Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and Fractionation of Agrimonia pilosa Ledeb. Extract
2.3. Isolation of Single Components from Agrimonia pilosa Ledeb. Extract
2.4. Experimental Animals
2.5. Production of MSU-Induced Gout Pain Model
2.6. Von-Frey Test
2.7. Acetic Acid-Induced Writhing and Intraplantar Formalin Tests
2.8. Spinal Pretreatment of Antagonists
2.9. Protein Extraction and Western Blot
2.10. Drugs
2.11. Statistical Analysis
3. Results
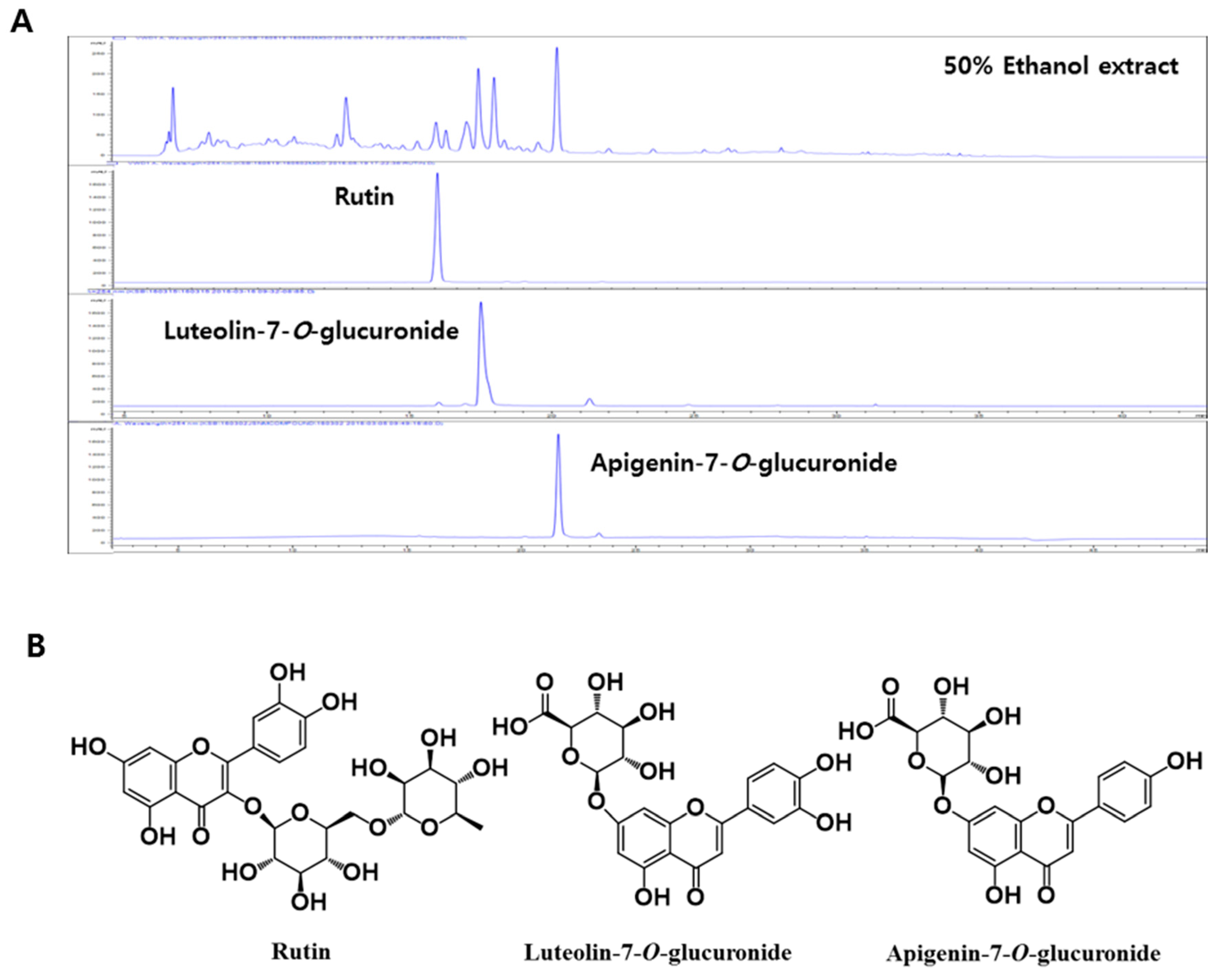
3.1. Identification of Isolated Compounds from Agrimonia pilosa Ledeb. Extract
3.2. Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract on Mechanical Pain Threshold in MSU-Treated Pain Model
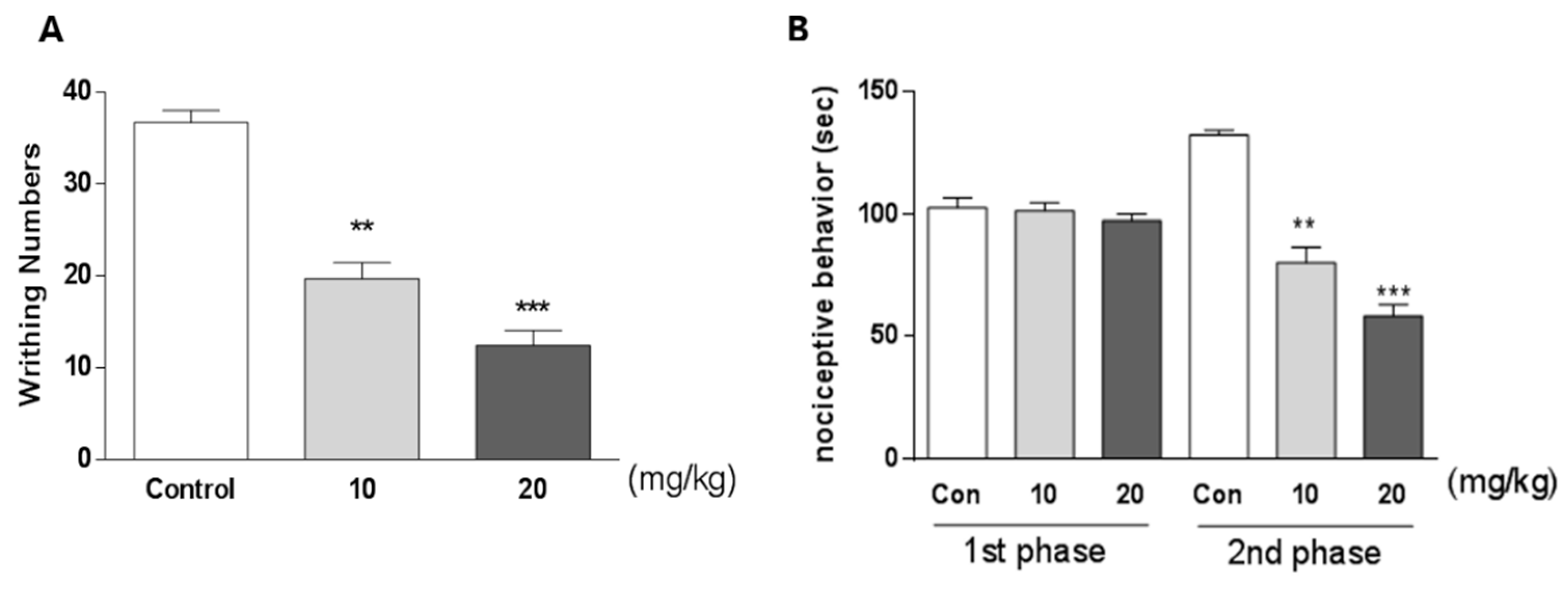
3.3. Effect of Apigenin-7-O-Glucuronide on Pain Behavior in Writhing and Formalin Tests
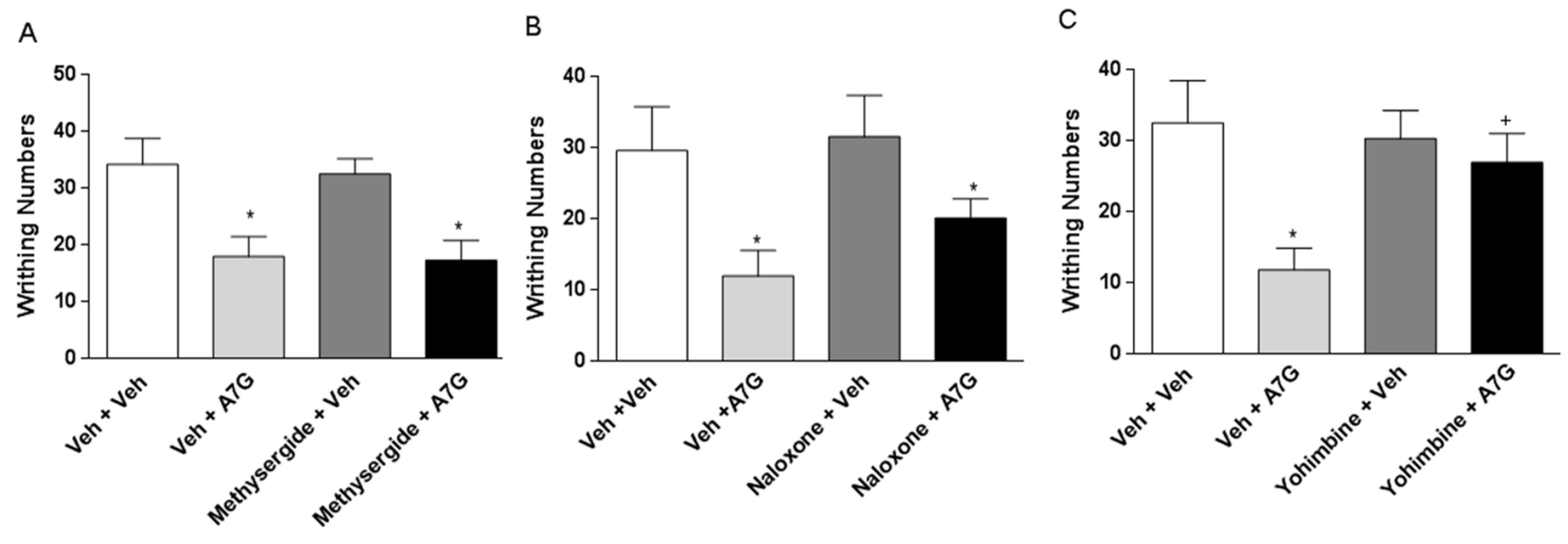
3.4. Effect of Serotonergic, Adrenergic, and Opioidergic System on the Inhibition of Writhing Response Induced by Apigenin-7-O-Glucuronide Administered Orally
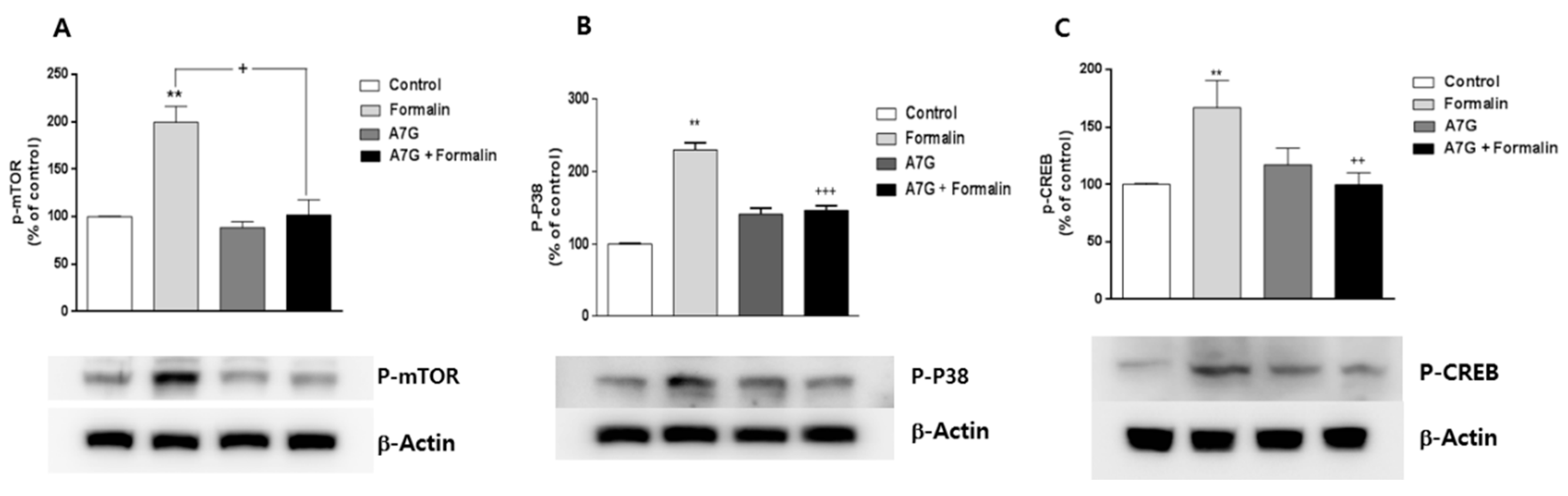
3.5. Changes of Phosphorylated mTOR, P38, and CREB Proteins in the Spinal Cord by Apigenin-7-O-Glucuronide in the Formalin Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kato, H.; Li, W.; Koike, M.; Wang, Y.; Koike, K. Phenolic glycosides from Agrimonia pilosa. Phytochemistry 2010, 71, 1925–1929. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kishi, N.; Koshiura, R. Antitumor effect of agrimoniin, a tannin of Agrimonia pilosa Ledeb., on transplantable rodent tumors. Jpn. J. Pharm. 1987, 43, 187–195. [Google Scholar] [CrossRef]
