Abstract
The World Health Organization (WHO) officially announced coronavirus disease 2019 (COVID-19) as a pandemic in March 2020. Unfortunately, there are still no approved drugs for either the treatment or the prevention of COVID-19. Many studies have focused on repurposing established antimalarial therapies, especially those that showed prior efficacy against Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), such as chloroquine and hydroxychloroquine, against COVID-19 combined with azithromycin. These classes of drugs potentially induce prolongation of the QT interval, which might lead to lethal arrhythmia. Beta-blockers, as a β-adrenergic receptor (β-AR) antagonist, can prevent an increase in the sympathetic tone, which is the most important arrhythmia trigger. In this literature review, we aimed to find the effect of administering azithromycin, chloroquine, and hydroxychloroquine on cardiac rhythm disorders and our findings show that bisoprolol, as a cardio-selective beta-blocker, is effective for the management of the QT (i.e., the start of the Q wave to the end of the T wave) interval prolongation in COVID-19 patients.
1. Introduction
On 31 December 2019, the China Health Authority reported cases of pneumonia with unknown etiology in Wuhan City, Central China, to the World Health Organization (WHO) [1,2]. On 30 January 2020, the WHO reported 82 confirmed cases in 18 other countries and announced coronavirus disease 2019 (COVID-19) as an International Emergency Health Care Community (PHEIC) [3,4].
On 11 March 2020, the WHO officially announced COVID-19 as a pandemic. By 22 March 2020, the worldwide confirmed number of cases had reached 303,000, with more than 12,900 deaths in 150 countries. The data from China showed that some patients suffered from respiratory failure, septic shock, and multi-organ dysfunction, resulting in the death of 4% of patients, even if case fatality rate has been lower in any other region in the world [5].
Presently, there are no approved drugs for either the treatment or the prevention of COVID-19 [6]. There has been significant enthusiasm for repurposing existing medications and speeding up formative antiviral medicines, for example, those for flu, hepatitis B (HBV), hepatitis C (HCV), and filoviruses, to permit a quick turn of events [7]. The quick genomic sequencing of COVID-19 encouraged this cycle, presenting a correlation in structural proteins with MERS-CoV, SARS-CoV, and other morbific infections [8].
Currently, there have been 35 trials [9] that have examined the utilization of the antimalarial drugs chloroquine and hydroxychloroquine for COVID-19 treatment [10]. Azithromycin was recommended as having in vitro antiviral activity against different infections and is viable for treating bacterial pneumonia [11]. The clinical results among COVID-19 patients indicate infection disposal after administration of these treatments; however, the risk of lethal arrhythmias was increased, such as QT prolongation, torsade de pointes (TdP), and sudden cardiac death (SCD) [12]. The QT interval is the time from the start of the Q wave to the end of the T wave, which represents the repolarization of the ventricle, while the corrected QT interval (QTc) estimates the QT interval at a standard heart rate. It is considered QT prolongation if the QTc is over 440 ms in men and 460 ms in women [12].
As chloroquine, hydroxychloroquine, and azithromycin are the only choices of current standard treatments, the risk of life-threatening arrhythmia from these medications is not anticipated. On the other hand, these COVID-19 antimicrobial medications decrease the mortality rate by 5%, thereby providing generous advantages in contrast to drug-induced sudden death, considering that the absolute levels of risk and benefits are unclear at present [13].
Beta-blockers broadly utilize particles that can alienate β-adrenergic receptors (β-AR); thus, they diminish the adrenergic tone/incitement of the heart muscle and pacemaker cells [14]. A diminished adrenergic tone shows less contractility of the heart muscle, brings down the pulse of the cardiac rhythm, and prevents arrythmia [15].
An integrated understanding of this complex system is essential to optimize therapies aimed to prevent arrhythmias. Therefore, we investigated the effect of azithromycin, chloroquine, and hydroxychloroquine as a treatment of COVID-19 on QT interval prolongation and the possible benefits of beta-blockers for the management of QT prolongation induced by said COVID-19 treatments.
2. Materials and Methods
We used the keywords “COVID-19”, “QT Prolongation”, and “Beta-blockers” to search for relevant reports on the PubMed and Google Scholar platforms. The available articles were reviewed based on the type of study (e.g., in vitro, in vivo, clinical use in hospitals, and clinical trials). All existing publications until 30 July 2020 were included in this study. To enrich our discussion, the latest studies evaluating the mechanism of beta-blockers in controlling the QT prolongation effect post-antiviral and/or antibiotic therapy for COVID-19 were also identified and reviewed.
