Abstract
3-O-ethyl l-ascorbic acid (EA), an ether derivative of Vitamin C, is widely used in skincare formulations. Previously, we reported the effects of neat solvents on EA percutaneous absorption and observed that 0.6–7.5% of the applied EA was delivered through the skin over 24 h. In this work, we designed complex formulations using combinations of solvents that may act synergistically and examined their impact on EA permeation in porcine skin in vitro under finite dose conditions. Binary combinations of propylene glycol (PG) with propylene glycol monolaurate (PGML) were effective in enhancing skin permeation of EA compared with individual solvents (p < 0.05). Combining PGML with 1,2-hexanediol (HEX) did not result in significantly higher EA permeation compared with the neat solvents (p > 0.05). Addition of the volatile solvent isopropyl alcohol (IPA) to PG solutions also did not improve EA skin delivery compared with neat PG. Ternary solvent systems containing PG:PGML were subsequently prepared by the addition of a lipophilic solvent, either isopropyl myristate (IPM), medium-chain triglycerides (MCT) or isostearyl isostearate (ISIS). The optimum vehicle, PG:PGML:IPM, promoted up to 70.9% skin delivery of EA. The PG:PGML:ISIS vehicles also promoted EA permeation across the skin, but to a significantly lesser extent than the IPM-containing vehicles. No enhancement of EA delivery was noted for the PG:PGML:MCT mixtures. These results will inform the development of targeted formulations for EA in the future.
1. Introduction
Vitamin C, also known as l-ascorbic acid, is a widely used water-soluble antioxidant that has demonstrated various beneficial actions on human skin following topical application in vivo. These effects include significant reduction of clinical signs of photo-ageing in sun-exposed skin [1] as well as promotion of collagen synthesis in skin sites treated with a vitamin C-containing formulation [2]. However, the molecule is unstable, readily undergoing oxidation in solutions and on exposure to air. This poses challenges for its use in topical formulations; also the physicochemical properties of the compound are also not optimal for skin delivery. 3-O-ethyl l-ascorbic acid (EA) is an ether derivative of vitamin C that is currently used in many personal care products [3,4]. Structurally, the molecule has an ethyl group on the 3-OH group of the ascorbic acid structure. EA has been found to have good stability in several commonly used solvents, with a reported recovery of greater than 90% after 120 h at 32 ± 1 °C [5].
Despite the improved stability of EA compared with the parent compound [6], topical delivery of this active still presents challenges, given its hydrophilic nature (logP(o/w) = −1.1) [5]. Even though the ability of some solvents to promote delivery of EA across porcine skin in vitro has been confirmed, the permeability of the compound was comparatively low, with 0.6% to 7.5% of the applied dose delivered after 24 h. Additionally, commonly used excipients and/or penetration enhancers such as ethoxydiglycol, dipropylene glycol, 1,5 pentanediol, and tripropylene glycol did not promote permeation of EA through porcine skin [5]. Generally, it is accepted that skin permeation of actives is vehicle-dependent [7,8] and combinations of solvents have been shown to act synergistically to enhance permeation of compounds [9,10,11].
The aims of the present work were therefore (i) to develop complex solvent systems for delivery of EA to the skin (ii) to assess EA solubility in these solvent mixtures and (iii) to investigate if synergistic effects on EA skin delivery from these vehicles could be achieved.
2. Materials and Methods
2.1. Materials
3-O-ethyl l-ascorbic acid (EA) (Et-VC™, Corum Inc., Taipei, Taiwan) was donated by GlaxoSmithKline (Weybridge, UK). 1,2 propanediol (PG), 1,2 hexanediol (HEX), isopropyl myristate (IPM) and 2-propanol (IPA) were supplied by Sigma-Aldrich (Dorset, UK). LauroglycolTM 90 or propylene glycol monolaurate (PGML) Type II and LabrafacTM lipophile WL 1349 or medium chain triglycerides of caprylic (C8) and capric (C10) acids (MCT) were kind donations from Gattefossé (St. Priest, France). CrodamolTM ISIS or isostearyl isosearate (ISIS) was kindly provided by Croda Europe Ltd. (Cowick Hall, UK). High-vacuum grease was purchased from Dow Corning (Seneffe, Belgium). Phosphate buffered saline (PBS) tablets were purchased from Oxoid Limited (Cheshire, UK). Ortho-phosphoric acid (H3PO4), HPLC grade water, and HPLC grade methanol were obtained from Fisher Scientific (Leicestershire, UK).
2.2. Methods
2.2.1. Miscibility, Solubility Parameter Calculation, and Solubility Studies
Miscibility studies were conducted as described by Haque et al [12]. Briefly, for both binary and ternary miscibility determinations, the solvents were mixed at different percentages, varying by 10%. All solutions were mixed using a Vortex mixer (IKA vortex mixer genius 3, VWR International Limited, Leicestershire, UK). The tubes were left to stand for at least 24 h at room temperature. Where necessary, the dyes Sudan III and methylene blue were used to confirm miscibility. For ternary systems, phase diagrams were constructed using Origin® Pro 2017 software (OriginLab Cooperation, Northampton, MA, USA). All ternary solvent mixtures presented partial miscibility, forming phase diagrams with miscible and immiscible areas defined by the various proportions of solvents.
The solubility parameters of the solvent mixtures were estimated by the Van-Krevelen and Hoftyzer approach using Molecular Modelling Pro® software (Version 7.0.8, 2016, Norgwyn Montgomery Software, Inc., North Wales, PA, USA), as explained previously [12]. Solubility studies were conducted at 32 ± 1 °C and samples were analysed using the previously reported and validated HPLC method [5].
2.2.2. Porcine Skin Permeation and Mass Balance Studies of EA
All formulations were prepared to contain EA 2% (w/w), because neat solvents containing EA at this concentration were previously reported to promote penetration of the active across pig skin [5]. Permeation studies were conducted in full thickness porcine ear skin in vitro, using Franz-type cells of a diffusion area of approximately 1 cm2 and were followed by mass balance studies. The experiments were performed as previously described [5,13,14]. For the receptor medium, freshly prepared PBS solution was used, with a pH value of 7.3. (Dulbecco A, pH 7.3 ± 0.2 at 25 °C). The temperature of the skin throughout the permeation studies was controlled and maintained at 32 ± 1 °C. Finite doses of formulations (5 μL) were applied to the donor compartment under non-occluded conditions. Samples from the receptor compartment were taken at several time points and EA was quantified using HPLC analysis. The number of replicate experiments was 5 ≥ n ≥ 3. The mass balance studies were conducted at the end of the permeation experiments to measure the amounts of EA on the surface and inside the skin. The procedure was previously validated and reported [5]. Briefly, at the end of the permeation studies, the receptor solution was removed from the Franz cells and the skin surface was washed once with 1 mL of methanol and three times with 1 mL of water:methanol (50:50), consecutively, followed by swabbing with a cotton bud. Subsequently, the skin membranes were cut into small pieces and placed in Eppendorf® tubes with 1 mL of water:methanol (50:50). The samples were extracted by incubation for at least 5 h in a thermostatically controlled orbital shaker (Orbital Mini shaker, VWR International Limited, Leicestershire, UK) with 1000× g rotation at 32 °C. All samples were centrifuged at 13,000× g for 20 min before sampling, and the supernatant solution was analysed using HPLC. The mass balance procedures were validated to ensure all of the active deposited on and inside the skin was recovered. The findings showed sufficient total recovery of EA following application to the skin for a short period of time i.e., 5 h (Figure S1). This suggests that both washing and extraction procedures were capable of recovering the active according to recommendations by official guidelines.
