Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Miscibility, Solubility Parameter Calculation, and Solubility Studies
2.2.2. Porcine Skin Permeation and Mass Balance Studies of EA
2.2.3. Data Analysis
3. Results and Discussion
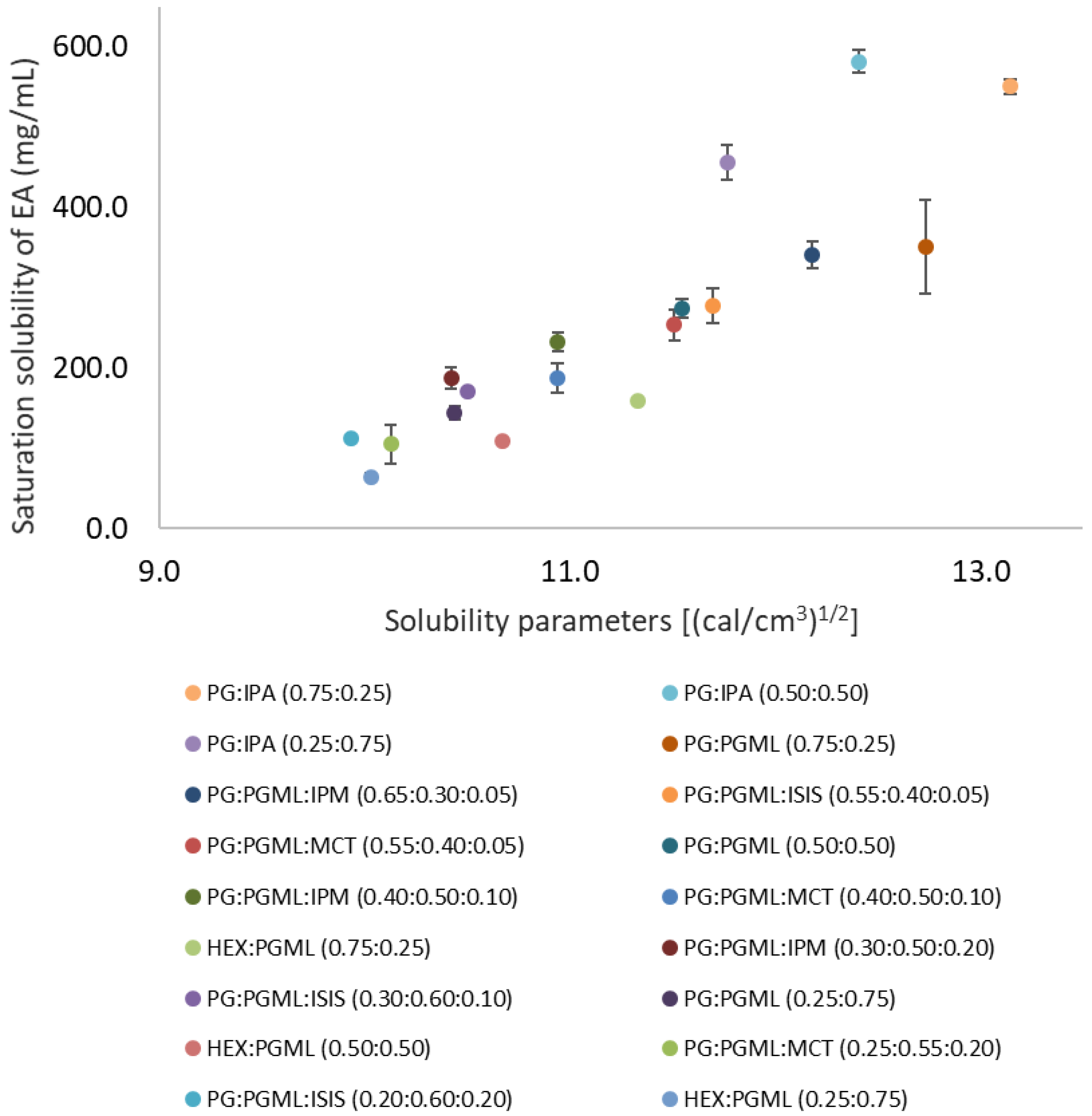
3.1. Miscibility of Solvents, Solubility Parameter Values, and Solubility of EA in Candidate Vehicles
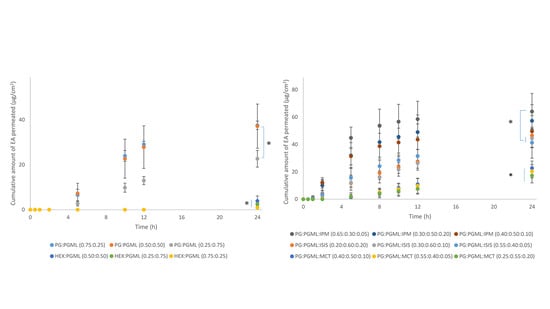
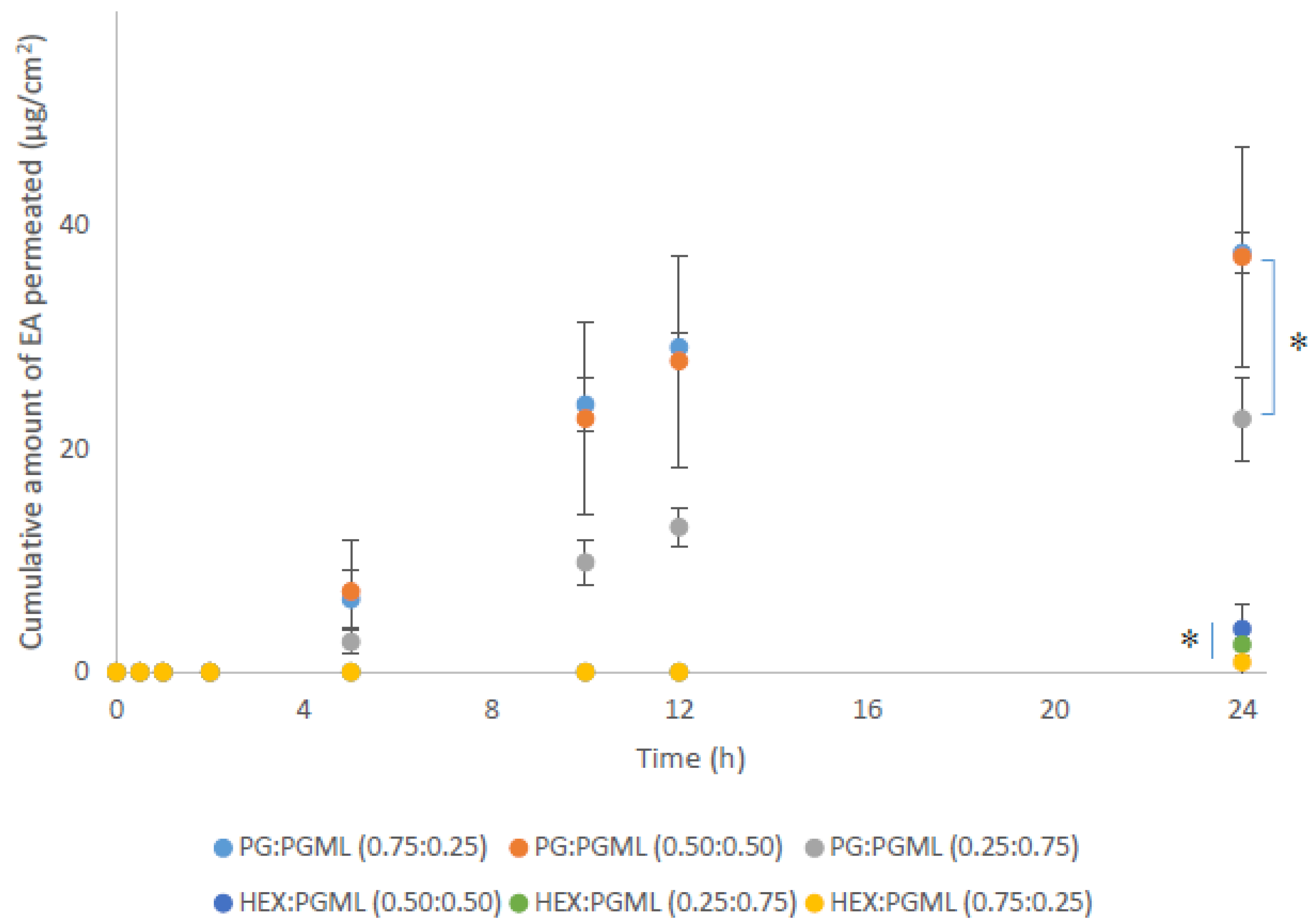
3.2. Porcine Skin Permeation and Mass Balance Studies of EA Using Binary Systems of PG and HEX with PGML
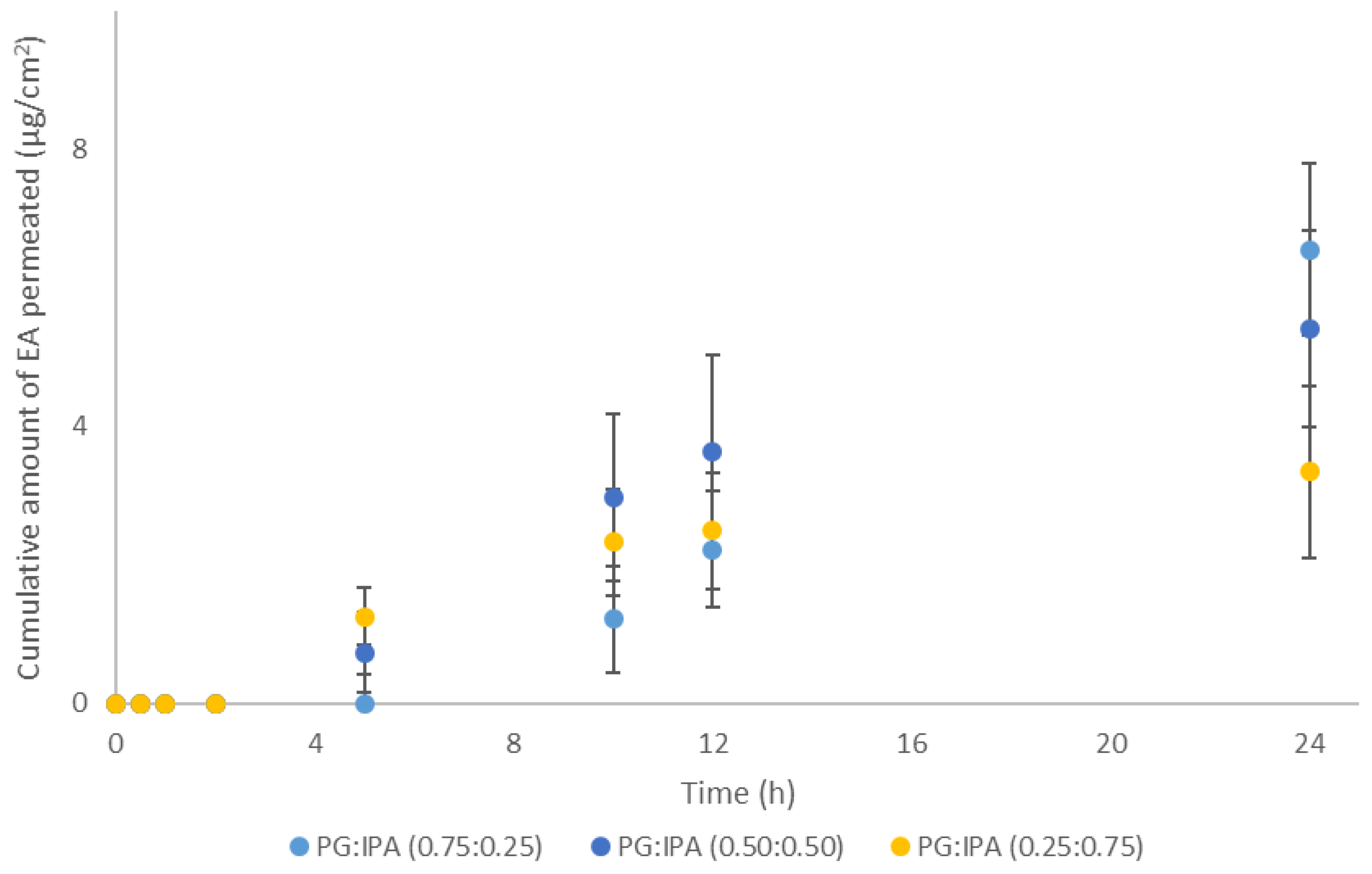
3.3. Porcine Skin Permeation and Mass Balance Studies of EA Using Binary Systems of PG and IPA
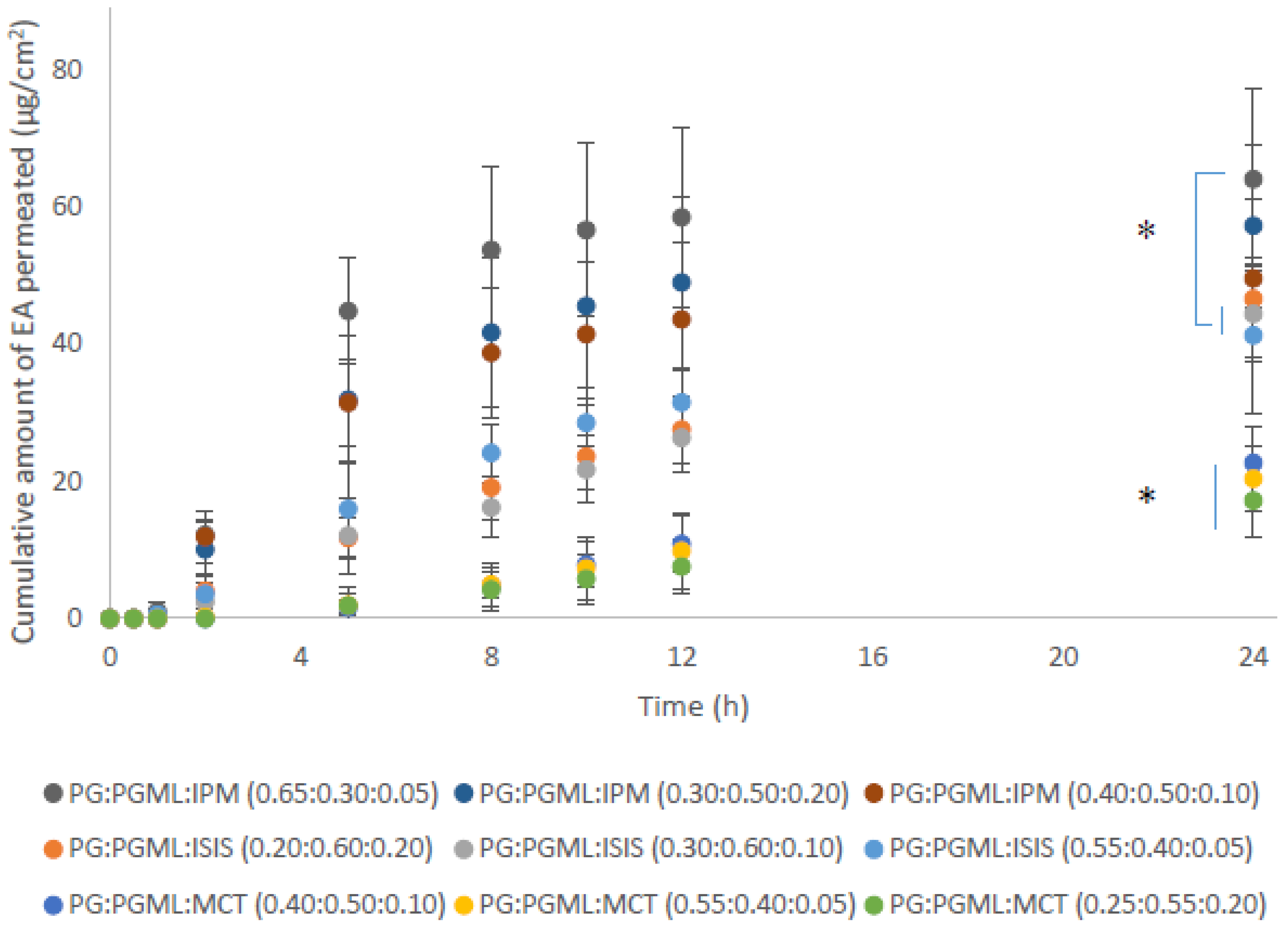
3.4. Porcine Skin Permeation and Mass Balance Studies of EA Using Ternary Systems of ISIS, MCT, and IPM with PG:PGML
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapiere, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapière, C.M. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef]
- EWG. Ethyl Ascorbic Acid. Available online: https://www.ewg.org/skindeep/ingredients/718948-ethyl-ascorbic-acid (accessed on 11 July 2019).
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhaes, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of antioxidants in anti-aging cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef]