3. COVID-19 Treatment and Arrhythmia
The single available option is to use broad-spectrum antiviral drugs, such as nucleoside analogs as well as HIV-protease inhibitors, which can attenuate specific viral infections, thereby resulting in antivirus availability [16]. The regiment of treatment includes twice daily oral administration of 75 mg of oseltamivir, 500 mg of lopinavir, 500 mg of ritonavir, and 0.25 g of intravenous ganciclovir for 3–14 days [17]. Other reports have suggested that the broad-spectrum antivirals remdesivir and chloroquine are effective enough to control 2019 novel coronavirus (2019-nCoV) infections in vitro. These antiviral regiments have been safely administered in human patients [18].
A study from France showed a higher rate of SARS-CoV-2 clearance by day 6 in 14 patients treated with hydroxychloroquine compared with the control group [19]. A result from a case series study suggested that the combination of hydroxychloroquine and azithromycin resulted in a higher level of virus eradication and clinical improvement than the hydroxychloroquine without azithromycin therapy group [20]. Safety problems were found using hydroxychloroquine and azithromycin, including the QTc prolongation potential, which reached a greater potential when both agents were used together. Additionally, another study reported the termination of high-dose chloroquine due to mortality issues [21,22].
3.1. Azithromycin
Azithromycin has antiviral activity against other viruses in vitro and is a medication for bacterial pneumonia [13]. According to a French case series study, to lower the amount of the viral load, it was more beneficial and effective to employ azithromycin than hydroxychloroquine in spite of the potential risk of QT prolongation, TdP, and SCD [23]. A rise in the action potential duration (APD) of ventricular myocytes, usually occurring due to a decrease in the net repolarizing current, resulted in the QT interval prolongation showing on an electrocardiogram (ECG) [24]. Ventricular APD prolongation as a result of the inhibition of the rapid component of the delayed rectifier potassium current (IKr) was the primary mechanism underlying the therapeutic effects of Class III antiarrhythmic agents (e.g., dofetilide and D-sotalol). In contrast, APD and QT interval prolongation can be arrhythmogenic and can cause malignant ventricular tachyarrhythmia [25]. However, azithromycin has been proven safe without cautions in routine clinical practice [26].
3.2. Chloroquine
Chloroquine has been investigated as a broad-spectrum antiviral drug. Chloroquine influences negative virus–receptor binding and invokes infections by impairing glycosylated angiotensin-converting enzyme 2 (ACE-2) terminals [27,28]. Chloroquine is associated with quinidine, and the latter is typically used as an antiarrhythmic drug in Brugada syndrome and idiopathic ventricular fibrillation forms. Quinidine also has a prolonged QT effect and is associated with QT-related malignant arrhythmias. Fortunately, the QT prolongation effect of chloroquine is mild and usually not clinically significant in patients without a history of long QT syndrome (LQTS) [29]. Hydroxychloroquine (HCQ) sulfate, a less toxic form of chloroquine, is widely used in the treatment of chronic autoimmune diseases without significant effects on the ECG parameters, and as many as 23,149 patients have demonstrated inhibition of the SARS-CoV-2 infection following this treatment [30,31].
HCQ potentially causes QT interval prolongation and TdP in high-risk individuals [32]. Not all patients who suffer from drug-induced QTc prolongation will develop TdP. These side effects are rare and can be prevented in combination with other drugs, such as azithromycin, which is also recommended for the management of COVID-19 [33]. When HCQ therapy is initiated in patients or those suspected to have COVID-19, clinicians must identify all individuals who are at high risk of developing QT prolongation. It is recommended to perform an ECG evaluation in all COVID-19 patients before beginning HCQ therapy, as not all patients who take HCQ will develop QT prolongation. This depends on the underlying diseases, genetic disorders associated with the QT interval, electrolyte disturbances, and drug interactions [34].
Patients can be categorized into the low-risk group if they have a normal QTc interval, into the moderate-risk group if they have a little prolongation (up to 500 ms), or into the high-risk group if they have a prolonged QTc interval (more than 500 ms). HCQ can be safely administered in the low-risk group. In the moderate-risk group (according to the QTC interval), HCQ should be cautiously administered while addressing the risk factors. The high-risk group will have one of the HCQ-induced arrhythmia risk factors, namely, the presence of structural heart disease or left ventricular hypertrophy (particularly ventricular dysfunction), a previous history of ventricular arrhythmias or syncope, the presence of implantable heart rhythm devices, co-treatment with drugs that can prolong the QT interval (macrolides, quinolones, antihistamines, antivirus, antiarrhythmics, antifungal drugs, etc.). The high-risk group is divided into two risk groups based on the COVID-19 risks. Lower-risk patients should not be treated with HCQ or it can be cautiously administered. Higher-risk patients may be treated using HCQ with close monitoring [35].