2.2.3. Data Analysis
The data were analysed using the Microsoft® Excel 2013 software (Microsoft Corporation, Redmond, Washington, DC, USA) and GraphPad Prism Statistics software (version 8.3.0, San Diego, CA, USA, 2019). Results are presented as the mean ± standard deviation (SD). For parametric data, statistical evaluation was performed by one-way analysis of variance (ANOVA) and multiple comparisons between groups by post hoc Tukey test. For non-parametric data, the Kruskal-Wallis test was performed. Statistical significance was assumed when the p value was less than 0.05. Solvent miscibility data were analysed using the Origin® Pro 2017 software (OriginLab Cooperation, Northampton, MA, USA). Molecular Modelling Pro® software (Version 7.0.8, 2016, Norgwyn Montgomery Software, Inc., North Wales, PA, USA) was used for solubility parameter determinations.
3. Results and Discussion
3.1. Miscibility of Solvents, Solubility Parameter Values, and Solubility of EA in Candidate Vehicles
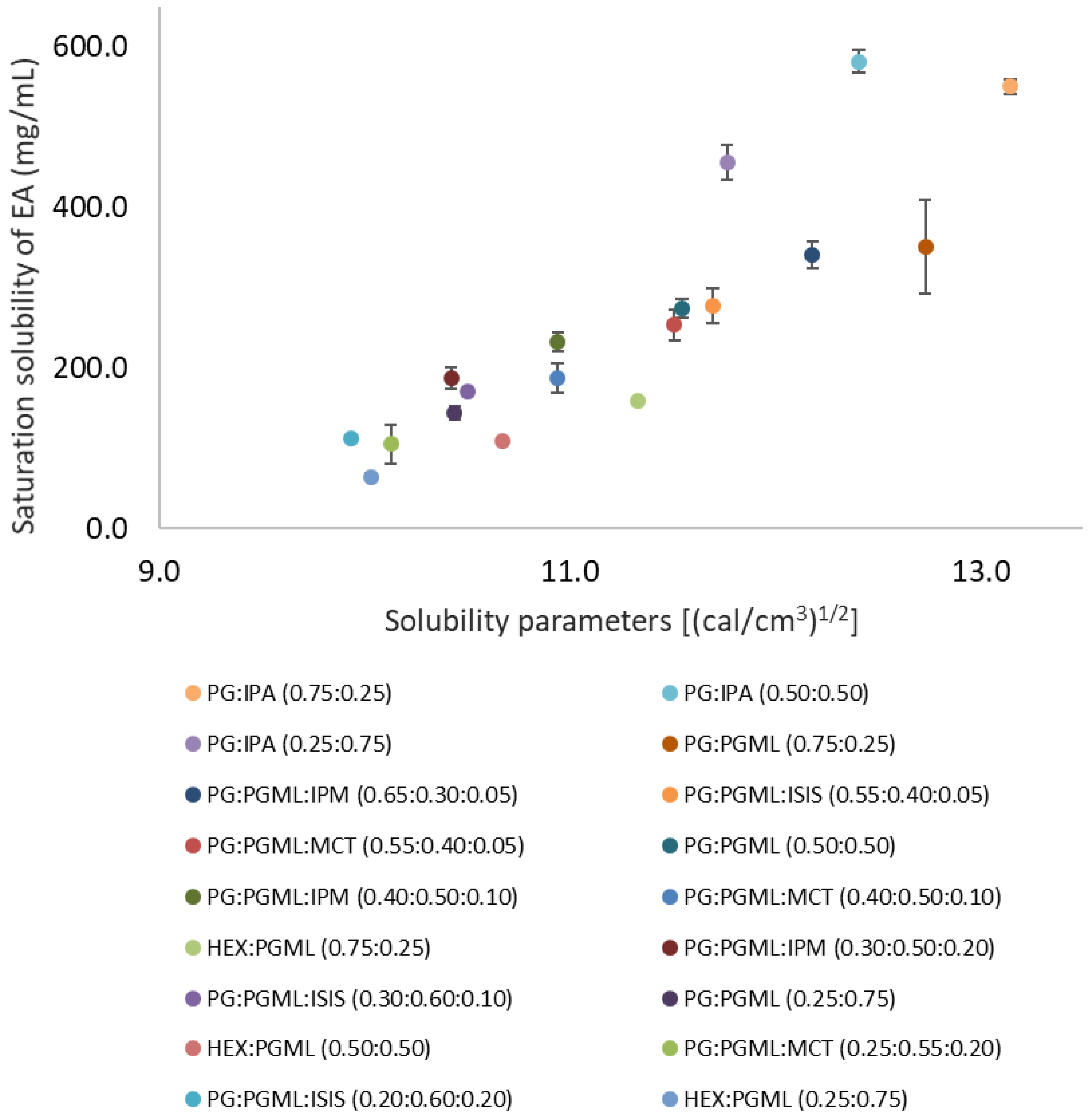
The binary solvent systems i.e., PG:PGML, HEX:PGML, and PG:IPA were miscible at all ratios. Ternary solvent vehicles consisting of PG:PGML:IPM, PG:PGML:ISIS, and PG:PGML:MCT were prepared based on their respective miscibility phase diagrams, as described previously [12]. The saturation solubility of EA in the mixtures as well as calculated solubility parameters of vehicles are shown in Figure 1.
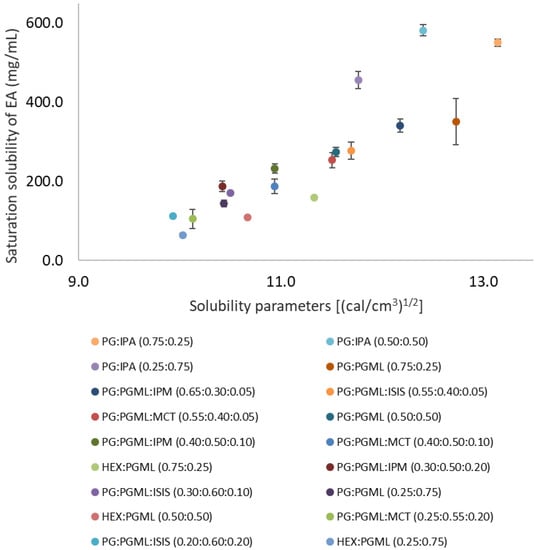
Figure 1.