- Bandelin, F.J.; Tuschhoff, J.V. The stability of ascorbic acid in various liquid media. J. Am. Pharm. Assoc. 1955, 44, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E.; Hadgraft, J.; Oliveira, G.; Vieira, R.; Mohammed, D.; Hirata, K. Rational formulation design. Int. J. Cosmet. Sci. 2012, 34, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In Vitro Evaluation of Sunscreen Safety: Effects of the Vehicle and Repeated Applications on Skin Permeation from Topical Formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Mitragotri, S. Synergistic Effect of Enhancers for Transdermal Drug Delivery. Pharm. Res. 2000, 17, 1354–1359. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin II. Influence of binary and ternary solvent systems. Eur. J. Pharm. Sci. 2018, 121, 59–64. [Google Scholar] [CrossRef]
- OECD. Test. No. 28: Guidance Document for the Conduct of Skin Absorption Studies; Organisation for Economic Cooperation and Development Publishing: Paris, France, 2004. [Google Scholar]
- Hossain, A.S.M.M.A.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Monjur Al Hossain, A.S.M.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical delivery of niacinamide: Influence of neat solvents. Int. J. Pharm. 2020, 579, 119137. [Google Scholar] [CrossRef] [PubMed]
- Irion, G.D.; Garrison, M.D.; Abraham, W. Effect of PGML Excipient Mixture in a Transdermal System on the in Vitro Transport of Estradiol across Skin. Pharm. Res. 1995, 12, 1618–1622. [Google Scholar] [CrossRef]
- Jasti, B.R.; Abraham, W. Fluorescence Spectroscopic Investigation of Effect of Excipients on Epidermal Barrier and Transdermal Systems. J. Investig. Dermatol. Symp. Proc. 1998, 3, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Parisi, N.; Paz-Alvarez, M.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Topical delivery of hexamidine. Int. J. Pharm. 2016, 506, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Guy, R.H.; Hadgraft, J. In vitro and in vivo enhancement of skin permeation with oleic and lauric acids. Int. J. Pharm. 1988, 48, 103–111. [Google Scholar] [CrossRef]
- Lane, M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Passive Skin Permeation Enhancement. In Topical and Transdermal Drug Delivery; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of percutaneous absorption by the use of volatile: Nonvolatile systems as vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef]
- Goates, C.Y.; Knutson, K. Enhanced permeation of polar compounds through human epidermis. I. Permeability and membrane structural changes in the presence of short chain alcohols. Biochim. Biophys. Acta 1994, 1195, 169–179. [Google Scholar] [CrossRef]