3.3. Hydroxychloroquine–Azithromycin Combination Therapy
Hydroxychloroquine–azithromycin combination therapy has a high probability of QT interval prolongation and of resulting lethal arrhythmia. In a previous study, 37 of 90 patients were treated with hydroxychloroquine monotherapy, and the other 53 patients were given hydroxychloroquine–azithromycin combination therapy. The hydroxychloroquine–azithromycin combination group had a greater median (interquartile range) change in the QT interval (23 (10–40) ms) compared with the hydroxychloroquine single therapy group (5.5 (−15.5 to 34.25) ms; p = 0.03). Seven patients (19%) who received monotherapy hydroxychloroquine developed a prolonged QTc of 500 ms or more, and three patients (3%) developed a QTc change of approximately 60 ms or more. In the hydroxychloroquine–azithromycin combination group, 11 of the 53 patients (21%) developed a QTc of 500 ms or more, and 7 of 53 (13%) developed a QTc of 60 ms or more [33].
Tisdale et al. developed a risk score tool to predict the use of QT prolongation, including the age, gender, diuretic drugs, serum potassium levels, QTc, QT extending drugs, acute myocardial infarction, heart failure, and sepsis conditions. The QT prolongation risk score is divided into low, moderate, and high. Scores of less than 7 are categorized as low risk and have a 15% incidence of QTc prolongation. Scores from 7 to 10 are categorized as moderate risk, reaching 37% of the incidence of QTc prolongation, and scores of more than 11 are categorized as high risk with an incidence of QTc prolongation of 73% [36,37]. COVID-19 patients treated with hydroxychloroquine or chloroquine with or without azithromycin were considered by evaluating the following parameters: calculating Tisdale′s risk scores, monitoring the QT interval using ECG, the termination of useless drugs that can potentially induce prolonged QT, and the level of serum electrolytes (potassium and magnesium) [38].
4. Beta-Blockers
4.1. β-Adrenergic Physiology
The sympathetic nervous system (SNS) has a key role in the neurohormonal control of cardiovascular functions. The SNS mediates the neural and hormonal responses of fear and stress, and exercises with the cardiovascular functions to meet the increasing demands of the body with a rapid increase in the cardiac output (fight or flight response) [39]. Sympathetic activation triggers an increase in heart rate (chronotropic), the force of contraction (inotropy), relaxation rate (lusitropy), and conduction (dromotopy) [40]. Positive chronotropy is generated by phosphorylation changes in intracellular Ca coupling similar to increasing a cyclic adenosine 3′,5′-cyclic monophosphate (cAMP) mediated response that, together, accelerate diastolic depolarization in the sinoatrial (SA) node through the membrane and Ca-coupled clocks, leading to faster impulse generation [41]. Faster rates of heart and K+ channel phosphorylation typically abbreviate cardiac repolarization, and it is necessary to facilitate a shorter cycle length to counterbalance the increase in ICal required to increase the contractility [42,43].
If the spontaneous diastolic depolarization reaches the threshold voltage, the action potential upstroke is activated. The “Funny” current (If) is a well-known ionic pacemaker current, responsible for phase 4 spontaneous depolarization. Channels that accommodate If currents are triggered by hyperpolarization (increasingly negative voltage) and primarily canalize sodium ions. After the membrane voltage becomes more negative than −50 mV, If is activated. Decreasing the If activity causes a delay in the time to reach the threshold potential (TP), which leads to a prolongation in phase 4 [44]. Phase 4 depolarization in a pacemaker cell is depicted in Figure 1.
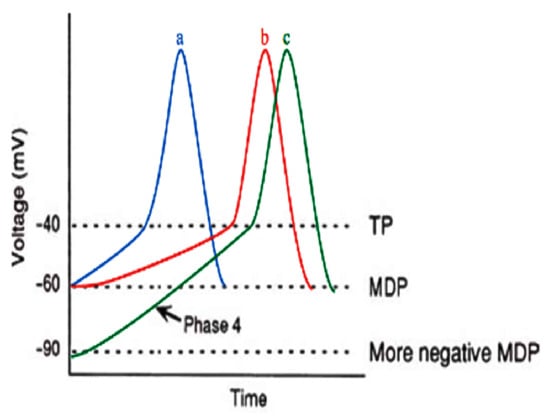
Figure 1.
Phase 4 depolarization in a pacemaker cell [45]. MDP, maximum diastolic potential; TP, threshold potential. Blue (a) curve is normal action potential; red (b) curve is reducing If, which renders the slope of phase 4 less steep, therefore, the time required to reach threshold TP is elevated; green (c) curve means the MDP is more negative, thus the time required to reach TP is augmented.
Parasympathetic (cholinergic) stimulation leads to antagonistic sympathetic stimulation, thereby decelerating the depolarization process [46]. Thus, the excitation rate is suppressed. In addition, the stimulation enhances acetylcholine-sensitive K+ channels that are accessible during the resting period. Positively charged K+ ions come out through this “inward rectifier” channel, which varies from the K+ channel that is favorable in phase 3 repolarization, generating an outward current that accommodates the diastolic potential to be more negative. The whole effect of If suppression, including the more negative maximum diastolic potential (MDP) and the less negative threshold level, decelerates the excitation level and leads to a reduced intrinsic heart rate [44].