Solubility parameter values of various solvents plotted against saturation solubility of 3-O-ethyl l-ascorbic acid in vehicles at 32 ± 1 °C (n = 3; mean ± SD).
Overall, combining the hydrophilic solvents PG and HEX with the fatty acid esters resulted in decreased EA solubility compared with the neat glycols. The saturation solubility of EA was higher in complex solvent systems with solubility parameters close to those of EA (15.4 ((cal/cm3)1/2). This is consistent with previous findings on EA saturation solubility in neat solvents, as well as with reports on the solubility of other compounds [5,14,15]. For binary solvent systems, EA solubility ranged from 64.4 ± 4.0 mg/mL to 550.4 ± 9.5 mg/mL, while for ternary solvent systems, EA solubility ranged from 104.6 ± 23.6 mg/mL to 340.9 ± 16.4 mg/mL (Figure 1). Saturation solubility of EA was not used for the permeation experiments but it was determined for identification of appropriate vehicles for formulations.
3.2. Porcine Skin Permeation and Mass Balance Studies of EA Using Binary Systems of PG and HEX with PGML
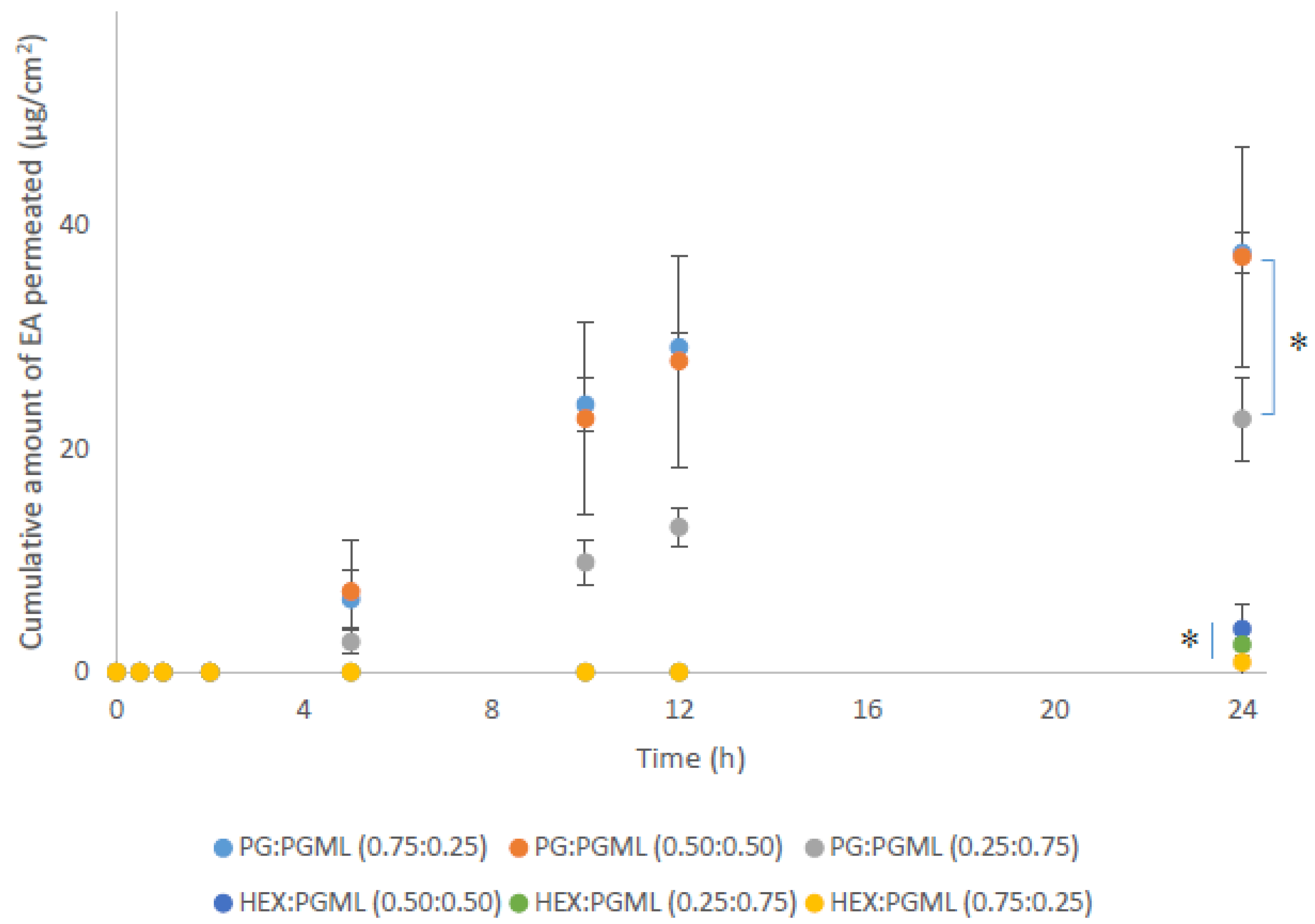
As shown in Figure 2, all PG:PGML vehicles significantly enhanced permeation of EA at 24 h across pig skin compared with the HEX:PGML combinations (p < 0.05).
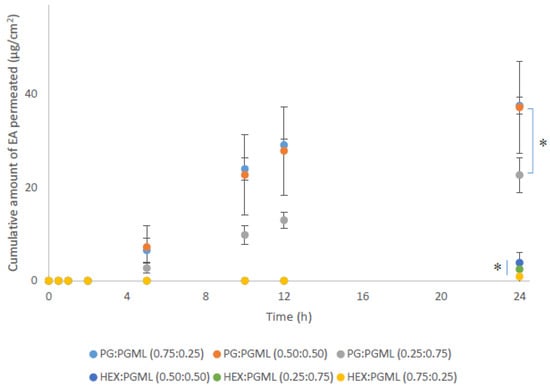
Figure 2.
Cumulative permeation of 3-O-ethyl l-ascorbic acid over time for various PG:PGML and HEX:PGML solvent systems (n ≥ 3; mean ± SD, * p < 0.05).
