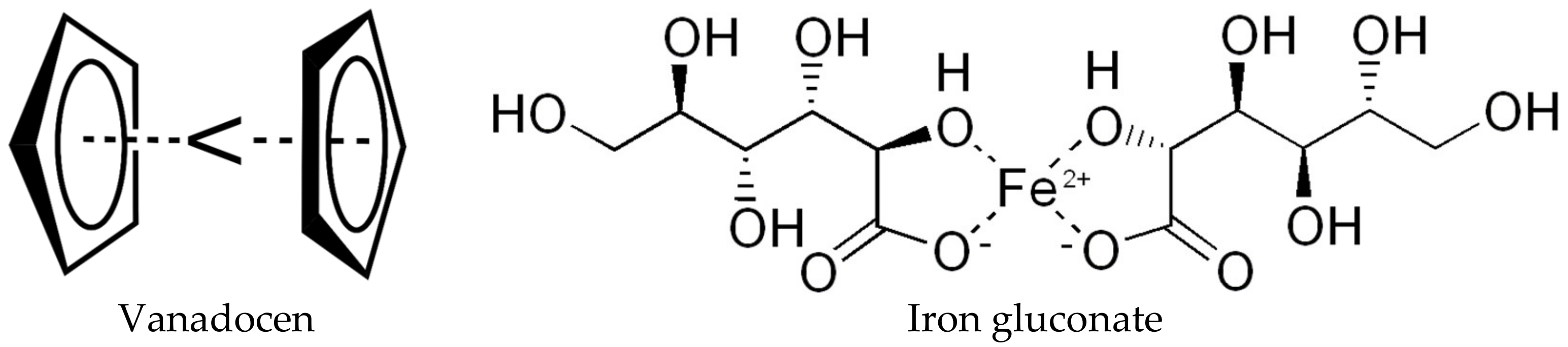
- D’Cruz, O.J.; Uckun, F.M. Vaginal contraceptive activity of a chelated vanadocene. Contraception 2005, 72, 146–156. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Dong, Y.; Uckun, F.M. Potent dual anti-HIV and spermicidal activities of novel oxovanadium(V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase. Biochem. Biophys. Res. Commun. 2003, 302, 253–264. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Ghosh, P.; Uckun, F.M. Spermicidal activity of metallocene complexes containing vanadium(IV) in humans. Biol. Reprod. 1998, 58, 1515–1526. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Vassilev, A.; Uckun, F.M. Evaluation of boar sperm as a model system to study the mechanism of spermicidal activity of vanadocenes. Biochem. Biophys. Res. Commun. 2000, 270, 826–830. [Google Scholar] [CrossRef]
- Less, G.B.; Ockwig, N.W.; Rasmussen, P.G.; Smith, G.D.; Keller, L.M.; Drach, J.C. Vanadium Complex of 2-(2′-Pyridyl)-4,5-dicyanoimidazole Showing Spermicidal and Cytotoxic Properties. Inorg. Chem. 2006, 45, 7105–7110. [Google Scholar] [CrossRef]
- Hong, C.Y.; Lee, M.F.; Lai, L.J.; Wang, C.P. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia 1994, 26, 61–65. [Google Scholar] [CrossRef]
- Calamera, J.C.; Giovenco, P.; Quiros, M.C.; Brugo, S.; Dondero, F.; Nicholson, R.F. Effect of lipid peroxidation upon human spermatic adenosinetriphosphate (ATP). Relationship with motility, velocity and linearity of the spermatozoa. Andrologia 1989, 21, 48–54. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Buckingham, D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J. Reprod. Fertil. 1993, 98, 257–265. [Google Scholar] [CrossRef]
- Han, Y.A.; Singh, M.; Saxena, B.B. Development of vaginal rings for sustained release of nonhormonal contraceptives and anti-HIV agents. Contraception 2007, 76, 132–138. [Google Scholar] [CrossRef]