Beta-blocker usage results in an alteration in the autonomic nervous system, which has an impact on the SA node′s firing rate. Beta-blockers stimulate an inhibition of the beta-adrenergic sympathetic effect. Therefore, they decelerate the SA node′s phase 4 depolarization, resulting in a decrease in heart rate [47]. Beta-blockers are well known as a first-line therapy for hypertension, although the mechanisms involved in reducing blood pressure are still not clear [48]. Reducing blood pressure is reached by decreasing the cardiac output related to lowering the heart rate and decreasing the contractility [49]. The cholinergic and sympathetic effects are depicted in Figure 2, and the beta-blockers are listed in Table 1.
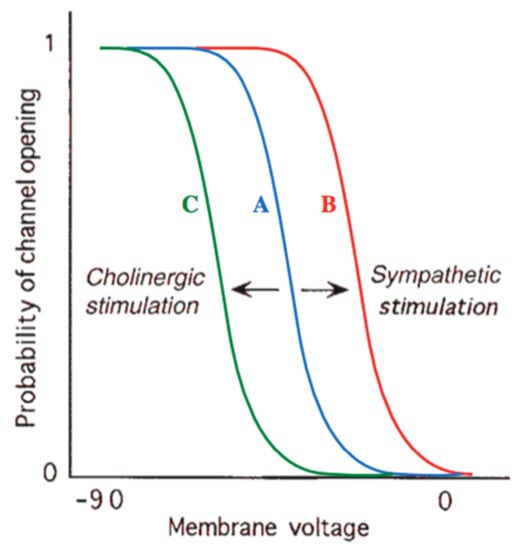
Figure 2.
Effects of cholinergic and sympathetic stimulation on pacemaker channel. At any given voltage, there exists a chance between 0 and 1 that a certain channel will be disclosed. Compared with normal baseline behavior (curve A), sympathetic stimulation (curve B) or treatment with anticholinergic drugs shifts this possibility to a higher value or any given level of membrane voltage, thus elevating the number of disclose channels and the rate at which the cell will free. Curve C shows that parasympathetic cholinergic stimulation (or treatment with a β-blocker, which antagonizes sympathetic stimulation) has the contradictory effect, lowering the probability of a channel being disclose and therefore blocking depolarization [45].

Table 1.
Βeta-blocker agents used worldwide.
One of the most important arrhythmia triggers is an increase in the sympathetic tone, which can be prevented by beta-blockers [50]. In addition to exerting positive tropic effects in response to physiological and pathological stressors, β-adrenergic stimulation influences cardiac electrophysiology and leads to disturbances of the heart rhythm and potentially lethal arrhythmias, particularly in pathological settings. Due to this, beta-blockers are utilized clinically as antiarrhythmics [51,52]. Cardiac-selected beta-blockers minimize the symptoms that may occur from the organization of vague beta-blockers where there is a blockage of the different adrenoreceptors (i.e., β2, β3, α1, and α2). Different receptors evoke an assortment of reactions in the body, and their blockage could cause a wide scope of responses; however, β1 adrenoreceptors are cardio-specific, which makes bisoprolol ideal for the treatment of cardiac events. Bisoprolol has a higher degree of β1 selectivity compared to other β1-selective beta-blockers, such as atenolol, metoprolol, and betaxolol [53].
4.2. Cardio-Selective Beta-Blockers Associated with Lung Function
One study involved 51 chronic obstructive pulmonary disease (COPD) patients and heart failure and directly compared the therapy of bisoprolol, metoprolol, and carvedilol for six weeks. From the study, the authors found that the carvedilol group had the lowest forced expiratory volume in one second (FEV1) and that the bisoprolol group had the highest FEV1 with metoprolol in between. In a randomized controlled trial (RCT) study comparing bisoprolol (mean dose of 6.4 mg) and carvedilol (mean dose of 47 mg) in patients with heart failure and COPD, there was a significantly improved FEV1 for approximately 137 mL in the bisoprolol group, but not in the carvedilol group [54].
In a subgroup involving 2712 patients who had conducted a series of spirometry tests in a cohort study for more than four years, there were no aggravating effects in either the FEV1 or forced vital capacity (FVC) with long-term beta-blocker consumption (88% were administered cardio-selective agents) [55]. Bisoprolol had the highest ratio of β1/β2 receptor selectivity among atenolol and metoprolol, with ratios of 14:1, 5:1, and 2:1, respectively [56]. Nebivolol possessed greater in vitro β1/β2 receptor selectivity than bisoprolol in the human myocardium and also extinguished nitric oxide in endothelial tests [53]. Terbutaline-induced hypokalemia was significantly greater with the bisoprolol and atenolol therapy groups compared to the nebivolol therapy group. Nebivolol generated significant blunting terbutaline-induced glucose and insulin responses compared to the placebo. In conclusion, cardio-selective beta-blockers can be cautiously prescribed for patients with COPD and cardiovascular disease (CVD) [57].