As regards the PG-containing vehicles, values for cumulative permeation of EA from the formulations PG:PGML (0.75:0.25) and PG:PGML (0.50:0.50) were 37.5 ± 1.9 μg/cm2 and 37.2 ± 9.8 μg/cm2, respectively (p > 0.05). Additionally, the vehicle PG:PGML (0.75:0.25) promoted significantly higher amounts of EA compared with PG:PGML (0.25:0.75), 22.7 ± 3.7 µg/cm2, (p < 0.05). All PG:PGML combinations delivered significantly higher amounts of EA compared with the neat PG (7.5 ± 3.2 µg/cm2, p < 0.05) or PGML (3.8 ± 0.5 µg/cm2, p < 0.05) [5]. All ratios of HEX:PGML vehicles delivered comparable amounts of EA over 24h, ranging from 0.9 ± 1.5 µg/cm2 to 3.9 ± 2.8 µg/cm2, p > 0.05. The values observed for these vehicles were not statistically different than from neat HEX (5.2 ± 2.1 µg/cm2, p > 0.05) or neat PGML (3.8 ± 0.5 µg/cm2, p > 0.05), as reported in previous work.
The solvent PGML was selected because it has been reported to have an influence on the skin barrier and acts as a penetration enhancer for certain compounds. Irion et al. used PGML as a vehicle for the transport of estradiol across human skin in vitro, and they reported good correlation of PGML penetration with drug transport through human epidermis [16]. In a later study, Jasti and Abraham [17] used frequency-domain fluorescence spectroscopy to investigate the influence of PGML on human epidermis in vitro. These researchers reported a high skin uptake of PGML, and they proposed that PGML is likely to affect skin delivery by altering the solubility of permeants in the skin. With regards to combinations of PG with PGML, previous studies in the literature have reported synergistic enhancement of topical delivery for a number of compounds. Mohammed et al. [18] reported that the vehicle PG:PGML (50:50) resulted in a significantly higher flux of niacinamide than PG across human skin in vitro and in vivo. More recently, Parisi et al. [19] showed that binary vehicles comprising PG and PGML at 50:50 ratios significantly increased topical delivery of both hexamidine diisethionate and the corresponding dihydrochloride salt in porcine skin in vitro compared with neat PG. The penetration enhancing effect of PGML was attributed to the presence of lauric acid in the solvent, a compound that was reported to increase the permeability of the skin to several compounds [20]. As regards the binary combinations of HEX with PGML, to our knowledge, there are no previous studies in the literature that have investigated the effects of these solvents on permeation of actives.
The results of the mass balance studies for EA binary formulations are shown in Table 1.

Table 1.
Cumulative permeation and percentage (%) recovery values of EA for various PG:PGML and HEX:PGML solvent systems following mass balance studies (n ≥ 3; mean ± SD).
Overall, the percentage permeation values of EA for the vehicles follow the same order as the cumulative amounts permeated. Significantly higher values for percentage permeation of applied EA were observed for all PG:PGML mixtures (30.9–45.9%) when compared with the HEX:PGML vehicles (0.9–4.5%, p < 0.05). Additionally, the percentage permeation of EA was comparable for PG:PGML (0.75:25) (45.9%) and PG:PGML (0.50:0.50) (41.1%, p > 0.05). However, the percentage permeation of EA for PG:PGML (0.75:0.25), 45.9%, was significantly higher than for PG:PGML (0.25:0.75), 30.9%, p < 0.05. For the HEX:PGML vehicles, similar percentages of applied EA permeated all solvent ratios (p > 0.05).
All combinations of PG:PGML resulted in similar values for percentage skin retention of applied EA, ranging from 16.7 to 21.6%, p > 0.05. These values were also comparable with the vehicles HEX:PGML (0.25:0.75), 8.7%, and HEX:PGML (0.50:0.50), 19.0%, p > 0.05. However, all PG:PGML mixtures promoted significantly higher EA skin retention compared with HEX:PGML (0.75:0.25); this vehicle delivered 2.1% of the applied EA to the skin (p < 0.05). Overall, it is apparent that PG:PGML vehicles promoted EA penetration to a greater extent compared with formulations consisting of HEX:PGML. The use of PGML was based on (i) its contrasting physicochemical properties to both PG and HEX, (ii) its miscibility with PG and HEX, and (iii) previous work demonstrating the ability of PGML to deliver significantly higher amounts of EA across the skin compared with a different lipophilic solvent with similar structure, namely propylene glycol mono-caprylate [5]. Finally, the total recovery values of EA for most formulations were lower than the recommended range in the OECD guidelines, namely 100 ± 20% [13]. This indicates that some breakdown of EA likely occurred as the compound permeated the skin, and this is consistent with our previous report [5]. Table 1 shows that total recovery values from the vehicles comprising PG:PGML were numerically greater; however, they were not significantly different to the HEX:PGML mixtures (p > 0.05).