- Saxena, B.B.; Singh, M.; Gospin, R.M.; Chu, C.C.; Ledger, W.J. Efficacy of nonhormonal vaginal contraceptives from a hydrogel delivery system. Contraception 2004, 70, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C 2020, 117, 111266. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.; Fellman, J.H.; Wolf, D.P. Human spermicidal activity of inorganic and organic oxidants. Fertil. Steril. 2001, 76, 157–162. [Google Scholar] [CrossRef]
- LeVeen, H.H.; LeVeen, R.F.; LeVeen, E.G. The mythology of povidone-iodine and the development of self-sterilizing plastics. Surg. Gynecol. Obstet. 1993, 176, 183–190. [Google Scholar]
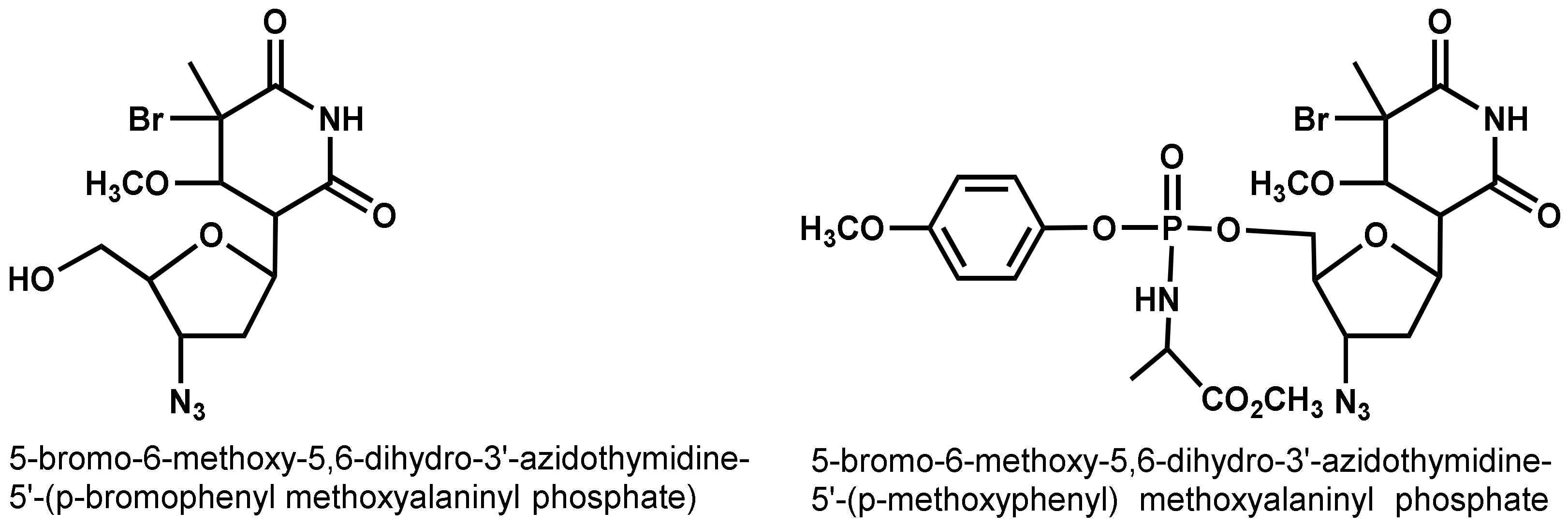
- Jan, S.T.; Shih, M.J.; Venkatachalam, T.K.; D’Cruz, O.J.; Chen, C.L.; Uckun, F.M. Synthesis of dual function (5R,6R)- and (5S,6S)-5-bromo-6-methoxy-5,6-dihydro-AZT-5′-(para-bromophenyl methoxyalaninyl phosphate) as novel spermicidal and anti-HIV agents. Antivir. Chem. Chemother. 1999, 10, 39–46. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Zhu, Z.; Yiv, S.H.; Chen, C.-L.; Waurzyniak, B.; Uckun, F.M. WHI-05, a novel bromo-methoxy substituted phenyl phosphate derivative of zidovudine, is a dual-action spermicide with potent anti-HIV activity. Contraception 1999, 59, 319–331. [Google Scholar] [CrossRef]
- Bharitkar, Y.P.; Banerjee, M.; Kumar, S.; Paira, R.; Meda, R.; Kuotsu, K.; Mondal, N.B. Search for a potent microbicidal spermicide from the isolates of Shorea robusta resin. Contraception 2013, 88, 133–140. [Google Scholar] [CrossRef]
- Paira, P.; Hazra, A.; Kumar, S.; Paira, R.; Sahu, K.B.; Naskar, S.; Saha, P.; Mondal, S.; Maity, A.; Banerjee, S.; et al. Efficient synthesis of 3,3-diheteroaromatic oxindole analogues and their in vitro evaluation for spermicidal potential. Bioorganic Med. Chem. Lett. 2009, 19, 4786–4789. [Google Scholar] [CrossRef]
- Jones, R.E.; Lopez, K.H. Chapter 13-Contraception. In Human Reproductive Biology, 4th ed.; Jones, R.E., Lopez, K.H., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 245–269. [Google Scholar]
| Contraceptive | Product Name | Chemical Composition | Protect against STD | Hormones/Drug | Effectiveness Duration | Ref |
|---|---|---|---|---|---|---|