4.3. Clinical Use of Beta-Blockers
Beta-blockers refer to a mixed group of drugs with diverse pharmacodynamic and pharmacokinetic properties. Beta-blockers are effective in preventing cardiovascular disease but are no longer suitable for the routine initial treatment of hypertension. Research has proven that β-blocker therapy may antagonize certain direct and indirect arrhythmogenic effects due to an increase in sympathetic activity. Depending on the type of arrhythmia, β-blockers reduce the risk of proarrhythmia by suppressing sympathetic-mediated triggers and functional reentrant substrates, and by suppressing the rate of the SA and AV nodes [58]. β-blockers are the selected drugs to manage arrhythmic conditions; they are commonly safe agents as they suppress ventricular ectopic beats and arrhythmias and prevent sudden cardiac death in a variety of heart diseases [59]. According to the guidelines, β-blockers are indicated in all patients except for those with AV blocks, bradycardia, or asthma and are recommended for all patients with heart failure no matter what baseline rhythm the patients have. β-blockers are also used to control ventricular rates in order to evade irregular ventricular activation during atrial fibrillation [60].
Beta-blockers, such as bisoprolol, are the drug of choice for LQTS. Beta-blockers are recommended in LQTS patients and should be administered in patients who carry LQTS mutations. The most common trigger of arrhythmias in long-QT syndrome type 1 (LQT1) (mostly caused by mutations in the KCNQ1 gene that leads to the production of drug-induced or slow-activating delayed rectifier potassium currents (IKs)) is an increased sympathetic tone (e.g., during exercise) and this can be prevented by using beta-blockers [61]. Clearly, the QT prolongation induced by hydroxychloroquine and azithromycin, such as in LQT1, can be prevented by using beta-blockers. Unlike in LQT1, beta-blockers are considered to be less effective in long-QT syndrome type 2 (LQT2) (caused by loss of function IKr). Recent studies have shown that propranolol is similarly effective compared to nadolol, whereas metoprolol has less efficacy as an antiarrhythmic therapy [62]. Nadolol has the greatest efficacy among other beta-blockers, such as in LQT1 and LQT2 treatment [63]. Beta-blockers can reduce the risk in patients with long-QT syndrome type 3 (LQT3) (caused by a lack of sodium flow), although previous studies have shown that beta-blockers are not effective in LQT3 treatment compared to LQT1 or LQT2 treatment [64].
Nebivolol, a selective β1-blocker, has vasodilating effects to reduce peripheral vascular resistance [65]. Nebivolol has been reported, in hypertension patients, to improve the indicators of myocardial repolarization heterogeneity and electrical instability, such as the QT dispersion, QTc, and corrected dispersion QT (QTcd) [66].
Bisoprolol therapy has been shown to lead to QTc shortening in LQT1 gene-positive and LQT2 patients and is quite well tolerated for long-term consumption [67]. A retrospective cohort study involved 114 consecutive gene-positive LQT1 and LQT2 patients who were administered bisoprolol, nadolol, or atenolol. The basic heart rate and QTc were equal between each therapy group. QTc shortening was found in the bisoprolol (ΔQTc −5 ± 31 ms; p = 0.049) and nadolol (ΔQTc −13 ± 16 ms; p = 0.02) groups, but not in the atenolol group (ΔQTc 9 ± 24 ms; p = 0.16) [68]. We conclude that bisoprolol is the only option for COVID-19 treatment due to its cardio-selective properties.
Similar to cluster of differentiation 147 (CD147), beta-adrenergic blockers block the entry of SARS-CoV-2 through the ACE2 receptor. Beta-blockers with negative regulation on juxtaglomerular cells in the kidney reduce the activity of both arms of the renin–angiotensin–aldosterone system (RAAS) pathway, and so the ACE2 levels decrease. ACE2 is known as the gate where SARS-CoV-2 enters a cell, and by the mechanism above, beta-adrenergic blockers can reduce SARS-CoV-2 entry. Propranolol triggers CD147 downregulation [69]. Therefore, beta-adrenergic blocker treatment in COVID-19 will decrease SARS-CoV-2 cell entry by the downregulation of both ACE2 and CD147 [70]. Activation of the beta-adrenergic receptors plays a role in interleukin (IL)-6 secretion, and beta-blockers in IL-4 reduce cardiac disorders in SARS-CoV-2 patients. Beta-adrenergic blockers have been shown to decrease a variety of pro-inflammatory cytokine expressions, including IL-1, IL-1β, IL-6, tumor necrosis factor-α (TNFα), and interferon-γ (IFNγ). Additionally, beta-adrenergic blockers reduce cytokine storms by decreasing pro-inflammatory cytokines (Figure 3) [70].