- Brinkmann, I.; Muller-Goymann, C.C. Role of isopropyl myristate, isopropyl alcohol and a combination of both in hydrocortisone permeation across the human stratum corneum. Skin Pharmacol. Physiol. 2003, 16, 393–404. [Google Scholar] [CrossRef]
- Engelbrecht, T.N.; Demé, B.; Dobner, B.; Neubert, R.H.H. Study of the Influence of the Penetration Enhancer Isopropyl Myristate on the Nanostructure of Stratum Corneum Lipid Model Membranes Using Neutron Diffraction and Deuterium Labelling. Skin Pharmacol. Physiol. 2012, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Eichner, A.; Stahlberg, S.; Sonnenberger, S.; Lange, S.; Dobner, B.; Ostermann, A.; Schrader, T.E.; Hauß, T.; Schroeter, A.; Huster, D.; et al. Influence of the penetration enhancer isopropyl myristate on stratum corneum lipid model membranes revealed by neutron diffraction and 2H NMR experiments. Biochim. Biophys. Acta 2017, 1859, 745–755. [Google Scholar] [CrossRef]
- EWG. Caprylic/Capric Triglyceride. Available online: https://www.ewg.org/skindeep/ingredient/701056/caprylic%3B%3B_capric_triglyceride/ (accessed on 2 November 2019).
- Leopold, C.S.; Lippold, B.C. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC). J. Pharm. Pharmacol. 1995, 47, 276–281. [Google Scholar] [CrossRef]
