Acute and Subchronic (28-day) Oral Toxicity Studies on the Film Formulation of k-Carrageenan and Konjac Glucomannan for Soft Capsule Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Substance Preparation
2.2. Experimental Animals
2.3. Acute Oral Toxicity Study
2.4. Subchronic Oral Toxicity Study
2.4.1. Body Weight and Clinical Observation
2.4.2. Relative Organ Weight Determination
2.4.3. Hematological Analysis
2.4.4. Biochemical Analysis
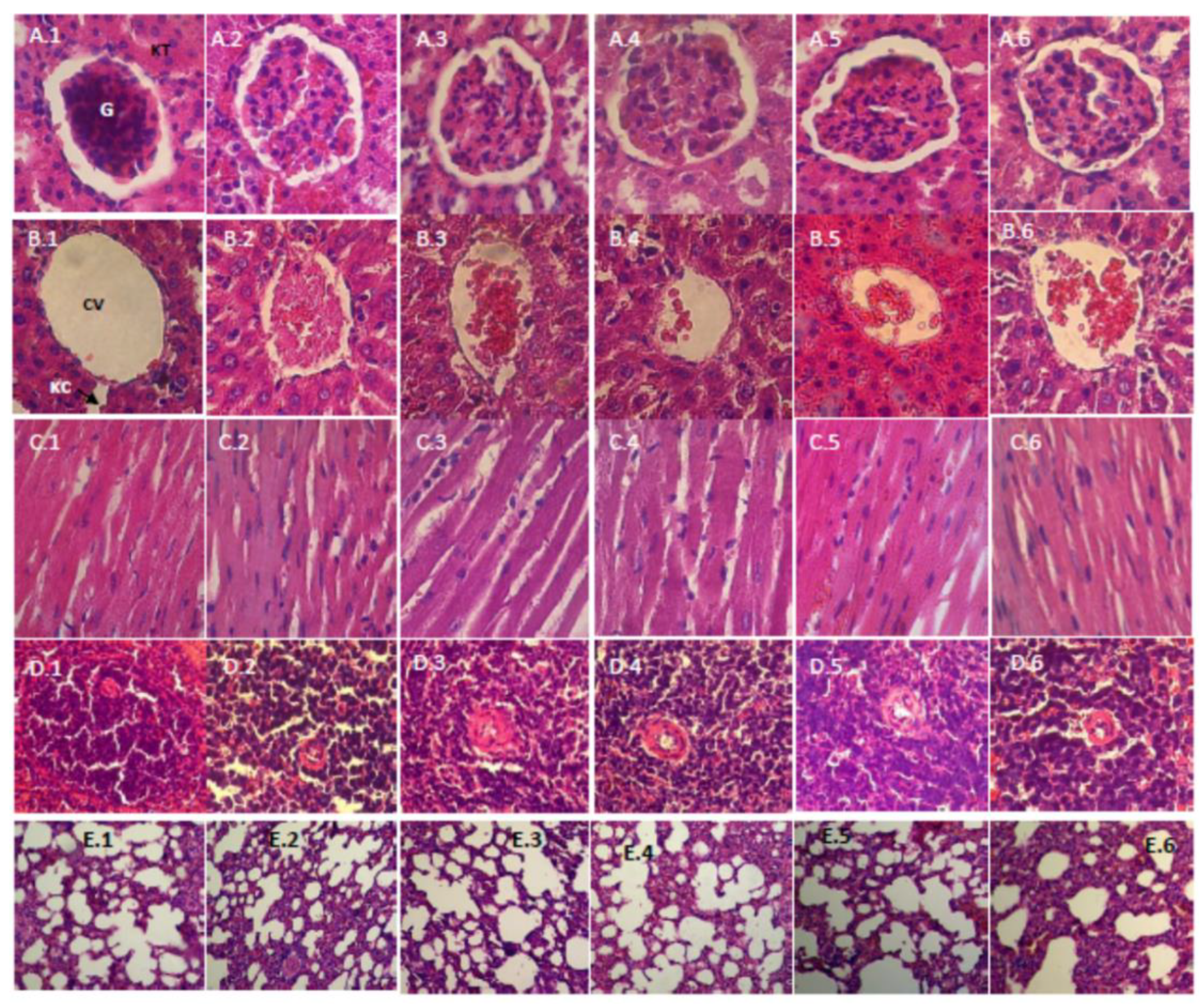
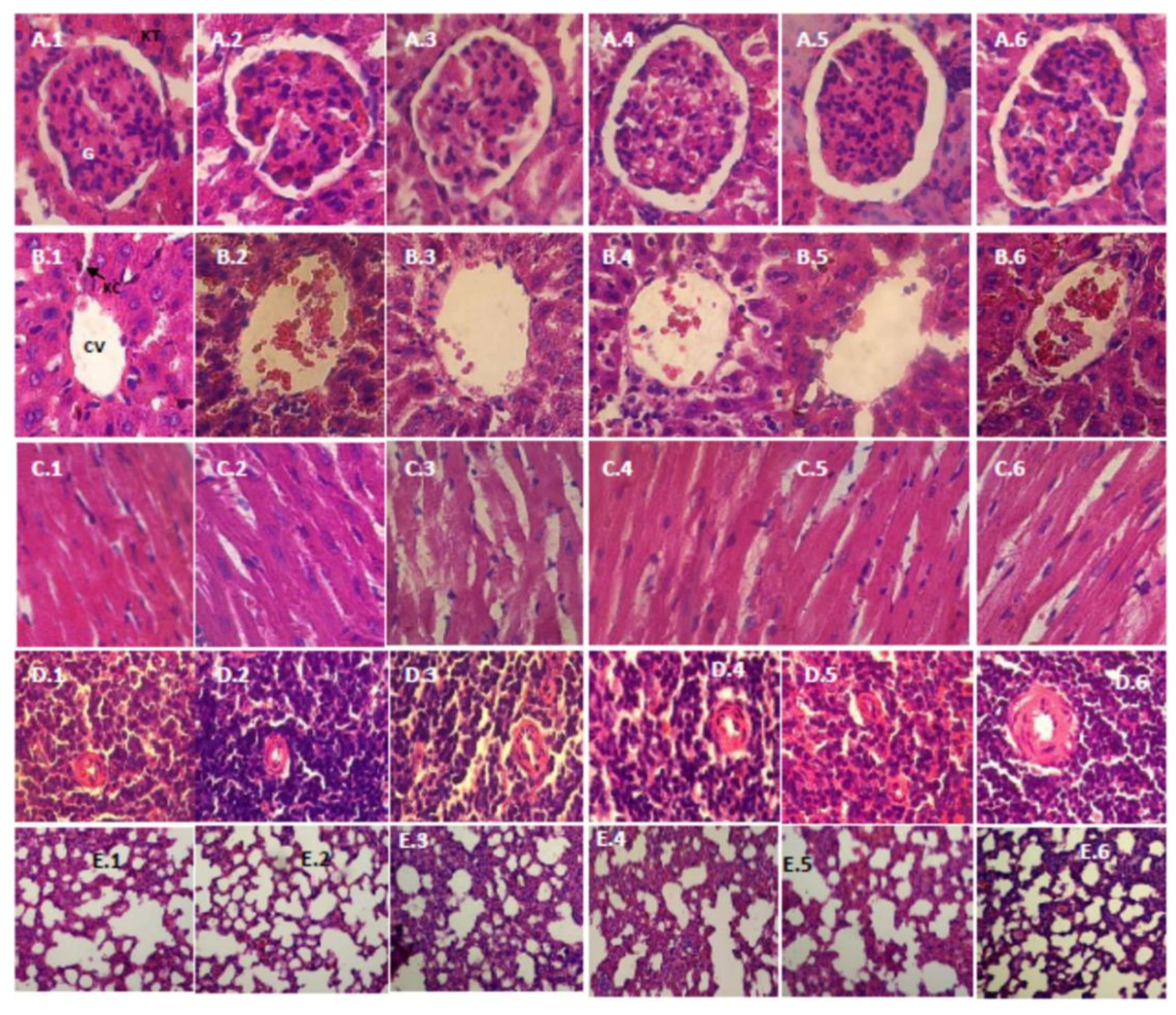
2.4.5. Histopathology Study
2.5. Statistical Analysis
3. Results
3.1. Acute Oral Toxicity Study
3.2. Subchronic 28-day Oral Toxicity Study
3.2.1. Body Weight and Clinical Observation
3.2.2. Relative Organ Weight Determination
3.2.3. Hematological Analysis
3.2.4. Biochemical Analysis
3.2.5. Histopathology Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gullapalli, R.P.; Mazzitelli, C.L. Gelatin and Non-Gelatin Capsule Dosage Forms. J. Pharm. Sci. 2017, 106, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Prosekov, A.; Petrov, A.; Ulrich, E.; Dyshlyuk, L.; Kozlova, O. Physical and Chemical Properties of Capsule Shell Based on Plant Analogues of Pharmaceutical Gelatin. Biol. Med. 2015, 7, BM-104-15. [Google Scholar]