3.3. Porcine Skin Permeation and Mass Balance Studies of EA Using Binary Systems of PG and IPA
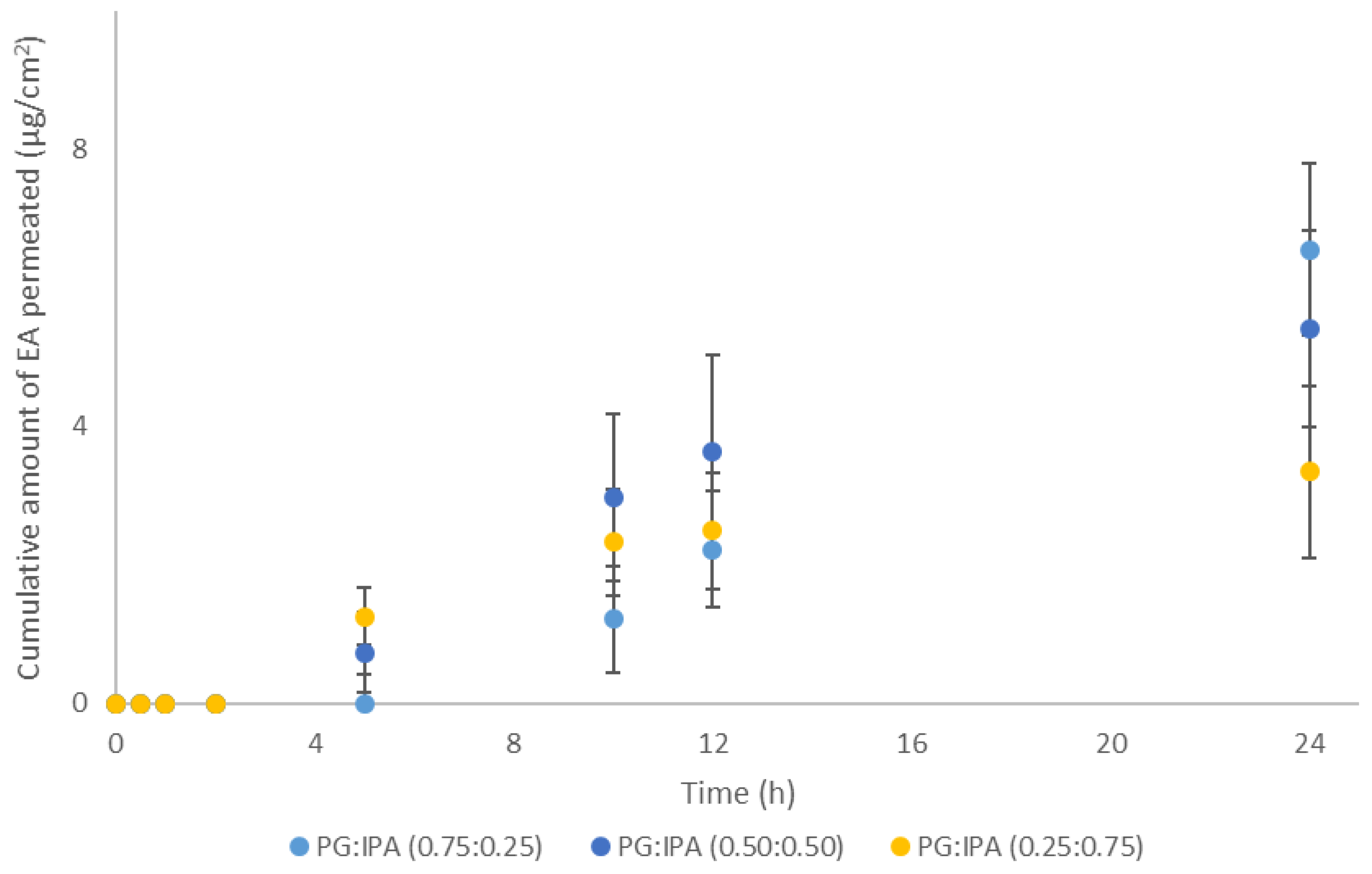
The use of volatile components as a strategy to influence thermodynamic activity of actives in formulations has previously been reported in the literature [21,22,23]. In the present work, binary solvent systems consisting of the short-chain alcohol 2-propanol (IPA) and the 1,2 glycol (PG) at ratios (0.75:0.25), (0.50:0.50), and (0.25:0.75) were prepared and their effects on EA skin delivery were investigated. The selection of these solvents was based on the use of IPA in topical formulations [14] as well as the effects of PG on EA skin permeation [5]. The volatile alcohol IPA is expected to evaporate rapidly from the skin surface following application of the PG:IPA vehicles, leaving a residual phase composed of increased concentrations of EA in the less volatile PG. The permeation results for these systems are shown in Figure 3.
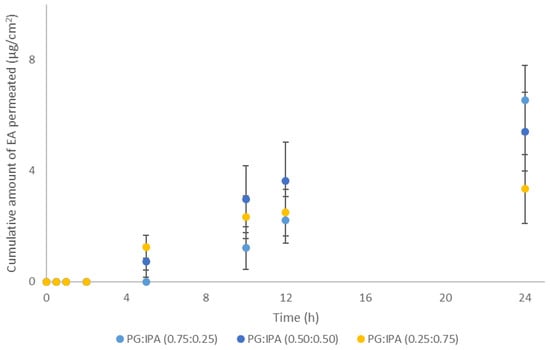
Figure 3.
Cumulative permeation of 3-O-ethyl l-ascorbic acid over time for solvent systems consisting of PG:IPA at various ratios (n ≥ 4; mean ± SD).
As shown in Figure 3, no statistical differences were apparent among the vehicles of different solvent ratios. The cumulative permeation of EA at 24 h ranged from 3.4 ± 1.2 µg/cm2 to 6.6 ± 3.9 µg/cm2, p > 0.05. However, only PG:IPA (0.75:0.25) promoted significantly higher amounts of EA permeation compared with neat IPA (0.5 ± 0.6 μg/cm2, p < 0.05), but not compared to PG alone (7.5 ± 3.2, p > 0.05), as reported elsewhere [5].
The effects of IPA on skin have been reported in the literature and this solvent has been reported to be a penetration enhancer for several compounds. Coldman et al. [24] used PG:IPA vehicles to investigate the penetration of a model compound, fluocinolone acetonide, as well as its acetate ester, in human skin in vitro. Permeation of drugs was enhanced compared to the pure PG and this was attributed to the increase of the drug concentration caused by the evaporation of the volatile component, rather than to the effects of IPA on the skin barrier. In a later study, Goates et al. [25] treated human stratum corneum with solutions of 75% (v/v) IPA in deuterium oxide and used FTIR to investigate the effect of IPA on the barrier structure. These researchers proposed that skin treatment with these solutions resulted in extraction of lipids from the membrane and suggested that permeation enhancement of polar compounds could be attributed to removal of stratum corneum lipid components. In a later study, Brinkmann and Muller-Goymann examined the effect of IPA on permeation of hydrocortisone across human skin in vitro [26]. These workers also conducted DSC, wide angle, and small angle X-ray diffraction studies following treatment of human stratum corneum with IPA to assess the effects of IPA on the skin. The solvent was reported to disturb the lipid bilayer structure and increase fluidity of skin lipids, thereby promoting permeation of the drug. Recently, Parisi et al. reported that combinations of PG:IPA (50:50) resulted in a significant increase of permeation of hexamidine diisethionate across porcine skin in vitro compared to the individual solvents [19]. In the present work, no PG:IPA combination outperformed neat PG, which delivered 7.5 ± 3.2 μg/cm2 of EA over 24 h (p > 0.05) [5].
The results for the mass balance studies for EA binary formulations are shown in Table 2.

Table 2.
Cumulative permeation and percentage (%) recovery values of EA for various PG:IPA solvent systems following mass balance studies (n ≥ 4; mean ± SD).
Overall, percentage permeation values of EA for the various vehicles ranged from 5.5% to 8.8% of the applied doses and no statistical differences were evident (p > 0.05). Additionally, all vehicles comprising of PG:IPA resulted in similar percentages of EA skin deposition, ranging from 18.2% to 22.5%, p > 0.05. These values were also comparable for the results observed for the neat solvents PG 19.5%, and IPA 20.3%, p > 0.05 [5].