| Vaginal rings | Nuvaring® (MSD, Oss, the Netherlands) | Ethylene-vinyl acetate copolymer and magnesium stearate | No | Ethinyl estradiol and etonogestrel | One month | [64] |
| Progering (Silesia; Santiago, Chile) | Silicone | No | Progesterone | Three months | [65] | |
| AnnoveraTM | Silicone elastomer | No | Nestorone® and ethinyl estradiol | One year | [66] | |
| Ornibel® (Exeltis Healthcare, Spain) | polyurethane core, and ethylene-vinyl acetate membrane | No | Etonogestrel and ethinyl estradiol | Three weeks | [67] | |
| Femring® | Silicone | No | 17β-estradiol-3-acetate | Three months | [68] | |
| Implants | Norplant® | Six flexible closed capsules made of silicone rubber tubing | No | Progestin levonorgestrel | Five years | [69] |
| Implanon® | Ethylene-vinyl acetate copolymer | No | Etonogestrel | Five years | [70] | |
| Uniplant | Silicone rubber | Nomegestrol acetate | One year | [71] | ||
| Patch | Ortho EvraTM | Polyethylene, polyester | No | Norelgestromin /ethinyl estradiol | Three weeks | [25] |
| Twirla | Polyacrylate, polyisobutylene adhesive layer | No | Levonorgestrel/ethinyl estradiol | Every seven days | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalalvandi, E.; Jafari, H.; Amorim, C.A.; Petri, D.F.S.; Nie, L.; Shavandi, A. Vaginal Administration of Contraceptives. Sci. Pharm. 2021, 89, 3. https://doi.org/10.3390/scipharm89010003
Jalalvandi E, Jafari H, Amorim CA, Petri DFS, Nie L, Shavandi A. Vaginal Administration of Contraceptives. Scientia Pharmaceutica. 2021; 89(1):3. https://doi.org/10.3390/scipharm89010003
Chicago/Turabian StyleJalalvandi, Esmat, Hafez Jafari, Christiani A. Amorim, Denise Freitas Siqueira Petri, Lei Nie, and Amin Shavandi. 2021. "Vaginal Administration of Contraceptives" Scientia Pharmaceutica 89, no. 1: 3. https://doi.org/10.3390/scipharm89010003
APA StyleJalalvandi, E., Jafari, H., Amorim, C. A., Petri, D. F. S., Nie, L., & Shavandi, A. (2021). Vaginal Administration of Contraceptives. Scientia Pharmaceutica, 89(1), 3. https://doi.org/10.3390/scipharm89010003