- Brenner, t.; Wang, Z.; Achayuthakan, P.; Nakajima, T.; Nishinari, K. Rheology and synergy of κ-carrageenan/locust bean gum/konjac glucomannan gels. Carbohydr. Polym. 2013, 98, 754–760. [Google Scholar] [CrossRef]
- He, X.J.; Wang, H.X.; Amadou, I.; Qin, X.J. Textural and rheological properties of hydrolyzed Konjac Glucomannan and Kappa-Carrageenan: Effect of molecular weight, total content, pH and temperature on the mixed system gels. Emir. J. Food Agric. 2012, 24, 200–207. [Google Scholar]
- WHO. Compendium of food additive specifications. Addendum 9. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives, 57th Session, Rome, Italy, 5–14 June 2001. [Google Scholar]
- Ariffin, S.H.Z.; Yeen, W.W.; Abidin, I.Z.Z.; Wahab, R.M.A.; Ariffin, Z.Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complementary Altern. Med. 2014, 14, 1–16. [Google Scholar]
- OECD Guidelines for The Testing of Chemicals: Acute Oral Toxicity—Fixed Dose Procedure, OECD/OCDE 420. Adopted: 17th December 2001.
- OECD Guidelines for The Testing of Chemicals: Repeated Dose 28-Day Oral Toxicity Study in Rodents, OECD/OCDE 407. Adopted: 3 October 2008.
- Barham, D.; Trinder, P. An Improved Color Reagent for the Determination of Blood Glucose by the Oxidase System. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determinationof Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar]
- Mc Gowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A Peroxidase-Coupled Method for the Colorimetric Determination of Serum Triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar]
- Tietz, N.W.; Rinker, A.D.; Shaw, L.M. IFCC Methods for the measurement of catalytic concentration of enzymes, IFCC Method for Alkaline Phosphatase. J. Clin. Chem. Clin. Biochem. 1983, 21, 731–748. [Google Scholar] [PubMed]
- Bergmeyer, H.U.; Horder, M.; Rey, J. Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes, IFCC method for Alanine Aminotransferase. J. Clin. Chem. Clin. Biochem. 1986, 24, 481–495. [Google Scholar] [PubMed]
- Bartels, H.; Bohmer, M. Micro-determination of Creatinine. Clin. Chim. Acta 1971, 32, 81–85. [Google Scholar]
- Guidelines for Hematoxylin and Eosin Staining. National Society for Histotechnology, July 2001.