- Koshiura, R.; Miyamoto, K.; Ikeya, Y.; Taguchi, H. Antitumor activity of methanol extract from roots of Agrimonia pilosa Ledeb. Jpn. J. Pharm. 1985, 38, 9–16. [Google Scholar] [CrossRef]
- Shin, W.J.; Lee, K.H.; Park, M.H.; Seong, B.L. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol. Immunol. 2010, 54, 11–19. [Google Scholar] [CrossRef]
- Li, Y.; Ooi, L.S.; Wang, H.; But, P.P.; Ooi, V.E. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother. Res. 2004, 18, 718–722. [Google Scholar] [CrossRef]
- Zhu, L.; Tan, J.; Wang, B.; He, R.; Liu, Y.; Zheng, C. Antioxidant activities of aqueous extract from Agrimonia pilosa Ledeb and its fractions. Chem. Biodivers. 2009, 6, 1716–1726. [Google Scholar] [CrossRef]
- Yamaki, M.; Kashihara, M.; Ishiguro, K.; Takagi, S. Antimicrobial Principles of Xian he cao (Agrimonia pilosa). Planta Medica 1989, 55, 169–170. [Google Scholar] [CrossRef]
- Jung, C.H.; Zhou, S.; Ding, G.X.; Kim, J.H.; Hong, M.H.; Shin, Y.C.; Kim, G.J.; Ko, S.G. Antihyperglycemic activity of herb extracts on streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2006, 70, 2556–2559. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, J.H.; Park, S.; Kweon, D.H.; Kim, S.H.; Ko, S.G. Inhibitory effect of Agrimonia pilosa Ledeb. on inflammation by suppression of iNOS and ROS production. Immunol. Investig. 2010, 39, 159–170. [Google Scholar] [CrossRef]