- Caussin, J.; Groenink, H.W.; de Graaff, A.M.; Gooris, G.S.; Wiechers, J.W.; van Aelst, A.C.; Bouwstra, J.A. Lipophilic and hydrophilic moisturizers show different actions on human skin as revealed by cryo scanning electron microscopy. Exp. Dermatol. 2007, 16, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Caussin, J.; Gooris, G.S.; Bouwstra, J.A. FTIR studies show lipophilic moisturizers to interact with stratum corneum lipids, rendering the more densely packed. Biochim. Biophys. Acta 2008, 1778, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Pennick, G.; Harrison, S.; Jones, D.; Rawlings, A.V. Superior effect of isostearyl isostearate on improvement in stratum corneum water permeability barrier function as examined by the plastic occlusion stress test. Int. J. Cosmet. Sci. 2010, 32, 304–312. [Google Scholar] [CrossRef] [PubMed]
| Binary System | Ratio of Solvents | Cumulative Permeation (µg/cm2) | Percentage (%) of Applied Dose | |||
|---|---|---|---|---|---|---|
| Extraction | Permeation | Washed from the Surface | Total Recovery | |||
| PG:PGML | 0.75:0.25 | 37.5 ± 1.9 | 16.7 ± 1.3 | 45.9 ± 2.3 | 17.5 ± 3.0 | 80.1 ± 4.2 |
| 0.50:0.50 | 37.2 ± 9.8 | 18.5 ± 6.5 | 41.1 ± 10.3 | 25.5 ± 4.7 | 85.1 ± 5.0 | |
| 0.25:0.75 | 22.7 ± 3.7 | 21.6 ± 2.1 | 30.9 ± 6.0 | 28.4 ± 8.0 | 80.8 ± 8.0 | |