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Martini, F.H.; Nath, J.L.; Bartholomew, E.F. Fundamental of Anatomy and Physiology, 9th ed.; Pearson: San Fransisco, CA, USA, 2012; pp. 642–645, 776. [Google Scholar]
- Sellers, R.S.; Morton, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Scafer, K. Society of Toxicologic Pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef]
- Myers, R.P.; Tainturier, M.H.; Ratziu, V.; Piton, A.; Thibault, V.; Imbert-Bismut, F.; Messous, D.; Charlotte, F.; Di Martino, V.; Benhamou, Y.; et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J. Hepatol. 2003, 39, 222–230. [Google Scholar] [CrossRef]
- Ghabril, M.; Chalasani, N.; Björnsson, E. Drug-induced liver injury: A clinical update. Curr. Opin. Gastroenterol. 2010, 26, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical Biochemistry of Hepatotoxicity. J. Clin. Toxicol. 2011, 1, 1–19. [Google Scholar]
- Paul, S.; Hossen, M.S.; Tanvir, E.M.; Islam, A.; Afroz, A.; Ahmed, I.; Saha, M.; Siew, H.G.; Khalil, I.; et al. “Antioxidant properties of citrus macroptera fruit and its in vivo effects on the liver, kidney and pancreas in wistar rats”. Int. J. Pharmacol. 2015, 11, 899–909. [Google Scholar]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Acute renal failure. Lancet 2005, 365, 417–430. [Google Scholar] [CrossRef]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Shen, G.X. Lipid disorders in diabetes mellitus and current management. Curr. Pharm. Anal. 2007, 3, 17–24. [Google Scholar] [CrossRef]
- Sood, N.; Baker, W.L.; Coleman, C.I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2008, 88, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.L.; Everds, N.E. Principles of clinical pathology for toxicity studies. In Principles and Methods of Toxicology, 5th ed.; Hayes, W.A., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1342–1348. [Google Scholar]
- Wua, J.; Deng, X.; Lin, X. Swelling characteristics of konjac glucomannan superabsobent synthesized by radiation-induced graft copolymerization. Radiat. Phys. Chem. 2013, 83, 90–97. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Zhao, X.; Xu, C.; Zhou, Y.; Peng, J. Soluble fiber with hing water-binding capacity, swelling capacity, and fermentability reduces food intake by promoting satiety rather than satiation in rats. Nutriens 2016, 8, 1–15. [Google Scholar]