- Bae, H.; Kim, H.J.; Shin, M.; Lee, H.; Yin, C.S.; Ra, J.; Kim, J. Inhibitory effect of Agrimoniae Herba on lipopolysaccharide-induced nitric oxide and proinflammatory cytokine production in BV2 microglial cells. Neurol. Research 2010, 32 (Suppl. 1), 53–57. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Kang, Y.J.; Lee, J.K.; Lim, S.S.; Suh, H.W. Effect of Agrimonia pilosa Ledeb Extract on the Antinociception and Mechanisms in Mouse. Korean J. Physiol. Pharmacol. 2012, 16, 119–123. [Google Scholar] [CrossRef]
- Meotti, F.C.; Posser, T.; Missau, F.C.; Pizzolatti, M.G.; Leal, R.B.; Santos, A.R. Involvement of p38MAPK on the antinociceptive action of myricitrin in mice. Biochem. Pharmacol. 2007, 74, 924–931. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Dun, L.; Xu, Y.; Jin, S.; Du, J.; Ma, L.; Li, J.; Zhou, R.; He, X. Antinociceptive effects of oxymatrine from Sophora flavescens, through regulation of NR2B-containing NMDA receptor-ERK/CREB signaling in a mice model of neuropathic pain. Phytomedicine 2013, 20, 1039–1045. [Google Scholar] [CrossRef]
- Asante, C.O.; Wallace, V.C.; Dickenson, A.H. Formalin-induced behavioral hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol. Pain 2009, 5, 27. [Google Scholar] [CrossRef]
- Lisi, L.; Aceto, P.; Navarra, P.; Dello Russo, C. mTOR kinase: A possible pharmacological target in the management of chronic pain. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Obara, I.; Tochiki, K.K.; Géranton, S.M.; Carr, F.B.; Lumb, B.M.; Liu, Q.; Hunt, S.P. Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice. Pain 2011, 152, 2582–2595. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 2014, 10, 26. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; Beer, D.E.J. Acetic acid for analgesic screening. Proc. Soc. Exp. Biol. Med. 1959, 18, 412–415. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Wang, M.; Kikuzaki, H.; Csiszar, K.; Boyd, C.D.; Maunakea, A.; Fong, S.F.; Ghai, G.; Rosen, R.T.; Nakatani, N.; Ho, C.T. Novel trisaccharide fatty acid ester identified from the fruits of Morinda citrifolia (Noni). J. Agric. Food Chem. 1999, 47, 4880–4882. [Google Scholar] [CrossRef]
- Ringl, A.; Prinz, S.; Huefner, A.; Kurzmann, M.; Kopp, B. Chemosystematic value of flavonoids from Crataegus × macrocarpa (Rosaceae) with special emphasis on (R)- and (S)-eriodictyol-7-O-glucuronide and luteolin-7-O-glucuronide. Chem. Biodivers. 2007, 4, 154–162. [Google Scholar] [CrossRef]
- Baris, O.; Karadayi, M.; Yanmis, D.; Guvenalp, Z.; Bal, T.; Gulluce, M. Isolation of 3 flavonoids from Mentha longifolia (L.) Hudson subsp. longifolia and determination of their genotoxic potentials by using the E. coli WP2 test system. J. Food Sci. 2011, 76, T212–T217. [Google Scholar] [CrossRef]
- Dalbeth, N.; Haskard, D. Mechanisms of inflammation in gout. Rheumatology 2005, 44, 1090–1096. [Google Scholar] [CrossRef]
- Marcotti, A.; Miralles, A.; Dominguez, E.; Pascual, E.; Gomis, A.; Belmonte, C.; de la Peña, E. Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan. Pain 2018, 159, 739. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, M. Antibacterial activity and mechanism of luteolin on Staphylococcus aureus. Acta Microbiologica Sinica 2010, 50, 1180–1184. [Google Scholar]