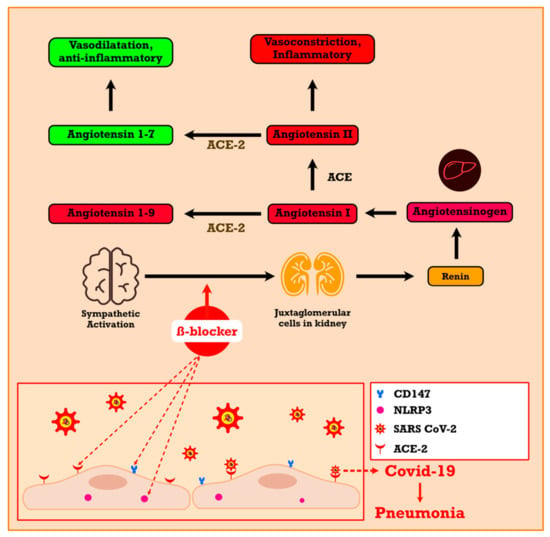
Figure 3.
The effect of beta-blockers in the renin–angiotensin–aldosterone system. (Modified from [70]). CD147, cluster of differentiation 147; COVID-19, coronavirus disease 2019.
Recent trends suggest that beta-adrenergic blockers are beneficial in septic shock and also reduce mortality in acute respiratory distress syndrome (ARDS). Norepinephrine for treating septic shock should be avoided because it increases the catecholamine level [70]. Critically ill COVID-19 patients may also experience a sympathetic storm, i.e., an increasing catecholamine level in the body. An increasing catecholamine level leads to an increase in renin release, which increases the activity of both of its arms, including an increase in ACE2 expression, thereby facilitating SARS-CoV-2 cellular entry and worsening the condition. Some severe COVID-19 patients may end up in septic shock [70].
5. Conclusions
Cardio-selective beta-blockers are established medications for treating arrhythmias and are a potential treatment in QT prolongation management due to COVID-19 protocol therapy. Beta-blockers reduce the risk of proarrhythmia by suppressing sympathetic-mediated triggers, the functioning reentrant substrate, and the rates of the SA and AV nodes. In addition to this main benefit, as beta-adrenergic blockers, these drugs are able to block the gate of SARS-CoV-2 entry through negative regulation on the juxtaglomerular cells in the kidneys, which reduces the activity of both arms of the RAAS pathway, thus lowering the ACE2 levels.
Author Contributions
Conceptualization, T.H.; validation, T.H. and T.A.W.; formal analysis and investigation, T.H. and T.A.W.; writing—original draft T.H., I.N.C., L.F., and T.F.H.; writing—review and editing; I.N.C., L.F., and T.F.H.; supervision, T.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, H.C.; Stratton, W.; Tang, Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. Outbreak of coronavirus disease 2019. Lancet Infect. Dis. 2020, 20, 292–293. [Google Scholar] [CrossRef]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Patil, V.M.; Singhal, S.; Masand, A. A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: Efficacy, safety and clinical trials. Life Sci. 2020, 254, 117775. [Google Scholar] [CrossRef]
- Saleh, M.; Gabriels, J.; Chang, D.; Kim, B.S.; Mansoor, A.; Mahmood, E.; Makker, P.; Ismail, H.; Goldner, B.; Willner, J.; et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circ. Arrhythm. Electrophysiol. 2020, 13, 496–504. [Google Scholar] [CrossRef]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Leon, W.R.M.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Antolović, R. From erythromycin to azithromycin and new potential ribosome-binding antimicrobials. Antibiotics 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Alqarawi, W.; MacIntyre, C.J.; Tadros, R.; Steinberg, C.; Roberts, J.D.; Laksman, Z.; Healey, J.S.; Krahn, A.D. Guidance on Minimizing Risk of Drug-Induced Ventricular Arrhythmia During Treatment of COVID-19: A Statement from the Canadian Heart Rhythm Society. Can. J. Cardiol. 2020, 36, 948–951. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta-blockers therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef] [PubMed]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef]
- Borba, M.G.S.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Brito, M.; Mourão, M.P.G.; Brito-Sousa, J.D.; Baía-Da-Silva, D.; Guerra, M.V.F.; et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e208857. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Lynch, J.B.; del Rio, C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012, 366, 1881–1990. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, S.; Assar, M.D.; Schussler, J.M. Drug-induced QT interval prolongation: Mechanisms and clinical management. Ther. Adv. Drug Saf. 2012, 3, 241–253. [Google Scholar] [CrossRef]
- Belardinelli, L.; Antzelevitch, C.; Vos, M.A. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol. Sci. 2003, 24, 619–625. [Google Scholar] [CrossRef]