- Chearskul, S.; Sangurai, S.; Nitiyanant, W.; Kriengsinyos, W.; Kooptiwut, S.; Harindhanavudhi, T. Glycemic and lipid responses to glucomannan in Thais with type 2 diabetes mellitus. J. Med. Assoc. Thai. 2007, 90, 2150–2156. [Google Scholar] [PubMed]
| Organs | Groups | |||||
|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | |
| Heart | 0.39 ± 0.04 | 0.36 ± 0.03 | 0.36 ± 0.04 | 0.43 ± 0.06 | 0.36 ± 0.04 | 0.34 ± 0.03 |
| Liver | 3.00 ± 0.28 | 3.32 ± 0.17 | 2.72 ± 0.24 | 2.98 ± 0.26 | 3.28 ± 0.34 | 3.22 ± 0.18 |
| Spleen | 0.24 ± 0.02 | 0.30 ± 0.06 | 0.20 ± 0.03 | 0.24 ± 0.03 | 0.24 ± 0.10 | 0.26 ± 0.07 |
| Kidneys | 0.68 ± 0.05 | 0.70 ± 0.09 | 0.66 ± 0.05 | 0.70 ± 0.03 | 0.69 ± 0.07 | 0.66 ± 0.05 |
| Lungs | 0.69 ± 0.18 | 0.67 ± 0.13 | 0.53 ± 0.11 | 0.60 ± 0.11 | 0.74 ± 0.15 | 0.71 ± 0.08 |
| Adrenal | 0.02 ± 0.009 | 0.02 ± 0.004 | 0.03 ± 0.011 | 0.02 ± 0.008 | 0.03 ± 0.005 | 0.02 ± 0.003 |
| Testicles | 1.05 ± 0.10 | 1.17 ± 0.08 | 1.03 ± 0.11 | 1.10 ± 0.05 | 1.25 ± 0.22 | 1.04 ± 0.09 |
| Seminal vesicles | 0.50 ± 0.10 | 0.35 ± 0.06 | 0.46 ± 0.06 | 0.44 ± 0.09 | 0.28 ± 0.04 * | 0.32 ± 0.05 |
| Organs | Groups | |||||
|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | |
| Heart | 0.40 ± 0.04 | 0.36 ± 0.05 | 0.42 ± 0.06 | 0.40 ± 0.06 | 0.38 ± 0.05 | 0.38 ± 0.03 |
| Liver | 3.25 ± 0.42 | 3.43 ± 0.34 | 2.91 ± 0.27 | 2.76 ± 0.27 | 3.91 ± 0.15 * | 3.42 ± 0.31 |
| Spleen | 0.39 ± 0.14 | 0.35 ± 0.09 | 0.38 ± 0.06 | 0.44 ± 0.09 | 0.42 ± 0.16 | 0.49 ± 0.03 |
| Kidneys | 0.66 ± 0.05 | 0.68 ± 0.05 | 0.67 ± 0.04 | 0.60 ± 0.17 | 0.67 ± 0.05 | 0.64 ± 0.07 |
| Lungs | 0.74 ± 0.18 | 0.72 ± 0.13 | 0.90 ± 0.37 | 1.02 ± 0.53 | 0.83 ± 0.07 | 0.85 ± 0.14 |
| Adrenal | 0.04 ± 0.010 | 0.04 ± 0.002 | 0.03 ± 0.008 | 0.04 ± 0.012 | 0.04 ± 0.005 | 0.04 ± 0.004 |
| Ovaries | 0.06 ± 0.015 | 0.06 ± 0.014 | 0.07 ± 0.014 | 0.05 ± 0.017 | 0.07 ± 0.007 | 0.07 ± 0.006 |
| Uterus | 0.22 ± 0.03 | 0.24 ± 0.10 | 0.23 ± 0.07 | 0.20 ± 0.03 | 0.21 ± 0.07 | 0.26 ± 0.09 |
| Parameter | Unit | Groups | |||||
|---|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | ||
| White blood cell counts | 103/mm3 | 8.72 ± 1.78 | 8.90 ± 2.34 | 5.60 ± 1.02 | 8.72 ± 3.01 | 6.00 ± 1.65 | 10.56 ± 2.33 |
| Hemoglobin (Hb) | g/dL | 8.40 ± 0.75 | 8.90 ± 1.32 | 8.16 ± 0.54 | 8.72 ± 0.59 | 9.04 ± 0.96 | 9.12 ± 0.59 |
| Red blood cell counts | 106/mm3 | 5.06 ± 0.93 | 4.62 ± 0.63 | 5.41 ± 0.73 | 6.48 ± 1.39 | 4.53 ± 0.60 | 5.07 ± 0.44 |
| Mean corpuscular volume (MCV) | fL | 37.04 ± 0.60 | 37.20 ± 0.41 | 37.27 ± 0.21 | 37.33 ± 0.58 | 36.42 ± 0.49 | 37.22 ± 0.47 |