- Gao, S.; Hu, M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Hara, K.; Haranishi, Y.; Terada, T.; Takahashi, Y.; Nakamura, M.; Sata, T. Effects of intrathecal and intracerebroventricular administration of luteolin in a rat neuropathic pain model. Pharmacol. Biochem. Behav. 2014, 125, 78–84. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.; Wong, D.V.; Lima-Junior, R.C.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 53. [Google Scholar] [CrossRef]
- Jeyadevi, R.; Sivasudha, T.; Rameshkumar, A.; Dinesh Kumar, L. Anti-arthritic activity of the Indian leafy vegetable Cardiospermum halicacabum in Wistar rats and UPLC-QTOF-MS/MS identification of the putative active phenolic components. Inflamm. Res. 2013, 62, 115–126. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Shim, E.-J.; Seo, Y.-J.; Choi, S.-S.; Lee, J.-Y.; Lee, H.-K.; Suh, H.-W. Effect of aspirin and acetaminophen on proinflammatory cytokine-induced pain behavior in mice. Pharmacology 2005, 74, 152–156. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Wilson, P.R. Spinal serotonin terminal system mediates antinociception. J. Pharmacol. Exp. Ther. 1979, 208, 446–453. [Google Scholar]
- Jensen, T.S.; Yaksh, T.L. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984, 321, 287–297. [Google Scholar] [CrossRef]
- Asante, C.O.; Wallace, V.C.; Dickenson, A.H. Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J. Pain 2010, 11, 1356–1367. [Google Scholar] [CrossRef]
- Xu, J.-T.; Xin, W.-J.; Wei, X.-H.; Wu, C.-Y.; Ge, Y.-X.; Liu, Y.-L.; Zang, Y.; Zhang, T.; Li, Y.-Y.; Liu, X.-G. p38 activation in uninjured primary afferent neurons and in spinal microglia contributes to the development of neuropathic pain induced by selective motor fiber injury. Exp. Neurol. 