3.4. Porcine Skin Permeation and Mass Balance Studies of EA Using Ternary Systems of ISIS, MCT, and IPM with PG:PGML
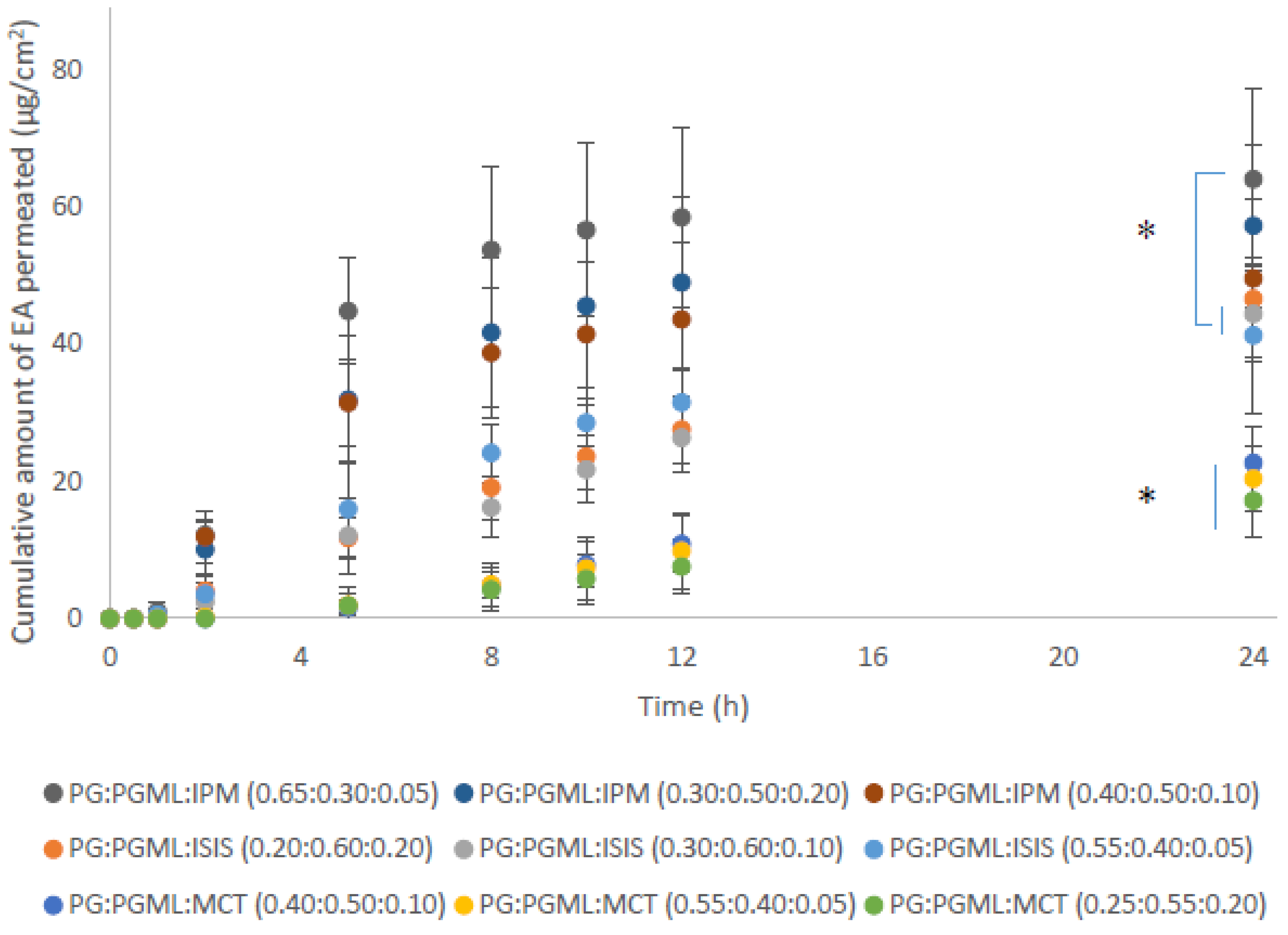
As reported above, combinations of PG with PGML resulted in superior permeation of EA compared with mixtures of HEX:PGML. Therefore, ternary solvent systems comprising PG:PGML and either isopropyl myristate (IPM), isostearyl isostearate (ISIS), or medium-chain triglycerides (MCT) were prepared at various solvent ratios. These solvents are fatty acid esters and their selection was based on the same principles as explained for the binary systems in the previous section. The ratios of the solvents were selected according to their mutual miscibility and the following vehicles were prepared: PG:PGML:IPM (0.65:0.30:0.05), PG:PGML:IPM (0.40:0.50:0.10), PG:PGML:IPM (0.30:0.50:0.20), PG:PGML:ISIS (0.55:0.40:0.05), PG:PGML:ISIS (0.30:0.60:0.10), PG:PGML:ISIS (0.20:0.60:0.20), PG:PGML:MCT (0.55:0.40:0.05), PG:PGML:MCT (0.40:0.50:0.10), and PG:PGML:MCT (0.25:0.55:0.20). Overall, the experiments showed that all ternary formulations promoted skin permeation of EA over 24 h and the cumulative EA permeation among the different solvent mixtures may be ranked as follows: PG:PGML:IPM > PG:PGML:ISIS > PG:PGML:MCT, as shown in Figure 4.
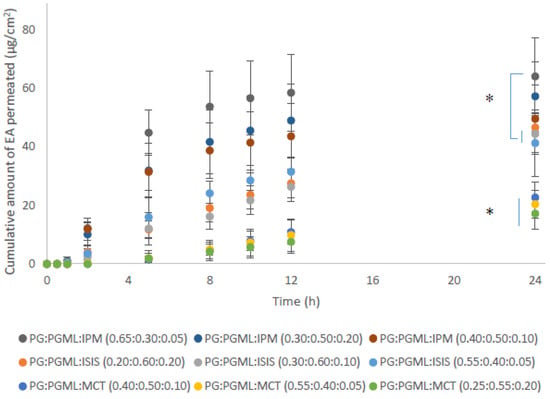
Figure 4.
Cumulative permeation of 3-O-ethyl l-ascorbic acid over time for various ternary solvent systems (n = 5; mean ± SD, * p < 0.05).
With regards to the IPM vehicles, similar cumulative permeation of EA was found for all ratios, ranging from 49.6 ± 11.6 μg/cm2 to 64.1 ± 13.3 μg/cm2 (p > 0.05). Additionally, all mixtures of PG:PGML:IPM resulted in significantly higher amounts of EA permeation when compared with all PG:PGML:MCT formulations (17.2 ± 5.3 μg/cm2 to 22.7 ± 5.2 μg/cm2, p < 0.05). The influence of IPM on the skin barrier has been investigated by several researchers. Brinkmann and Muller-Goymann [21] used differential scanning calorimetry, wide and small angle X- ray diffraction to investigate the effects of this solvent on human stratum corneum. IPM was reported to be inserted into the membrane and to cause a disorder of the lipid structure. This theory was further supported in a later study by Engelbrecht et al. [27], who examined the impact of IPM on stratum corneum lipid model membranes by using neutron diffraction. In a more recent study, Eichner et al. [28] proposed that IPM interacted with the stratum corneum lipid head groups and disturbed the lipid structures by forming a micellar or cubic phase in the membrane.