- Damle, B.; Vourvahis, M.; Wang, E.; Leaney, J.; Corrigan, B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020, 108, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Di Trani, L.; Donatelli, I.; Cauda, R.; Cassone, A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006, 6, 67–69. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, S.M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020, 75, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 2007, 7, 549–558. [Google Scholar] [CrossRef]
- Costedoat-Chalumeau, N.; Hulot, J.-S.; Amoura, Z.; Leroux, G.; Lechat, P.; Funck-Brentano, C.; Piette, J.-C. Heart conduction disorders related to antimalarials toxicity: An analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology 2007, 46, 808–810. [Google Scholar] [CrossRef]
- Wu, C.-I.; Postema, P.G.; Arbelo, E.; Behr, E.R.; Bezzina, C.R.; Napolitano, C.; Robyns, T.; Probst, V.; Schulze-Bahr, E.; Remme, C.A.; et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Hear. Rhythm. 2020, 17, 1456–1462. [Google Scholar] [CrossRef]
- Marquardt, K.; Albertson, T.E. Treatment of hydroxychloroquine overdose. Am. J. Emerg. Med. 2001, 19, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, N.J.; Yen, C.F.; Shim, D.J.; Maher, T.R.; McCoy, C.M.; Zimetbaum, P.J.; Gold, H.S. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or without Concomitant Azithromycin among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1036–1041. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Wang, Y.; Chu, J.; Liu, Y.; Chen, X.; Chen, X. QTc prolongation during antiviral therapy in two COVID-19 patients. J. Clin. Pharm. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Pandurangi, U.; Arora, V.; Gupta, A.; Jaswal, A.; Nabar, A.; Naik, A.; Naik, N.; Namboodiri, N.; Vora, A.; et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: A scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol. J. 2002, 20, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E. Drug-induced QT interval prolongation and torsade de pointes: Role of the pharmacist in risk assessment, prevention and management. Can. Pharm. J. 2020, 149, 139–152. [Google Scholar] [CrossRef]
- Purwowiyoto, S.L.; Hermanto, D.Y.; Muhammad, I. Managing QT prolongation in the Era of Coronavirus Disease 2019 (COVID-19). Indones. J. Cardiol. 2020, 41, 108–111. [Google Scholar] [CrossRef]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement; Cannon Press: Idaho, Moscow, 2008. [Google Scholar]
- DiFrancesco, D.; Tortora, P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 1991, 351, 145–147. [Google Scholar] [CrossRef]
- Lakatta, E.G.; DiFrancesco, D. What keeps us ticking: A funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 2009, 47, 157–170. [Google Scholar] [CrossRef]
- Grandi, E.; Sanguinetti, M.C.; Bartos, D.C.; Bers, D.M.; Chen-Izu, Y.; Chiamvimonvat, N.; Colecraft, H.M.; Delisle, B.P.; Heijman, J.; Navedo, M.F.; et al. Potassium channels in the heart: Structure, function and regulation. J. Physiol. 2017, 595, 2209–2228. [Google Scholar] [CrossRef] [PubMed]
- Bartos, D.C.; Grandi, E.; Ripplinger, C.M. Ion channels in the heart. Compr. Physiol. 2015, 5, 1423–1464. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.L. Pathophysiology of the Heart Disease: A Collaborative Project of Medical Students and Faculty, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Difrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef]
- Vincent, G.M.; Schwartz, P.J.; Denjoy, I.; Swan, H.; Bithell, C.; Spazzolini, C.; Crotti, L.; Piippo, K.; Lupoglazoff, J.-M.; Villain, E.; et al. High efficacy of β-blockers in long-QT syndrome type 1: Contribution of noncompliance and QT-prolonging drugs to the occurrence of β-blocker treatment failures. Circulation 2009, 119, 215–221. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Lux, R.L.; Khahera, A.; Kwon, O.; Aliotta, E.; Ennis, D.B.; Fishbein, M.C.; Ardell, J.L.; Shivkumar, K. Sympathetic modulation of electrical activation in normal and infracted myocardium: Implications for arrhythmogenesis. Am. J. Physiol. Hear. Circ. Physiol. 2017, 312, H608–H621. [Google Scholar] [CrossRef]
- Westfall, D.; Macarthur, H.; Westfall. The Autonomic and Somatic Motor Nervous Systems. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Mc.Graw Hill: New York, NY, USA, 2018. [Google Scholar]
- Jamali, H.K.; Waqar, F.; Gerson, M.C. Cardiac autonomic innervation. J. Nucl. Cardiol. 2017, 24, 1558–1570. [Google Scholar] [CrossRef]