2007, 204, 355–365. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ishida, M.; Arita, J.; Mitsushima, D.; Takahashi, T.; Kimura, F.; Funabashi, T. The cAMP response element-binding protein in the bed nucleus of the stria terminalis modulates the formalin-induced pain behavior in the female rat. Eur. J. Neurosci. 2009, 30, 2379–2386. [Google Scholar] [CrossRef]
- Liang, L.; Tao, B.; Fan, L.; Yaster, M.; Zhang, Y.; Tao, Y.-X. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 2013, 1513, 17–25. [Google Scholar] [CrossRef]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.-L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-D-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.H.; Lee, H.J.; Kim, S.B.; Jung, J.S.; Lim, S.S.; Suh, H.W. Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract. Sci. Pharm. 2019, 87, 18. https://doi.org/10.3390/scipharm87030018
Feng JH, Lee HJ, Kim SB, Jung JS, Lim SS, Suh HW. Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract. Scientia Pharmaceutica. 2019; 87(3):18. https://doi.org/10.3390/scipharm87030018
Chicago/Turabian StyleFeng, Jing Hui, Hee Jung Lee, Set Byeol Kim, Jeon Sub Jung, Soon Sung Lim, and Hong Won Suh. 2019. "Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract" Scientia Pharmaceutica 87, no. 3: 18. https://doi.org/10.3390/scipharm87030018
APA StyleFeng, J. H., Lee, H. J., Kim, S. B., Jung, J. S., Lim, S. S., & Suh, H. W. (2019). Antinociceptive Effect of Single Components Isolated from Agrimonia pilosa Ledeb. Extract. Scientia Pharmaceutica, 87(3), 18. https://doi.org/10.3390/scipharm87030018