LabrafacTM (MCT) is a mixture of medium-chain triglycerides of caprylic (C8) and capric (C10) acids and has been widely used as a lipophilic ingredient in personal care products [29]. As regards its potential to enhance permeation of actives, contrasting reports are found in the literature. Leopold and Lippold [30] used differential scanning calorimetry to examine the impact of various lipophilic solvents on human stratum corneum in vitro. The authors reported that MCT did not induce apparent changes in the lipid-phase transitions of the stratum corneum compared with the untreated stratum corneum samples and proposed that MCT did not interact with the skin lipids. However, in a recent study, a ternary solvent vehicle consisting of MCT, PG and PGML was examined both in vitro and in vivo in human skin and resulted in significantly higher flux values of niacinamide compared with other vehicles [18]. In the present work, all PG:PGML:MCT mixtures delivered significantly lower cumulative amounts of EA compared with all other ternary solvent systems, (p < 0.05), as shown in Figure 4. Additionally, EA permeation from the vehicle PG:PGML:MCT (0.25:0.55:0.20) was comparable to values observed for the binary system PG:PGML (0.25:0.75) (17.2 ± 5.3 μg/cm2 and 22.7 ± 3.7 μg/cm2 respectively, p > 0.05). The EA penetration amount from PG:PGML:MCT (0.40:0.50:0.10) was 22.7 ± 5.2 μg/cm2, which was not statistically different than from the PG:PGML (0.50:0.50) (37.2 ± 9.8, p > 0.05). However, PG:PGML:MCT vehicles at ratios (0.25:0.55:0.20) and (0.55:0.40:0.05) delivered significantly lower cumulative amounts of EA compared with PG:PGML (0.75:0.25) (37.5 ± 1.9 μg/cm2, p < 0.05). Overall, the presence of MCT in the formulations resulted in either lower or similar cumulative permeation compared with the various ratios of the binary solvent systems PG:PGML (p > 0.05).
As regards vehicles containing the solvent isostearyl isostearate (ISIS), the findings of this study showed that all combinations of PG:PGML:ISIS resulted in higher permeation of EA compared with the vehicles PG:PGML:MCT (0.25:0.55:0.20) (17.2 ± 5.3 μg/cm2, p < 0.05) and PG:PGML:MCT (0.55:0.40:0.05) (20.4 ± 4.9 μg/cm2, p < 0.05). ISIS is a lipophilic and commonly used ingredient in cosmetic preparations, and is believed to have a moisturising effect on the skin barrier. This has been examined in various in vitro and in vivo studies. For example, in 2007, Caussin et al. [31] applied various neat lipophilic solvents to dermatomed human skin in Franz-diffusion cells and examined their impact on the skin samples by cryo-scanning electron microscopy. They reported that stratum corneum thickness of both ISIS-treated and petrolatum-treated samples was increased compared with other lipophilic solvents, and this was attributed to an increase of the water content in the stratum corneum. The influence of ISIS on the skin barrier was also examined in vitro using isolated lipid mixtures that mimic the lipid composition of stratum corneum [32]. These researchers used Fourier transform infrared spectroscopy (FTIR) to investigate the influence of various lipophilic solvents, including ISIS, on the structure of the lipids and compared it with a control where no solvent was added. It was reported that ISIS was incorporated into the lipid domains, and the thermotropic stability of their orthorhombic lateral packing was increased. It was suggested that ISIS could promote an orthorhombic rather than a hexagonal lipid organisation and thus contribute to improved skin barrier function. This hypothesis was later investigated in an in vivo study, using a plastic occlusion stress test on human subjects following application of various neat lipophilic solvents [33]. ISIS significantly increased hydration of the skin compared with both the control and the sites that were treated with other lipophilic moisturisers i.e., petrolatum, isopropyl isostearate, and glyceryl mono-isostearate. In the present work, ISIS-containing vehicles enhanced the cumulative permeation of the water-soluble active ingredient EA, with values ranging from 41.3 ± 11.3 μg/cm2 to 46.7 ± 5.1 μg/cm2, which were similar for all ratios of PG:PGML:ISIS (p > 0.05). However, both PG:PGML:ISIS (0.30:0.60:0.10) (44.4 ± 6.9 μg/cm2) and PG:PGML:ISIS (0.55:0.40:0.05) (41.3 ± 11.3 μg/cm2) were outperformed by PG:PGML:IPM (0.65:0.30:0.05), (64.1 ± 13.3 μg/cm2, p < 0.05), indicating that the presence of IPM had a greater impact on EA skin delivery.
The percentage permeation values of EA among the different formulations followed the same trend as described for the cumulative permeation and are shown in Table 3. PG:PGML:IPM (0.65:0.30:0.05) delivered 70.9% of the applied EA, which was significantly higher compared to all ratios of the binary solvent systems PG:PGML (30.9–45.9%, p < 0.05). This value was also significantly higher than the percentage permeation of applied EA from all ratios of PG:PGML:MCT (20.5–27.8%, p < 0.05) as well as the vehicles PG:PGML:ISIS (0.55:0.40:0.05) (49.5%, p < 0.05) and PG:PGML:ISIS (0.30:0.60:0.10) (48.9%, p < 0.05). The findings suggest that the addition of IPM led to synergistic enhancement of EA skin delivery, which was greater compared with either ISIS or MCT.

Table 3.
Cumulative permeation and percentage (%) recovery values of EA for ternary solvent systems following mass balance studies (n = 5; mean ± SD).
As regards the retention of the active inside the skin, higher percentages of EA were extracted for the combinations of PG:PGML:MCT (13.3–21.6%) compared to all other formulations (3.3–8.0%, p < 0.05). Similar percentages of EA were deposited in skin for all PG:PGML:ISIS (6.2–8.0%) and PG:PGML:IPM vehicles (3.3–4.1%, p > 0.05). The total recovery for all formulations ranged from 68.7% to 88.2%. As noted in the previous section, some of these values are outside the recommended recovery limits (100 ± 20%) established by the OECD guidelines [13] and indicate that EA is subject to partial breakdown in the skin. This is likely to reflect conversion of the molecule to Vitamin C. Low total recovery of EA warrants further investigation for identification of potential conversion products and this will be the subject of future work.
4. Conclusions
EA is widely used as a more stable derivative of l-ascorbic acid. Previous work examined the influence of commonly used excipients on EA skin permeation in vitro. The present study reported the rational design of complex formulations and evaluated the effect of several solvent systems on EA skin delivery. Among the binary solvent systems, combinations of PG with PGML did enhance penetration of EA, with higher delivery observed when PG was present at higher amounts (75%). In contrast, HEX:PGML systems did not improve EA penetration compared with the individual solvents (p > 0.05). Addition of various amounts of the volatile solvent IPA to PG solutions did not improve EA skin delivery compared with the neat PG. With regards to the ternary solvent systems, the vehicles containing a fatty acid ester, either IPM or ISIS, were more efficacious than those containing MCT. Overall, PG:PGML:IPM (0.65:0.30:0.05) was found to be the most effective formulation, indicating that, as in binary solvent systems, high amounts of PG are related to higher EA delivery. The low recovery of EA following finite dose permeation studies was previously reported and again confirmed in this study. It is likely that EA converts to the parent compound, l-ascorbic acid, in the skin. In future work, identification of conversion products will give further insights into the fate of EA inside the skin. Additionally, EA topical delivery will be investigated in vivo using well-established techniques such as confocal Raman spectroscopy and tape stripping. The in vivo studies will also enable the investigation of the impact of topical EA on the skin by measuring parameters indicative of the condition of the skin barrier, such as the stratum corneum thickness and the trans-epidermal water loss.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-0532/88/2/19/s1.