| Mean corpuscular hemoglobin (MCH) | pg/sel | 16.95 ± 2.55 | 19.28 ± 1.18 | 15.25 ± 1.83 | 14.08 ± 3.78 | 20.02 ± 0.63 | 18.03 ± 1.06 |
| Mean corpuscular hemoglobin concentration (MCHC) | g/dL | 45.79 ± 7.11 | 51.85 ± 3.56 | 40.91 ± 4.85 | 37.67 ± 9.70 | 54.98 ± 1.87 | 48.47 ± 3.32 |
| Hematocrit | % | 37.44 ± 6.85 | 34.40 ± 4.98 | 40.32 ± 5.47 | 48.32 ± 10.21 | 32.96 ± 4.17 | 37.76 ± 3.32 |
| Platelet | 103/mL | 536.00 ± 106.73 | 509.25 ± 74.00 | 519.60 ± 43.64 | 556.33 ± 135.88 | 609.80 ± 81.23 | 543.80 ± 58.26 |
| Parameter | Unit | Groups | |||||
|---|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | ||
| White blood cell counts | 103/mm3 | 9.68 ± 2.46 | 7.30 ± 1.89 | 12.24 ± 4.54 | 10.72 ± 3.81 | 6.00 ± 1.17 | 6.88 ± 2.67 |
| Hemoglobin (Hb) | g/dL | 7.36 ± 1.08 | 8.90 ± 0.38 | 8.40 ± 0.57 | 8.40 ± 0.63 | 7.84 ± 1.08 | 8.48 ± 0.52 |
| Red blood cell counts | 106/mm3 | 3.89 ± 0.77 | 5.28 ± 0.81 | 4.69 ± 0.54 | 4.13 ± 0.64 | 5.17 ± 0.64 | 5.62 ± 1.37 |
| Mean corpuscular volume (MCV) | fL | 36.62 ± 0.80 | 37.89 ± 0.70 | 37.20 ± 0.75 | 37.20 ± 0.33 | 37.44 ± 0.69 | 36.34 ± 1.15 |
| Mean corpuscular hemoglobin (MCH) | pg/sel | 19.41 ± 3.65 | 17.10 ± 2.15 | 18.05 ± 1.67 | 20.56 ± 1.87 | 15.47 ± 3.39 | 15.78 ± 3.55 |
| Mean corpuscular hemoglobin concentration (MCHC) | g/dL | 53.08 ± 10.33 | 45.12 ± 5.41 | 48.49 ± 4.08 | 55.29 ± 5.31 | 41.33 ± 9.14 | 43.25 ± 8.65 |
| Hematocrit | % | 28.48 ± 5.81 | 40.00 ± 6.13 | 34.88 ± 4.14 | 30.72 ± 4.85 | 38.72 ± 5.11 | 40.64 ± 9.09 |
| Platelet | 103/mL | 548.75 ± 63.62 | 397.75 ± 66.53 | 551.20 ± 56.92 | 591.20 ± 68.43 | 492.00 ± 31.77 | 413.67 ± 90.84 |
| Parameter | Unit | Groups | |||||
|---|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | ||
| Glucose | mg/dL | 117.80 ± 34.30 | 168.25 ± 19.53 | 77.00 ± 18.21 * | 85.00 ± 13.02 | 109.00 ± 9.03 | 141.40 ± 16.56 |
| Cholesterol | mg/dL | 95.40 ± 13.43 | 88.75 ± 4.03 | 87.00 ± 5.48 | 77.60 ± 9.50 | 71.40 ± 13.05 | 87.20 ± 6.46 |
| Triglyceride | mg/dL | 40.80 ± 9.78 | 53.55 ± 3.12 | 48.60 ± 3.29 | 51.00 ± 5.61 | 82.60 ± 19.88 * | 76.60 ± 8.79 * |
| Aspartate amino transferase (AST) | U/L | 209.60 ± 30.88 | 259.75 ± 13.38 | 231.20 ± 30.74 | 269.60 ± 7.93 * | 238.20 ± 18.75 | 258.60 ± 1.95 |
| Alanine amino transferaase (ALT) | U/L | 105.40 ± 27.66 | 113.50 ± 11.03 | 106.80 ± 15.43 | 118.60 ± 13.69 | 122.20 ± 10.71 | 111.80 ± 16.04 |
| Urea | mg/dL | 46.24 ± 13.16 | 53.55 ± 3.12 | 42.76 ± 2.92 | 51.40 ± 10.69 | 45.56 ± 10.72 | 48.86 ± 1.41 |