- Laitinen, P.J.; Brown, K.M.; Piippo, K.; Swan, H.; Devaney, J.M.; Brahmbhatt, B.; Donarum, E.A.; Marino, M.; Tiso, N.; Viitasalo, M.; et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001, 103, 185–190. [Google Scholar] [CrossRef]
- Myles, R.C.; Wang, L.; Kang, C.; Bers, D.M.; Ripplinger, C.M. Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ. Res. 2012, 110, 154–164. [Google Scholar] [CrossRef]
- Bundkirchen, A.; Brixius, K.; Bölck, B.; Nguyen, Q.; Schwinger, R.H.G. β1-adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies. Eur. J. Pharmacol. 2003, 460, 19–26. [Google Scholar] [CrossRef]
- Lipworth, B.; Wedzicha, J.; Devereux, G.; Vestbo, J.; Dransfield, M.T. Beta-blockers in COPD: Time for reappraisal. Eur. Respir. J. 2016, 48, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Short, P.M.; Lipworth, S.I.; Elder, D.H.; Schembri, S.; Lipworth, B.J. Effect of β blockers in treatment of chronic obstructive pulmonary disease: A retrospective cohort study. BMJ 2011, 342, d2549. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.G. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br. J. Pharmacol. 2010, 160, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Kamp, O.; Metra, M.; Bugatti, S.; Bettari, L.; Cas, A.D.; Petrini, N.; Cas, L.D.; Metra, P.M. Nebivolol: Haemodynamic effects and clinical significance of combined β-blockade and nitric oxide release. Drugs 2010, 70, 41–56. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, J.; Hu, N.N.; George, A.L.; Murray, K.T. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ. Res. 2000, 87, 33–38. [Google Scholar] [CrossRef]
- Coppola, S.; Froio, S.; Chiumello, D. β-blockers in critically ill patients: From physiology to clinical evidence. Crit. Care 2015, 19, 83. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.; Ezekowitz, M.D.; Field, M.E.; et al. AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Priori, S.G.; Spazzolini, C.; Moss, A.J.; Vincent, G.M.; Napolitano, C.; Denjoy, I.; Guicheney, P.; Breithardt, G.; Keating, M.T.; et al. Genotype-Phenotype Correlation in the Long-QT Syndrome. Circulation 2001, 103, 89–95. [Google Scholar] [CrossRef]
- Chatrath, R.; Bell, C.M.; Ackerman, M.J. β-blocker therapy failures in symptomatic probands with genotyped long-QT syndrome. Pediatr. Cardiol. 2004, 25, 459–465. [Google Scholar] [CrossRef]
- Hockalingam, P.; Crotti, L.; Girardengo, G.; Johnson, J.N.; Harris, K.M.; Van Der Heijden, J.F.; Hauer, R.N.; Beckmann, B.M.; Spazzolini, C.; Rordorf, R.; et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: Higher recurrence of events under metoprolol. J. Am. Coll. Cardiol. 2012, 60, 2092–2099. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Moss, A.J.; Kaufman, E.S.; Shimizu, W.; Peterson, D.R.; Benhorin, J.; Lopes, C.; Towbin, J.A.; Spazzolini, C.; Crotti, L.; et al. Clinical Aspects of Type 3 Long-QT Syndrome: An International Multicenter Study. Circulation 2016, 134, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Zanchetti, A. Clinical pharmacodynamics of nebivolol: New evidence of nitric oxide-mediated vasodilating activity and peculiar haemodynamic properties in hypertensive patients. Blood Press 2004, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.M.; Cay, S.; Cagirci, G.; Sen, N. Nebivolol therapy improves QTc and QTcd parameters in heart failure patients. Cardiovasc. J. Afr. 2012, 23, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Myerburg, R.J.; Quinones, M.A.; Roden, D.M.; et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death-Executive Summary. J. Am. Coll. Cardiol. 2006, 48, e385–e484. [Google Scholar] [CrossRef]
- Steinberg, C.; Padfield, G.J.; Al-Sabeq, B.; Adler, A.; Yeung-Lai-Wah, J.A.; Kerr, C.R.; Deyell, M.W.; Andrade, J.G.; Bennett, M.T.; Yee, R.; et al. Experience with bisoprolol in long-QT1 and long-QT2 syndrome. J. Interv. Card. Electrophysiol. 2016, 47, 163–170. [Google Scholar] [CrossRef]
- Xie, W.; Xie, H.; Liu, F.; Li, W.; Dan, J.; Mei, Y.; Dan, L.; Xiao, X.; Li, J.; Chen, X. Propranolol induces apoptosis of human umbilical vein endothelial cells through downregulation of CD147. Br. J. Dermatol. 2013, 168, 739–748. [Google Scholar] [CrossRef]
- Natesan, V. Beta-adrenergic blocker treatment for COVID-19. Hypothesis 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).