Anti-Inflammatory and Antioxidant Activities of Medicinal Plants Used by Traditional Healers for Antiulcer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of the Extracts
2.2. Phytochemical Screening Test
2.3. Total Phenolic Content (TPC)
2.4. Total Flavonoid Content (TFC)
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.6. 2,2’-Azino-bis-3-ethylbenzthiazoline-6-sulfonic Acid (ABTS) Radical Scavenging Assay
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. NO Production Assay
2.10. Total RNA Extraction and cDNA Preparation
2.11. Quantitative Real-Time PCR (qPCR)
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Screening
3.2. TPC and TFC of the Extracts
3.3. DPPH and ABTS Radical Scavenging Assay
3.4. Effects of the Extracts on Cell Viability
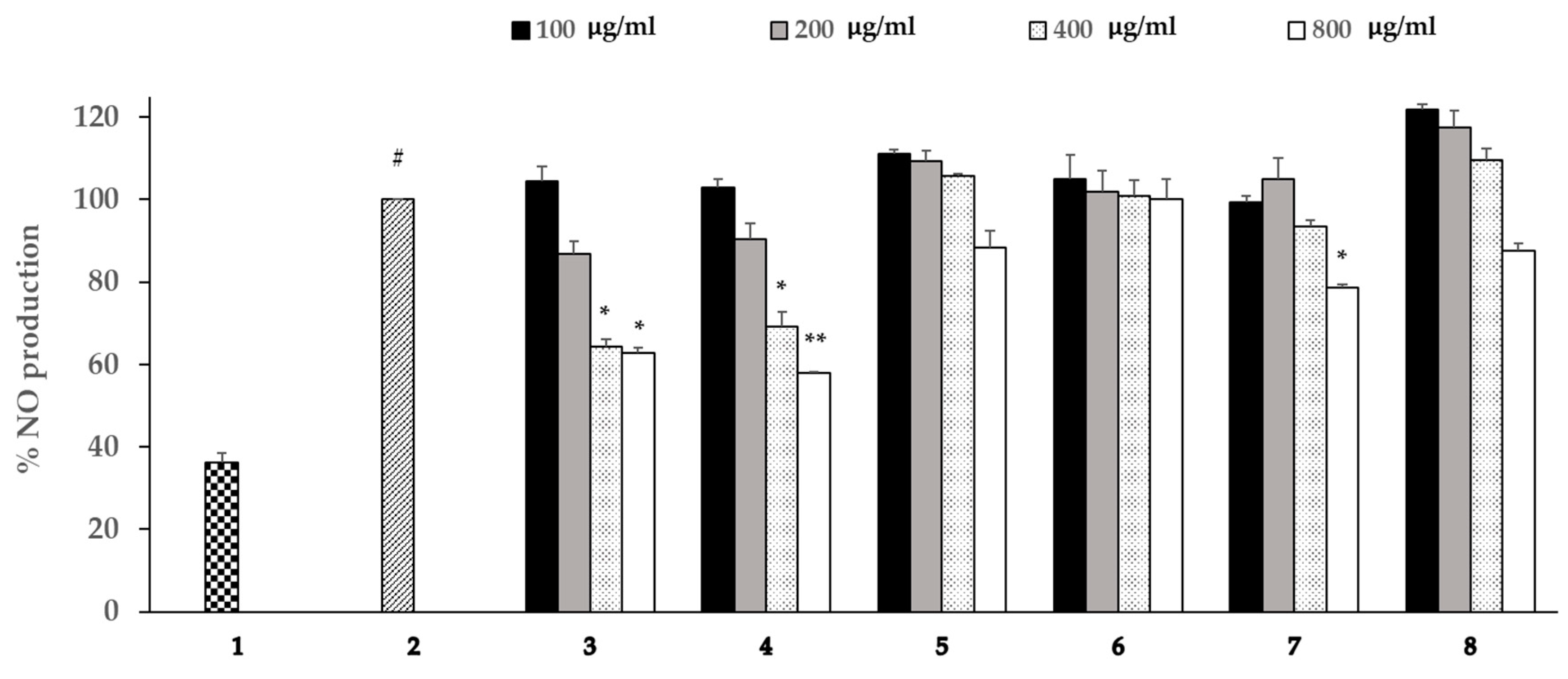
3.5. Effects of the Extracts on the Production of NO
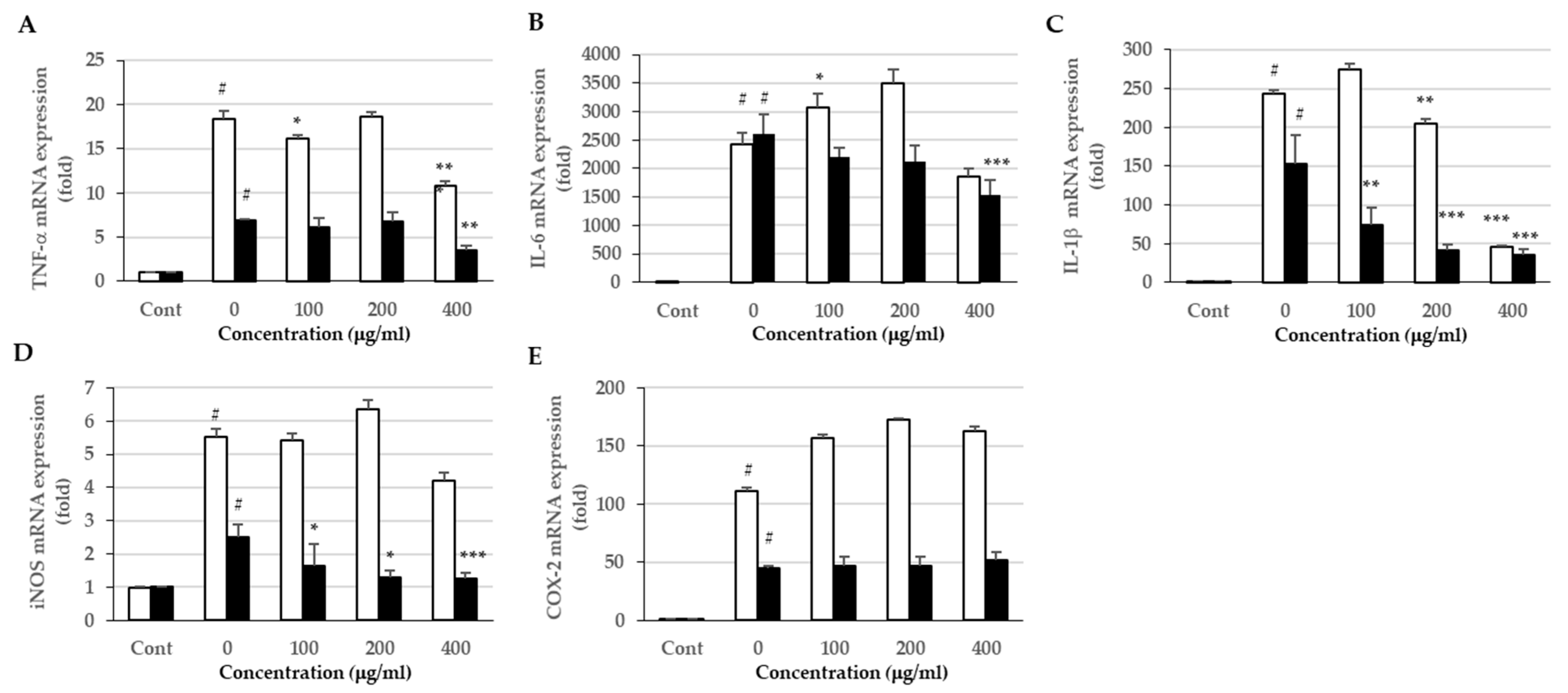
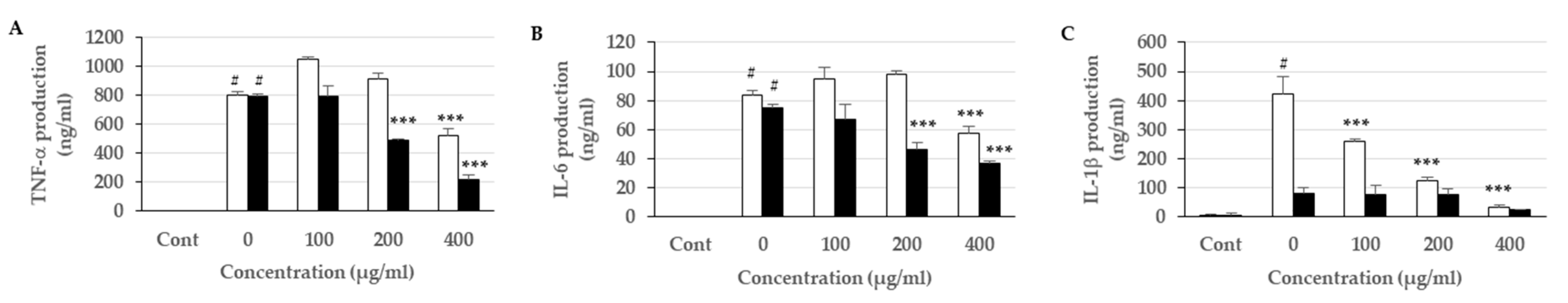
3.6. Effects of the Extracts on Proinflammatory Cytokine Expression and Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.-U.; Song, E.-K.; Yoo, C.-H.; Kwak, Y.-K.; Han, M.-K. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol. Cells 2012, 34, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, E.-J.; Kim, S.-K.; Jeong, P.; Cho, Y.-H.; Yun, S.J.; Kim, S.; Kim, G.-Y.; Choi, Y.H.; Cha, E.-J.; et al. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PloS ONE 2012, 7, e0040267. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Beckert, S.; Class, N.; Farrahi, F.; Coerper, S. Growth hormone enhances gastric ulcer healing in rats. Med. Sci. Monit. 2004, 10, Br255–Br258. [Google Scholar] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut--their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar]
- Leung, F.W.; Su, K.C.; Pique, J.M.; Thiefin, G.; Passaro, E., Jr.; Guth, P.H. Superior mesenteric artery is more important than inferior mesenteric artery in maintaining colonic mucosal perfusion and integrity in rats. Dig. Dis. Sci. 1992, 37, 1329–1335. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Ginter, G.; Ptak-Belowska, A.; Dembinski, A. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J. Physiol. Pharmacol. 2014, 65, 95–106. [Google Scholar]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kownacki, P.; Konturek, P.C. Role of sensory nerves in gastroprotective effect of anandamide in rats. J. Physiol. Pharmacol. 2011, 62, 207–217. [Google Scholar]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Pretreatment with obestatin reduces the severity of ischemia/reperfusion-induced acute pancreatitis in rats. Eur. J. Pharmacol. 