| HEX:PGML | 0.75:0.25 | 0.9 ± 1.5 | 2.1 ± 1.9 | 0.9 ± 1.6 | 66.5 ± 3.9 | 69.5 ± 4.5 |
| 0.50:0.50 | 3.9 ± 2.8 | 19.0 ± 5.3 | 4.5 ± 3.3 | 49.7 ± 19.3 | 73.2 ± 16.1 | |
| 0.25:0.75 | 2.5 ± 0.6 | 8.7 ± 2.9 | 2.6 ± 0.8 | 56.8 ± 0.6 | 68.1 ± 4.1 | |
| Binary System | Ratio of Solvents | Cumulative Permeation (µg/cm2) | Percentage (%) of Applied Dose | |||
|---|---|---|---|---|---|---|
| Extraction | Permeation | Washed from the Surface | Total Recovery | |||
| PG:IPA | 0.75:0.25 | 6.6 ± 3.9 | 22.5 ± 3.6 | 8.8 ± 5.2 | 58.5 ± 9.4 | 89.8 ± 6.5 |
| 0.50:0.50 | 5.4 ± 1.4 | 19.9 ± 3.6 | 6.4 ± 1.7 | 63.5 ± 8.7 | 90.4 ± 4.9 | |
| 0.25:0.75 | 3.4 ± 1.2 | 18.2 ± 4.4 | 5.5 ± 2.1 | 70.1 ± 11.4 | 93.8 ± 6.0 | |
| Ternary System | Ratio of Solvents | Cumulative Permeation (µg/cm2) | Percentage (%) of Applied Dose | |||
|---|---|---|---|---|---|---|
| Extraction | Permeation | Washed from the Surface | Total Recovery | |||
| PG:PGML:IPM | 0.65:0.30:0.05 | 64.1 ± 13.3 | 3.9 ± 1.1 | 70.9 ± 10.5 | 6.9 ± 2.5 | 81.8 ± 9.8 |
| 0.40:0.50:0.10 | 49.6 ± 11.6 | 3.3 ± 0.9 | 57.9 ± 12.7 | 7.6 ± 5.8 | 68.7 ± 9.7 | |
| 0.30:0.50:0.20 | 57.3 ± 11.8 | 4.1 ± 0.4 | 62.3 ± 13.7 | 6.1 ± 3.8 | 72.5 ± 12.3 | |
| PG:PGML:ISIS | 0.55:0.40:0.05 | 41.3 ± 11.3 | 6.2 ± 1.6 | 49.5 ± 10.4 | 30.3 ± 5.3 | 85.9 ± 6.1 |