Author Contributions
Conceptualization, F.I., M.E.L., and R.A.L.; methodology, F.I. and M.E.L.; validation, M.E.L. and F.I.; formal analysis, F.I.; investigation, F.I.; data curation, F.I. and M.E.L.; writing—original draft preparation, F.I.; writing—review and editing, F.I., M.E.L., A.S.M.M.A.H., B.C.S., R.A.L., and D.J.M.; visualization, F.I., M.E.L., A.S.M.M.A.H., and B.C.S.; supervision, M.E.L., R.A.L., and D.J.M.; project administration, M.E.L. and F.I.; funding acquisition, M.E.L., F.I., R.A.L., and D.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
Fotis Iliopoulos is grateful for financial support from the Engineering and Physical Sciences Research Council (EPSRC) and GlaxoSmithKline (GSK, UK).
Acknowledgments
We thank our colleagues from the UCL Skin Research Group who provided insight and expertise that greatly assisted the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapiere, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapière, C.M. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef]
- EWG. Ethyl Ascorbic Acid. Available online: https://www.ewg.org/skindeep/ingredients/718948-ethyl-ascorbic-acid (accessed on 11 July 2019).
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhaes, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of antioxidants in anti-aging cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef]
- Bandelin, F.J.; Tuschhoff, J.V. The stability of ascorbic acid in various liquid media. J. Am. Pharm. Assoc. 1955, 44, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E.; Hadgraft, J.; Oliveira, G.; Vieira, R.; Mohammed, D.; Hirata, K. Rational formulation design. Int. J. Cosmet. Sci. 2012, 34, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In Vitro Evaluation of Sunscreen Safety: Effects of the Vehicle and Repeated Applications on Skin Permeation from Topical Formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Mitragotri, S. Synergistic Effect of Enhancers for Transdermal Drug Delivery. Pharm. Res. 2000, 17, 1354–1359. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin II. Influence of binary and ternary solvent systems. Eur. J. Pharm. Sci. 2018, 121, 59–64. [Google Scholar] [CrossRef]
- OECD. Test. No. 28: Guidance Document for the Conduct of Skin Absorption Studies; Organisation for Economic Cooperation and Development Publishing: Paris, France, 2004. [Google Scholar]
- Hossain, A.S.M.M.A.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Monjur Al Hossain, A.S.M.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical delivery of niacinamide: Influence of neat solvents. Int. J. Pharm. 2020, 579, 119137. [Google Scholar] [CrossRef] [PubMed]
- Irion, G.D.; Garrison, M.D.; Abraham, W. Effect of PGML Excipient Mixture in a Transdermal System on the in Vitro Transport of Estradiol across Skin. Pharm. Res. 1995, 12, 1618–1622. [Google Scholar] [CrossRef]
- Jasti, B.R.; Abraham, W. Fluorescence Spectroscopic Investigation of Effect of Excipients on Epidermal Barrier and Transdermal Systems. J. Investig. Dermatol. Symp. Proc. 1998, 3, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Parisi, N.; Paz-Alvarez, M.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Topical delivery of hexamidine. Int. J. Pharm. 2016, 506, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Guy, R.H.; Hadgraft, J. In vitro and in vivo enhancement of skin permeation with oleic and lauric acids. Int. J. Pharm. 1988, 48, 103–111. [Google Scholar] [CrossRef]
- Lane, M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Passive Skin Permeation Enhancement. In Topical and Transdermal Drug Delivery; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of percutaneous absorption by the use of volatile: Nonvolatile systems as vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef]
- Goates, C.Y.; Knutson, K. Enhanced permeation of polar compounds through human epidermis. I. Permeability and membrane structural changes in the presence of short chain alcohols. Biochim. Biophys. Acta 1994, 1195, 169–179. [Google Scholar] [CrossRef]
- Brinkmann, I.; Muller-Goymann, C.C. Role of isopropyl myristate, isopropyl alcohol and a combination of both in hydrocortisone permeation across the human stratum corneum. Skin Pharmacol. Physiol. 2003, 16, 393–404. [Google Scholar] [CrossRef]
- Engelbrecht, T.N.; Demé, B.; Dobner, B.; Neubert, R.H.H. Study of the Influence of the Penetration Enhancer Isopropyl Myristate on the Nanostructure of Stratum Corneum Lipid Model Membranes Using Neutron Diffraction and Deuterium Labelling. Skin Pharmacol. Physiol. 2012, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Eichner, A.; Stahlberg, S.; Sonnenberger, S.; Lange, S.; Dobner, B.; Ostermann, A.; Schrader, T.E.; Hauß, T.; Schroeter, A.; Huster, D.; et al. Influence of the penetration enhancer isopropyl myristate on stratum corneum lipid model membranes revealed by neutron diffraction and 2H NMR experiments. Biochim. Biophys. Acta 2017, 1859, 745–755. [Google Scholar] [CrossRef]
- EWG. Caprylic/Capric Triglyceride. Available online: https://www.ewg.org/skindeep/ingredient/701056/caprylic%3B%3B_capric_triglyceride/ (accessed on 2 November 2019).
- Leopold, C.S.; Lippold, B.C. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC). J. Pharm. Pharmacol. 1995, 47, 276–281. [Google Scholar] [CrossRef]
- Caussin, J.; Groenink, H.W.; de Graaff, A.M.; Gooris, G.S.; Wiechers, J.W.; van Aelst, A.C.; Bouwstra, J.A. Lipophilic and hydrophilic moisturizers show different actions on human skin as revealed by cryo scanning electron microscopy. Exp. Dermatol. 2007, 16, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Caussin, J.; Gooris, G.S.; Bouwstra, J.A. FTIR studies show lipophilic moisturizers to interact with stratum corneum lipids, rendering the more densely packed. Biochim. Biophys. Acta 2008, 1778, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Pennick, G.; Harrison, S.; Jones, D.; Rawlings, A.V. Superior effect of isostearyl isostearate on improvement in stratum corneum water permeability barrier function as examined by the plastic occlusion stress test. Int. J. Cosmet. Sci. 2010, 32, 304–312. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).