2015, 760, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Kusnierz-Cabala, B.; Konturek, P.; Ambrozy, T.; Dembinski, A. Obestatin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 2834386. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. Biomed. Res. Int. 2015, 2015, 718314. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, R.S.M.; Wieslawpawlik, W.; Stanislawkonturek, J. Deleterious Effect of Helicobacter pylori Infection on the Course of Acute Pancreatitis in Rats. Pancreatology 2002, 2, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori infection. Mol. Med. 2018, 115, 219–224. [Google Scholar]
- Lanza, F.L.; Chan, F.K.; Quigley, E.M. Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol. 2009, 104, 728–738. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef]
- Somasundaram, S.; Sigthorsson, G.; Simpson, R.J.; Watts, J.; Jacob, M.; Tavares, I.A.; Rafi, S.; Roseth, A.; Foster, R.; Price, A.B.; et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharmacol. Ther. 2000, 14, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Brzozowska, I.; Pawlik, T. Role of prostaglandins in gastroprotection and gastric adaptation. J. Physiol. Pharmacol. 2005, 56 (Suppl. 5), 33–55. [Google Scholar]
- Peskar, B.M.; Maricic, N. Role of prostaglandins in gastroprotection. Dig. Dis. Sci. 1998, 43, 23s–29s. [Google Scholar] [PubMed]
- Tripathi, K. Essentials of Medical Pharmacology, 6th ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2008; pp. 453–474. [Google Scholar]
- Bennett, P.N.; Brown, M.J. Clinical Pharmacology; Churchill Livingstone: London, UK, 2005. [Google Scholar]
- Abbas, A.M.; Sakr, H.F. Effect of selenium and grape seed extract on indomethacin-induced gastric ulcers in rats. J. Physiol. Biochem. 2013, 69, 527–537. [Google Scholar] [CrossRef]
- Nagori, B.P.; Solanki, R. Role of Medicinal Plants in Wound Healing. J. Med. Plants Res. 2011, 5, 392–405. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.; Bamagous, G.; Omran, G. Anti-ulcerogenic effect of genistein against indomethacin-induced gastric ulcer in rats. Asian J. Pharm. Clin. Res. 2016, 9, 58–63. [Google Scholar]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J. Trop. Med. Public Health 2001, 32, 208–215. [Google Scholar]
- Deb Roy, S.; Chakraborty, J.; Shil, D.; Das, S.; Begum, N. Herbs Used In Peptic Ulcer: A Review. Int. J. Pharm. Res. Allied Sci. 2013, 2, 9–23. [Google Scholar]
- Boggula, N.; Peddapalli, H. Phytochemical analysis and evaluation of in vitro anti oxidant activity of Punica granatum leaves. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1110–1118. [Google Scholar] [CrossRef]