| Creatinine | mg/dL | 0.90 ± 0.21 | 0.51 ± 0.12 | 0.59 ± 0.24 | 0.61 ± 0.25 | 1.00 ± 0.11 | 0.53 ± 0.15 |
| Parameter | Unit | Groups | |||||
|---|---|---|---|---|---|---|---|
| Control | Satellite Control | 10 mg/kg | 30 mg/kg | 75 mg/kg | Satellite 75 mg/kg | ||
| Glucose | mg/dL | 118.20 ± 14.52 | 119.25 ± 27.69 | 96.40 ± 24.15 | 105.80 ± 25.64 | 161.40 ± 26.66 | 107.20 ± 17.24 |
| Cholesterol | mg/dL | 122.40 ± 29.71 | 94.50 ± 15.33 | 99.20 ± 29.39 | 109.80 ± 18.75 | 96.60 ± 15.14 | 92.00 ± 8.12 |
| Triglyceride | mg/dL | 53.80 ± 6.02 | 40.25 ± 4.57 | 44.40 ± 4.22 | 30.40 ± 6.19 * | 69.80 ± 9.96 * | 40.40 ± 12.44 |
| Aspartate amino transferase (AST) | U/L | 159.20 ± 9.09 | 293.00 ± 23.92 | 195.80 ± 38.62 | 205.60 ± 31.37 | 242.40 ± 16.80 * | 278.00 ± 22.67 |
| Alanine amino transferaase (ALT) | U/L | 104.60 ± 17.26 | 93.00 ± 23.40 | 97.00 ± 12.27 | 87.20 ± 10.64 | 120.80 ± 25.06 | 81.40 ± 21.22 |
| Urea | mg/dL | 48.24 ± 5.22 | 39.80 ± 6.59 | 44.06 ± 9.15 | 35.86 ± 2.77 * | 46.82 ± 6.40 | 42.12 ± 4.97 |
| Creatinine | mg/dL | 0.42 ± 0.18 | 0.41 ± 0.04 | 0.57 ± 0.18 | 0.71 ± 0.22 | 1.12 ± 0.10 * | 0.42 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutrisni, N.N.W.; Soewandhi, S.N.; Adnyana, I.K.; Sasongko, L.D.N. Acute and Subchronic (28-day) Oral Toxicity Studies on the Film Formulation of k-Carrageenan and Konjac Glucomannan for Soft Capsule Application. Sci. Pharm. 2019, 87, 9. https://doi.org/10.3390/scipharm87020009
Sutrisni NNW, Soewandhi SN, Adnyana IK, Sasongko LDN. Acute and Subchronic (28-day) Oral Toxicity Studies on the Film Formulation of k-Carrageenan and Konjac Glucomannan for Soft Capsule Application. Scientia Pharmaceutica. 2019; 87(2):9. https://doi.org/10.3390/scipharm87020009
Chicago/Turabian StyleSutrisni, Ni Nyoman Wiwik, Sundani Nurono Soewandhi, I Ketut Adnyana, and Lucy D N Sasongko. 2019. "Acute and Subchronic (28-day) Oral Toxicity Studies on the Film Formulation of k-Carrageenan and Konjac Glucomannan for Soft Capsule Application" Scientia Pharmaceutica 87, no. 2: 9. https://doi.org/10.3390/scipharm87020009
APA StyleSutrisni, N. N. W., Soewandhi, S. N., Adnyana, I. K., & Sasongko, L. D. N. (2019). Acute and Subchronic (28-day) Oral Toxicity Studies on the Film Formulation of k-Carrageenan and Konjac Glucomannan for Soft Capsule Application. Scientia Pharmaceutica, 87(2), 9. https://doi.org/10.3390/scipharm87020009