| 0.30:0.60:0.10 | 44.4 ± 6.9 | 8.0 ± 1.0 | 48.9 ± 5.4 | 28.4 ± 9.6 | 85.4 ± 4.3 | |
| 0.20:0.60:0.20 | 46.7 ± 5.1 | 7.1 ± 2.5 | 52.6 ± 6.5 | 26.8 ± 3.6 | 86.5 ± 7.8 | |
| PG:PGML:MCT | 0.55:0.40:0.05 | 20.4 ± 4.9 | 13.3 ± 4.0 | 26.9 ± 5.9 | 39.4 ± 5.4 | 79.7 ± 10.0 |
| 0.40:0.50:0.10 | 22.7 ± 5.2 | 17.0 ± 4.2 | 27.8 ± 6.1 | 41.5 ± 5.1 | 86.3 ± 6.3 | |
| 0.25:0.55:0.20 | 17.2 ± 5.3 | 21.6 ± 5.4 | 20.5 ± 6.1 | 46.1 ± 13.6 | 88.2 ± 5.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliopoulos, F.; Hossain, A.S.M.M.A.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Sci. Pharm. 2020, 88, 19. https://doi.org/10.3390/scipharm88020019
Iliopoulos F, Hossain ASMMA, Sil BC, Moore DJ, Lucas RA, Lane ME. Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Scientia Pharmaceutica. 2020; 88(2):19. https://doi.org/10.3390/scipharm88020019
Chicago/Turabian StyleIliopoulos, Fotis, A. S. M. Monjur Al Hossain, Bruno C. Sil, David J. Moore, Robert A. Lucas, and Majella E. Lane. 2020. "Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems" Scientia Pharmaceutica 88, no. 2: 19. https://doi.org/10.3390/scipharm88020019
APA StyleIliopoulos, F., Hossain, A. S. M. M. A., Sil, B. C., Moore, D. J., Lucas, R. A., & Lane, M. E. (2020). Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Scientia Pharmaceutica, 88(2), 19. https://doi.org/10.3390/scipharm88020019