- Guo, X.; Yoshitomi, H.; Gao, M.; Qin, L.; Duan, Y.; Sun, W.; Xu, T.; Xie, P.; Zhou, J.; Huang, L.; et al. Guava leaf extracts promote glucose metabolism in SHRSP.Z-Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement. Altern. Med. 2013, 13, 52. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.; Carneiro, J.N.; Machado, A.J.; Dos Santos, A.T.; Sales, D.L.; Lima, L.F.; Figueredo, F.G.; Coutinho, H.D. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J. Ethnopharmacol. 2016, 194, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Sharma, M.; Pillai, K.K.; Aeri, V.; Sheliya, M.A. Inhibitory effect of Careya arborea on inflammatory biomarkers in carrageenan-induced inflammation. Pharm. Biol. 2015, 53, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.C.; Rao, C.V. Gastroprotective effect of standardized leaf extract from Careya arborea on experimental gastric ulcers in rats. Pharm. Biol. 2014, 52, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Chea, A.; Jonville, M.C.; Bun, S.S.; Laget, M.; Elias, R.; Dumenil, G.; Balansard, G. In vitro antimicrobial activity of plants used in Cambodian traditional medicine. Am. J. Chin. Med. 2007, 35, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dai, W.; Li, B.; Zhang, J.; Chen, Q.; Zhang, M. Chemical Constituents from Gochnatia decora Barks and Their Anti-inflammatory Activities. J. Trop. Subtrop. Bot. 2017, 10. [Google Scholar]
- Zhang, M.; Zhao, C.; Dai, W.; He, J.; Jiao, S.; Li, B. Anti-inflammatory ent-kaurenoic acids and their glycosides from Gochnatia decora. Phytochemistry 2017, 137, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bopage, N.S.; Gunaherath, G.M.K.B.; Jayawardena, K.H.; Wijeyaratne, S.C.; Abeysekera, A.M.; Somaratne, S. Dual function of active constituents from bark of Ficus racemosa L in wound healing. BMC Complement. Altern. Med. 2018, 18, 29. [Google Scholar] [CrossRef]
- Tanvi, P.; Pallavi, D.; Sunita, O. Antibacterial and Antifungal Approaches of Ficus racemosa. Pharmacogn. J. 2019, 11. [Google Scholar]
- Mohammadi, M.; Alaei, M.; Bajalan, I. Phytochemical screening, total phenolic and flavonoid contents and antioxidant activity of Anabasis setifera and Salsola tomentosa extracted with different extraction methods and solvents. Orient. Pharm. Exp. Med. 2016, 16, 31–35. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis, 3rd ed.; SpringerScience & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Chumphukam, O.; Pintha, K.; Khanaree, C.; Chewonarin, T.; Chaiwangyen, W.; Tantipaiboonwong, P.; Suttajit, M.; Khantamat, O. Potential anti-mutagenicity, antioxidant, and anti-inflammatory capacities of the extract from perilla seed meal. J. Food Biochem. 2018, 42, e12556. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J. Anti-aging and tyrosinase inhibition effects of Cassia fistula flower butanolic extract. BMC Complement. Altern. Med. 2016, 16, 497. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein activity and reversal of cancer multidrug resistance by Momordica charantia extract. Cancer Chemother. Pharmacol. 2004, 54, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Budluang, P.; Pitchakarn, P.; Ting, P.; Temviriyanukul, P.; Wongnoppawich, A.; Imsumran, A. Anti-inflammatory and anti-insulin resistance activities of aqueous extract from Anoectochilus burmannicus. Food Sci. Nutr. 2017, 5, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, S.; Arsenijevic, J.; Bozic, D.; Milenkovic, M.; Tesevic, V.; Maksimovic, Z. Antioxidant, anti-inflammatory and gastroprotective activity of Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench. J. Ethnopharmacol. 2018, 213, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Unsicker, S.B.; Fellenberg, C.; Constabel, C.P.; Schmidt, A.; Gershenzon, J.; Hammerbacher, A. Flavan-3-ols Are an Effective Chemical Defense against Rust Infection. Plant Physiol. 2017, 175, 1560–1578. [Google Scholar] [CrossRef]
- Franklin, R.; Bispo, R.F.M.; Sousa-Rodrigues, C.F.; Pires, L.A.S.; Fonseca, A., Jr.; Babinski, M.A. Grape Leucoanthocyanidin Protects Liver Tissue in Albino Rabbits with Nonalcoholic Hepatic Steatosis. Cells Tissues Organs 2018, 205, 129–136. [Google Scholar] [CrossRef]
- Howe, E.; Keiwkarnka, B.; Khan, M.I. Traditional medicine and medicinal plants: utilization, policy and research in Thailand. J. Public Health 2004, 2, 102. [Google Scholar]
- Panthong, A.; Kanjanapothi, D.; Taylor, W.C. Ethnobotanical review of medicinal plants from thai traditional books, Part I: Plants with anti-inflammatory, anti-asthmatic and antihypertensive properties. J. Ethnopharmacol. 1986, 18, 213–228. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological and therapeutic effects of Jasminum sambac-A review. Indo Am. J. Pharm. Sci. 2018, 5, 1766–1778. [Google Scholar]
- Li, H.; Zhang, Z.; He, D.; Xia, Y.; Lliu, Q.; Li, X. Ultrasound-assisted aqueous enzymatic extraction of oil from perilla seeds and determination of its physicochemical properties, fatty acid composition and antioxidant activity. J. Food Sci. Technol. 2017, 37, 71–77. [Google Scholar] [CrossRef]
- Mohammad Sharrif, M.; Kashani Hamed, H. Chemical composition of the plant Punica granatum L. (Pomegranate) and its effect on heart and cancer. J. Med. Plants Res. 2012, 6, 5306–5310. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; Trovatti, E.; Marto, J.; Almeida-Cincotto, M.G.J.d.; Melero, A.; Corrêa, M.A.; Chiavacci, L.A.; Ribeiro, H.; Garrigues, T.; Isaac, V.L.B. Guava: phytochemical composition of a potential source of antioxidants for cosmetic and/or dermatological applications. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Ikegami, F.; Okonogi, S. Antioxidant Active Principles Isolated from Psidium guajava Grown in Thailand. Sci. Pharm. 2007, 75, 179–193. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Amjad, L.; Shafighi, M. Antioxidant activity of leaf different extracts in Punica granatum. Int. J. Biol. Med. Res. 2012, 3, 2065–2067. [Google Scholar]
- Fernandes, M.R.V.; Dias, A.L.T.; Carvalho, R.R.; Souza, C.R.F.; Oliveira, W.P. Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. Ind. Crop Prod. 2014, 60, 39–44. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Badami, S.; Cherian, M.M.; Hariharapura, R.C. Potent in vitro cytotoxic and antioxidant activity of Careya arborea bark extracts. Phytother. Res. 2007, 21, 492–495. [Google Scholar] [CrossRef]
- Tinker, A.C.; Wallace, A.V. Selective inhibitors of inducible nitric oxide synthase: potential agents for the treatment of inflammatory diseases? Curr. Top. Med. Chem. 2006, 6, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Jeong, S.W.; Cho, S.K.; Ahn, K.S.; Lee, J.H.; Yang, D.C.; Kim, J.C. Anti-inflammatory effects of an ethanolic extract of guava (Psidium guajava L.) leaves in vitro and in vivo. J. Med. Food 2014, 17, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Berköz, M.; Allahverdiyev, O. Punicalagin isolated from Punica granatum husk can decrease the inflammatory response in RAW 264.7 macrophages. East. J. Med. 2017, 22, 57–64. [Google Scholar]
- Glauser, M.P. The inflammatory cytokines. New developments in the pathophysiology and treatment of septic shock. Drugs 1996, 52 (Suppl. 2), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Ham, Y.M.; Yoo, B.S.; Moon, J.Y.; Koh, J.; Hyun, C.G. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J. Biosci. Bioeng. 2009, 107, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bera, E.; Bhattacharya, S.; Biswas, M. Evaluation of acute anti-inflammatory activity of Psidium guajava leaf extracts in Wistar albino rats. J. Adv. Pharm. Educ. Res. 2013, 3. [Google Scholar]
- Pinheiro, A.J.M.C.R.; Gonalves, J.S.; Dourado, A.W.A.; de Sousa, E.M.; Brito, N.M.; Silva, L.K.; Batista, M.C.A.; de Sá, J.C.; Monteiro, C.R.A.; Soares, E.; et al. Punica granatum L. Leaf Extract Attenuates Lung Inflammation in Mice with Acute Lung Injury. J. Immunol. Res. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
| Scientific Name | Used Part | Polyphenols | Tannins | Leuco-Anthocyanins |
|---|---|---|---|---|
| Punica granatum L. | Leaf | † | † | − |
| Psidium guajava L. | Leaf | † | † | − |
| Careya arborea Roxb. | Bark | † | † | † |
| Gochnatia decora (Kurz) Cabr. | Bark | † | − | − |
| Shorea obtusa Wall. ex Blume | Bark | † | † | − |
| Ficus hispida L.f. | Bark | † | − | † |
| Medicinal Herbs | TPC (mg GAE/g Extract) | TFC (mg CAE/g Extract) | ||
|---|---|---|---|---|
| EtOH | Water | EtOH | Water | |
| P. granatum/leaf | 410.04 ± 2.06 e | 235.79 ± 0.89 c | 23.39 ± 2.10 b | 13.06 ± 1.74 a |
| P. guajava/leaf | 315.77 ± 1.41 d | 274.78 ± 9.51 d | 90.83 ± 1.15 d | 59.58 ± 3.56 c |
| C. arborea/bark | 252.34 ± 11.88 c | 208.58 ± 10.36 c | 27.32 ± 1.52 b | 34.54 ± 5.51 b |
| G. decora/bark | 62.99 ± 4.89 a | 98.64 ± 7.17 b | 28.24 ± 3.2 b | 25.68 ± 3.08 b |
| S. obtusa/bark | 332.15 ± 24.01 d | 301.81 ± 21.36 d | 4.54 ± 1.32 a | 8.25 ± 1.39 a |
| F. hispida/bark | 151.49 ± 5.70 b | 58.91 ± 4.13 a | 77.45 ± 4.51 c | 26.28 ± 0.80 b |
| Medicinal Herbs | IC50 (µg/ml) | |
|---|---|---|
| DPPH Assay | ABTS Assay | |
| P. granatum/leaf | 8.72 ± 0.64 b | 2.21 ± 0.01 a |
| P. guajava/leaf | 11.62 ± 0.49 b | 3.77 ± 0.16 c |
| C. arborea/bark | 10.15 ± 0.05 b | 2.75 ± 0.08 b |
| G. decora/bark | 94.21 ± 3.12 e | 20.52 ± 0.56 e |
| S. obtusa/bark | 19.75 ± 0.45 c | 2.85 ± 0.03 b |
| F. hispida/bark | 46.88 ± 0.82 d | 10.00 ± 0.04 d |
| Ascorbic acid | 6.81 ± 0.01 a | 2.44 ± 0.10 a |
| Trolox | 8.83 ± 0.07 b | 3.42 ± 0.01 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phromnoi, K.; Sinchaiyakij, P.; Khanaree, C.; Nuntaboon, P.; Chanwikrai, Y.; Chaiwangsri, T.; Suttajit, M. Anti-Inflammatory and Antioxidant Activities of Medicinal Plants Used by Traditional Healers for Antiulcer Treatment. Sci. Pharm. 2019, 87, 22. https://doi.org/10.3390/scipharm87030022
Phromnoi K, Sinchaiyakij P, Khanaree C, Nuntaboon P, Chanwikrai Y, Chaiwangsri T, Suttajit M. Anti-Inflammatory and Antioxidant Activities of Medicinal Plants Used by Traditional Healers for Antiulcer Treatment. Scientia Pharmaceutica. 2019; 87(3):22. https://doi.org/10.3390/scipharm87030022
Chicago/Turabian StylePhromnoi, Kanokkarn, Puksiri Sinchaiyakij, Chakkrit Khanaree, Piyawan Nuntaboon, Yupa Chanwikrai, Thida Chaiwangsri, and Maitree Suttajit. 2019. "Anti-Inflammatory and Antioxidant Activities of Medicinal Plants Used by Traditional Healers for Antiulcer Treatment" Scientia Pharmaceutica 87, no. 3: 22. https://doi.org/10.3390/scipharm87030022
APA StylePhromnoi, K., Sinchaiyakij, P., Khanaree, C., Nuntaboon, P., Chanwikrai, Y., Chaiwangsri, T., & Suttajit, M. (2019). Anti-Inflammatory and Antioxidant Activities of Medicinal Plants Used by Traditional Healers for Antiulcer Treatment. Scientia Pharmaceutica, 87(3), 22. https://doi.org/10.3390/scipharm87030022





